Abstract
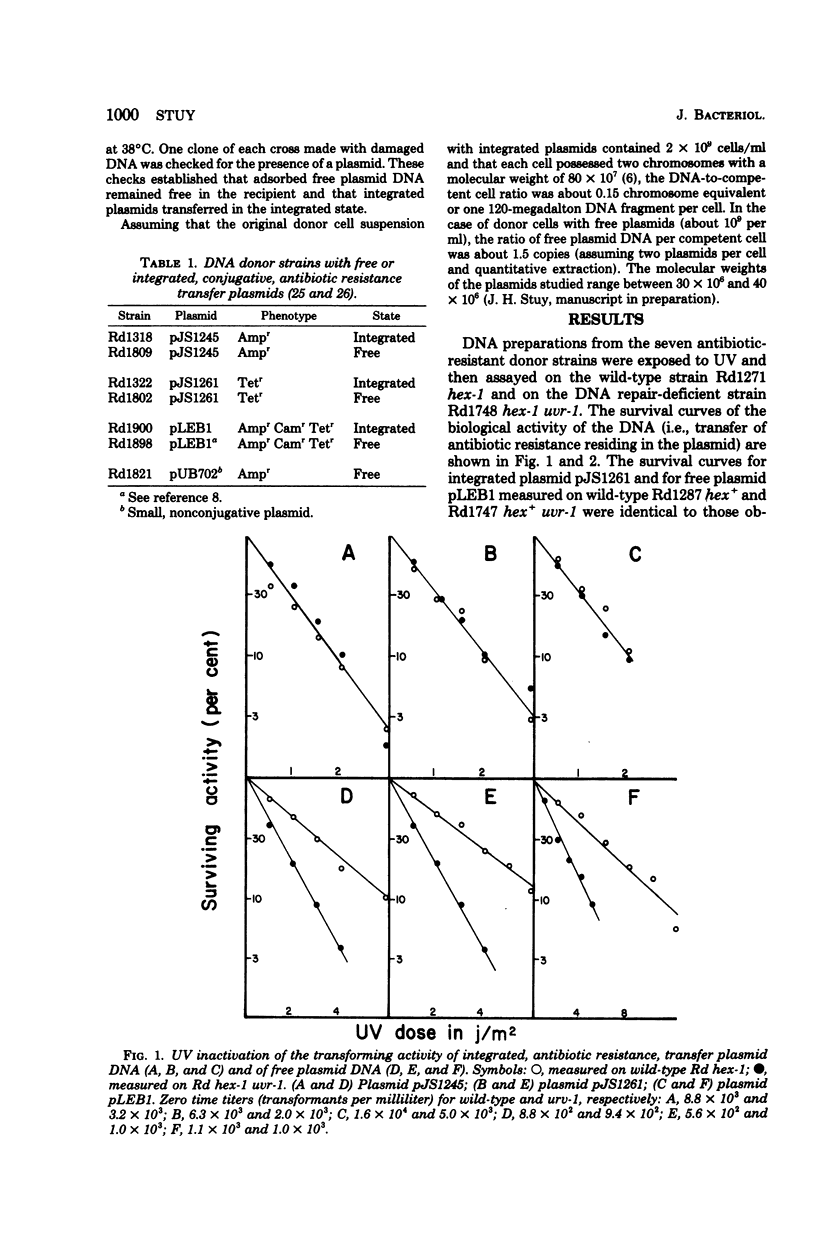
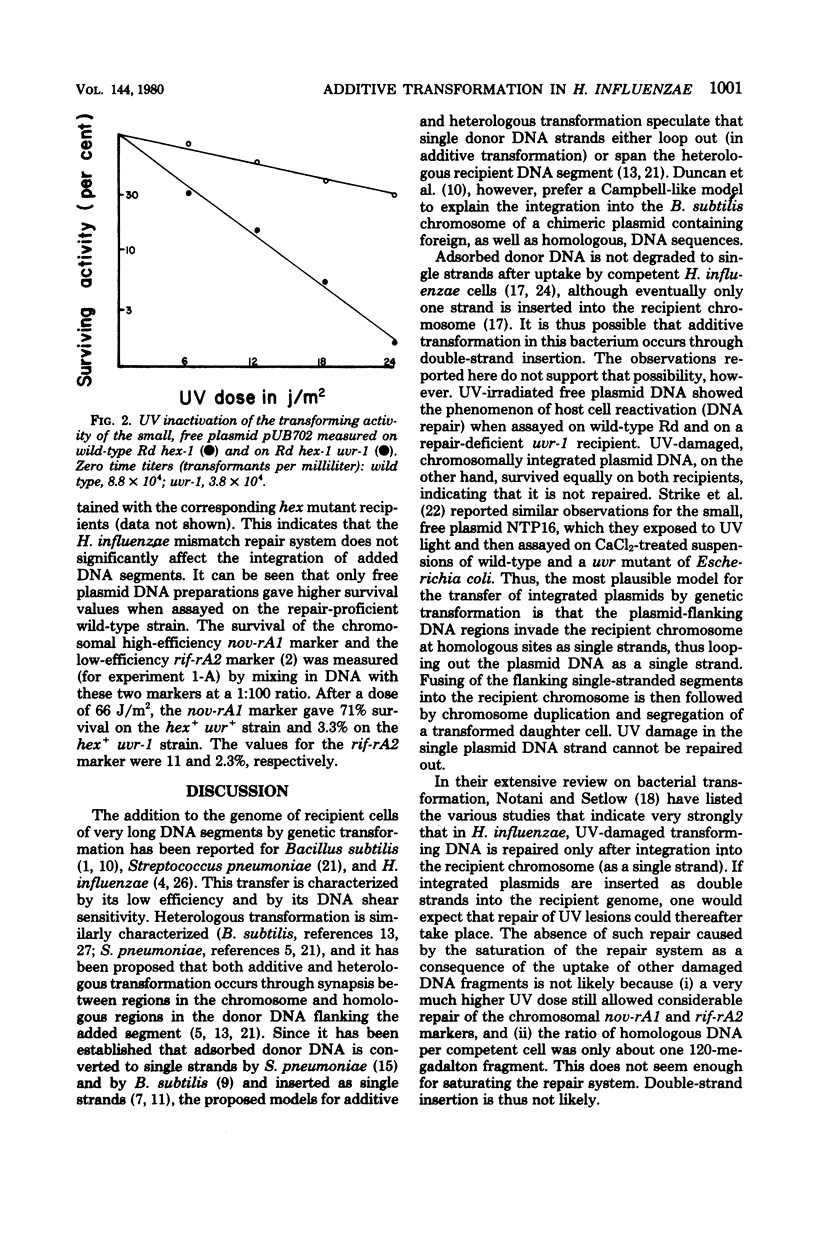
Transforming deoxyribonucleic acid (DNA) preparations from Haemophilus influenzae Rd strains carrying a chromosomally integrated, conjugative, antibiotic resistance transfer (R) plasmid were exposed to ultraviolet radiation and then assayed for antibiotic resistance transfer on sensitive wild-type Rd competent suspensions and on similar suspensions of a uvr-1 mutant unable to excise pyrimidine dimers. No host cell reactivation of resistance transfer (DNA repair) was observed. Parallel experiments with ethanol-precipitated, heated, free R plasmid DNA preparations gave much higher survival when assayed on the wild-type strain compared to the survival on the uvr-1 strain. These observations indicate that additive genetic transformation (in this case, the addition of the integrated R plasmid to the recipient genome) involves single-strand insertion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. Transformation and transduction of a large deletion mutation in Bacillus subtilis. Mol Gen Genet. 1972;118(4):311–320. doi: 10.1007/BF00333566. [DOI] [PubMed] [Google Scholar]
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- BODMER W. F., GANESAN A. T. BIOCHEMICAL AND GENETIC STUDIES OF INTEGRATION AND RECOMBINATION IN BACILLUS SUBTILIS TRANSFORMATION. Genetics. 1964 Oct;50:717–738. doi: 10.1093/genetics/50.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagci H., Stuy J. H. A hex mutant of Haemophilus influenzae. Mol Gen Genet. 1979 Sep;175(2):175–179. doi: 10.1007/BF00425533. [DOI] [PubMed] [Google Scholar]
- Barnhart B. J., Cox S. H. Radiation-sensitive and radiation-resistant mutants of Haemophilus influenzae. J Bacteriol. 1968 Jul;96(1):280–282. doi: 10.1128/jb.96.1.280-282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendler J. W., 3rd Physical size of the donor locus and transmission of Haemophilus influenzae ampicillin resistance genes by deoxyribonucleic acid-mediated transformation. J Bacteriol. 1976 Jan;125(1):197–204. doi: 10.1128/jb.125.1.197-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E. Homology in capsular transformation reactions in Pneumococcus. Mol Gen Genet. 1972;116(1):68–83. doi: 10.1007/BF00334261. [DOI] [PubMed] [Google Scholar]
- Bryan L. E. Transferable chloramphenicol and ampicillin resistance in a strain of Haemophilus influenzae. Antimicrob Agents Chemother. 1978 Jul;14(1):154–156. doi: 10.1128/aac.14.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Conditions affecting the isolation from transformed cells of Bacillus subtilis of high-molecular-weight single-stranded deoxyribonucleic acid of donor origin. J Bacteriol. 1973 Oct;116(1):146–153. doi: 10.1128/jb.116.1.146-153.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. H., Wilson G. A., Young F. E. Mechanism of integrating foreign DNA during transformation of Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3664–3668. doi: 10.1073/pnas.75.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Lederberg J. Interspecies transformation in Bacillus: mechanism of heterologous intergenote transformation. J Bacteriol. 1978 Mar;133(3):1246–1253. doi: 10.1128/jb.133.3.1246-1253.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., Setlow J. K. Similarity in properties and mapping of three Rec mutants of Haemophilus influenzae. J Bacteriol. 1976 Jul;127(1):327–333. doi: 10.1128/jb.127.1.327-333.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K. Mechanism of bacterial transformation and transfection. Prog Nucleic Acid Res Mol Biol. 1974;14(0):39–100. doi: 10.1016/s0079-6603(08)60205-6. [DOI] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUY J. H. Transformability of Haemophilus influenzae. J Gen Microbiol. 1962 Nov;29:537–549. doi: 10.1099/00221287-29-3-537. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E. Bacteriophage of Haemophilus influenzae. II. Repair of ultraviolet-irradiated phage DNA and the capacity of irradiated cells to make phage. J Mol Biol. 1972 Feb 14;63(3):349–362. doi: 10.1016/0022-2836(72)90432-9. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. Organization and transfer of heterologous chloramphenicol and tetracycline resistance genes in pneumococcus. J Bacteriol. 1979 Aug;139(2):432–441. doi: 10.1128/jb.139.2.432-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike P., Humphreys G. O., Roberts R. J. Nature of transforming deoxyribonucleic acid in calcium-treated Escherichia coli. J Bacteriol. 1979 Jun;138(3):1033–1035. doi: 10.1128/jb.138.3.1033-1035.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Chromosomally integrated conjugative plasmids are common in antibiotic-resistant Haemophilus influenzae. J Bacteriol. 1980 Jun;142(3):925–930. doi: 10.1128/jb.142.3.925-930.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Fate of transforming DNA in the Haemophilus influenzae transformation system. J Mol Biol. 1965 Sep;13(2):554–570. doi: 10.1016/s0022-2836(65)80117-6. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Plasmid transfer in Haemophilus influenzae. J Bacteriol. 1979 Aug;139(2):520–529. doi: 10.1128/jb.139.2.520-529.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Tetracyclin-stimulated expression of ampicillin resistance in Haemophilus influenzae. J Bacteriol. 1980 Nov;144(2):823–825. doi: 10.1128/jb.144.2.823-825.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Intergenotic transformation of the Bacillus subtilis genospecies. J Bacteriol. 1972 Sep;111(3):705–716. doi: 10.1128/jb.111.3.705-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



