Abstract
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the United States and a leading cause of cancer related mortality. Routine screening decreases incidence and mortality; however rates of screening remain low. Physician recommendation is a key determinant of screening rates; thus, physician availability may also influence CRC incidence and mortality.
METHODS
Data on CRC incidence and stage at diagnosis was obtained for each county in Pennsylvania from the Pennsylvania cancer registry. Physician density (per 100,000 population) was calculated for each county using physician counts from the American Medical Association. Pearson correlation coefficients and linear regression models were used to examine the association between physician density and CRC incidence and outcomes.
RESULTS
Primary care physician density (Pearson’s correlation coefficient: -0.25, p = 0.05) and gastroenterologist density (correlation coefficient -0.25, p = 0.04) inversely correlated with county-level incidence of late-stage CRC. However, this association was seen only in non-metropolitan counties or those with low population density. On linear regression, non-metropolitan counties which had a high density of gastroenterologists had an incidence of late-stage CRC that was lower by 4/100,000 (reduction of 14%). Low population density counties had lower incidence of late-stage CRC by 5/100,000 (reduction of 17%) when they had at least 3.3 gastroenterologists/100,000 population compared to counties with a lower gastroenterologist-per-population ratio. Gastroenterologist density did not correlate with reduced late-stage CRC incidence prior to institution of Medicare coverage for colonoscopy for routine CRC screening.
CONCLUSION
Higher gastroenterologist or PCP density is associated with 14-17% lower incidence of late-stage CRC in non-metropolitan counties or those with low population density. Efforts at increasing physician supply should target these underserved areas.
KEY WORDS: colon cancer, colorectal cancer, physician supply, screening, incidence, physician density
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the United States (US), and the second leading cause of cancer related death with an estimated 146,970 new cases and 49,920 deaths in 2009 (www.cancer.gov). CRC is one of the most preventable cancers. Several professional societies, including the American College of Gastroenterology (ACG)1, American Cancer Society2, American Gastroenterological Association3, and the US Preventive Services Task Force4,5 recommend routine screening for CRC from age 50 in average-risk individuals with the ACG recommending consideration of screening African-Americans from age 451. Screening allows removal of adenomatous polyps, thus decreasing cancer risk. It may also help in early diagnosis of established cancer6,7, leading to improved overall survival. However despite these benefits, the rate of CRC screening remains low8-13.
Health disparities are an important public health concern with several studies documenting racial and socioeconomic disparities in screening, incidence, stage at diagnosis, and mortality6,8,10,14-23. As physician recommendation is a key determinant of patients’ acceptance of screening, adequate access may be an important contributor to disparity. Frequency of physician contact as well as time spent during individual visits may influence screening24-27. Higher physician-per-population ratio may improve access, decrease waiting times and increase the opportunity for contact between the patient and physician. Compared to individuals with no physician contact, there is a nearly twofold increase in the odds of CRC screening for individuals with 1-2 physician contacts, rising to a 2.75-fold increase with more than four contacts in a year27.
Higher physician density has been associated with earlier stage of diagnosis of malignant melanoma28, lower incidence of cervical cancer29 and earlier stage at diagnosis of breast cancer30,31. There has been limited prior research examining the relation between physician density and CRC. In one study, supply of primary care physicians (PCP) negatively correlated with CRC incidence and mortality in Florida32. A 10-percentile increase in PCP density was associated with a 3% decrease in the odds of late-stage diagnosis33. However, it is likely that not just the supply of PCPs, but also gastroenterologists may be an important factor. The prior studies examining the correlation between physician supply and CRC outcomes were either conducted prior to Medicare coverage of colonoscopy for routine CRC screening in 2001 or did not specifically examine the availability of gastroenterologists32-34.
We performed this study with the following aims: (1) To examine the correlation between county-level physician-per-population ratio (‘physician density’) and CRC incidence, stage at diagnosis and mortality; (2) To specifically analyze the impact of density of PCPs and gastroenterologists on CRC outcomes; (3) To determine if the density of PCPs and gastroenterologists impacted CRC outcomes differentially in relation to Medicare coverage of colonoscopy for average-risk CRC screening, and (4) To determine if the physician density–CRC relationship varies by county characteristics, specifically degree of urbanization and population density.
METHODS
Study Population
Our study design was ecological and comprised county-level summary data from the state of Pennsylvania. We selected Pennsylvania as it has a high annual incidence rate of CRC (54.6/100,000), high CRC related mortality and provides a good mix of rural and urban population distribution.
Outcomes
Our primary outcomes of interest were county-level CRC incidence rate, overall and by stage at diagnosis obtained from the Pennsylvania Cancer Registry and Pennsylvania Department of Health Epidemiologic Query and Mapping System (EPIQMS) (http://app2.health.state.pa.us/epiqms/). Incidence data was obtained for the period 2004-06 for cancers involving the colon and rectum (International Classification of Diseases for Oncology (ICD-O) codes C180–C209, C260 excluding 9590–9989) and expressed as an age-adjusted rate standardized to the 2000 US census population. Age-adjusted rate was also obtained separately for early (American Joint Committee on Cancer (AJCC) Stage I and II) and late-stage CRC (AJCC Stage III and IV). Age-adjusted CRC-related mortality rate was also recorded.
To examine if the impact of physician density on CRC outcomes was different before and after Medicare coverage of colonoscopy for CRC screening in average risk individuals, we also examined the association between physician density and CRC incidence for 1997–1999.
Physician Density
The key predictor of interest was the county physician-per-population ratio. The count of physicians per county was obtained from the American Medical Association (AMA) physician resources file (http://www.ama-assn.org/cgi-bin/sserver/datalist.cgi, Accessed June 30, 2009) which includes both member and non-member US physicians. The county is assigned based on the preferred professional mailing address of the physician. Physician density was expressed as the number of physicians per 100,000 population. In addition to overall physician counts, we specifically examined the density of physicians of specialties which were felt to relate closely to CRC diagnosis and management. This included PCPs (general practice, family practice, family medicine, internal medicine, and medicine/pediatrics), gastroenterologists, general surgeons and oncologists (medical oncology, radiation oncology). Physician density was examined as a linear variable for the correlation analysis and as a dichotomous variable in the regression models. Counties were classified as having low or high physician density depending on values below and above the median density respectively (Table 1).
Table 1.
County-level Characteristics for the State of Pennsylvania (2000 United States Census Data)
| Characteristic | Mean (Standard deviation) | Range |
|---|---|---|
| Population density | 434.8 / sq. mile (1273.7) | 11.6–9999.9 / sq. mile |
| Age over 65 years | 16.3% (2.1) | 11.1–23.5% |
| Race | ||
| White | 91.2% (9.2) | 39.4–98.3 |
| Black | 4.3% (6.5) | 0.2–45% |
| Hispanic | 2.7% (3.2) | 0.1–15.1% |
| Socioeconomic status | ||
| High school graduates+ | 80.9% (3.9) | 71. 2–89.3% |
| Median per capita income | $44,582 (9637) | $ 30,501–82,979 |
| Living in poverty* | 11.9% (3.5) | 5.2–23.5% |
| No health insurance | 12.9% (3.2) | 7.0–23.0% |
| Median physician density (/100,000 population) | ||
| All physicians | 185 | 138-313 |
| Primary care physicians | 72 | 61-103 |
| Gastroenterologists | 3.3 | 0–4.4 |
| General surgeons | 10.8 | 6.6–14.3 |
| Oncologists (medical and radiation oncologists) | 3.4 | 0–5.7 |
| Colorectal cancer incidence and mortality (/100,000 population)++ | ||
| Overall incidence | 54.9 | 33.8–73.5 |
| Early stage (AJCC stage I and II) | 28.4 | 17.2–41.7 |
| Late stage (AJCC stage III and IV) | 29.0 | 17.8–46.2 |
| Mortality | 19.6 | 12.2–31.2 |
AJCC—American Joint Committee on Cancer
+ % adults over the age of 25 who graduated from high school
*poverty threshold determined based on size of household and number of children (http://www.census.gov/hhes/www/poverty/threshld.html)
++ age-adjusted incidence rate (per 2000 US Census population)
Other Variables
Other predictors of interest included county-level demographic (population density, proportion of patients of black race or Hispanic ethnicity in the county), educational (proportion of residents over the age of 25 who are high school graduates) and socioeconomic (median income, proportion of residents living in poverty, and % uninsured) variables obtained from the 2000 US census (www.census.gov). Because of the co-linearity between the various socio-economic variables, we created a composite variable comprising four variables that had previously been shown to be important markers of socioeconomic status (SES)35 (median income, % living in poverty, % uninsured, and % high school graduates). These were categorized into tertiles with 0, 1 or 2 points being assigned based on the tertile, higher points representing unfavorable distribution. A cumulative tertile sum score obtained by adding scores for each of the 4 variables and ranged from 0–8 with higher scores representing greater socioeconomic deprivation. Counties with scores of 0-3, 4-5, and 6-8 were classified as having a low, moderate, and high level of socioeconomic deprivation respectively. Counties were also labeled with the corresponding rural-urban continuum scores issued by the Office of Management and Budget (http://www.ers.usda.gov/briefing/rurality/ruralurbcon) based on the degree of urbanization and adjacency to a metro area. Counties were classified as metropolitan counties for continuum codes of 1-3 and as non-metropolitan counties for codes of 4-9.
Statistical Analysis
Data were analyzed using Stata 9.2 (StataCorp, College Station, TX). Normally distributed variables were summarized using means and standard deviations while skewed variables were expressed as medians with interquartile ranges. The t-test was used to compare continuous variables between different groups while the chi-square test or Fisher’s exact test were used for the comparison of categorical variables. Pearson correlation coefficients were calculated for associations between physician density and CRC incidence after excluding one county with a skewed density of physicians that was adjudged to be an influential outlier. This excluded county was Montour county which had a PCP (802/100,000) and gastroenterologist density (85/100,000) far in excess of the range for the other counties (PCP 0–194/100,000; gastroenterologists—0–13/100,000) primarily related to the small population size (n = 18,239). The CRC incidence in this county was comparable to the other counties. Linear regression was used to examine the relationship between the outcomes of interest and the independent variables. There was a strong co-linearity between the different physician density variables (i.e. overall physician and individual specialty physician densities); consequently only one physician density variable at a time was incorporated into each model; simultaneous adjustment for different physician specialties was not performed. A p-value <0.05 was considered statistically significant.
The main analysis and the subgroup analyses were planned a priori. The multivariate models were repeated after stratification by population density and county metropolitan status. To examine the specificity of the relationship for CRC, we examined the relationship between physician density and incidence of all cancers.
The study was approved by the Institutional Review Board of the Medical College of Wisconsin.
RESULTS
Study Population
Data from all 67 counties in Pennsylvania were included in our study. The mean proportion of population over the age of 65 years was 16.3%; 91.2% of the population was white (Table 1). The median per capita income was $44,582; about 12% of the population lived in poverty and 13% were uninsured.
The median county-level age-adjusted incidence of CRC was 54.9/100,000. This was almost equally distributed between early (28.1/100,000) and late-stage CRC (29/100,000). The CRC mortality rate was 19.6/100,000. There was a median of 189 physicians per 100,000 population (interquartile range (IQR) 138–313/100,000). PCPs formed the largest proportion with a median of 71/100,000 (IQR 61–103/100,000). There was approximately a median of 11 general surgeons (IQR 7–32), 3.3 gastroenterologists (IQR 0–4), and 3.5 oncologists (IQR 0–6) per 100,000 population.
Predictors of High Physician Density
Table 2 compares characteristics of counties with low and high overall physician density. High density counties had a higher minority population, greater median income, higher proportion of high school graduates and a lower proportion uninsured. Three-quarters of low socioeconomic deprivation counties had a high physician density while only one-third of high deprivation counties had a high density of physicians. Analysis of predictors of PCPs and gastroenterologists density separately showed similar results.
Table 2.
County-level Predictors of High Physician Density (All Physicians)
| Low physician density (0–185 / 100,000 population) | High physician density (≥185 / 100,000 population) | p-value | |
|---|---|---|---|
| Population density | 102.7 / sq. mile | 776.9 / sq. mile | 0.03 |
| % blacks | 2.8 | 5.9 | 0.04 |
| % Hispanic | 1.9 | 3.5 | 0.04 |
| % high school graduates | 79.5 | 82.4 | 0.002 |
| Median income (in $) | $40,957 | $48,316 | 0.001 |
| % living below poverty level | 12.6 | 11.3 | 0.14 |
| % uninsured | 13.8 | 12 | 0.02 |
| Socioeconomic deprivation | 0.001 | ||
| Low | 23.1% | 76.9% | |
| Moderate | 73.7% | 26.3% | |
| High | 63.6% | 36.4% |
Physician Density and CRC Incidence
Overall physician density (Pearson’s Correlation coefficient (rho) -0.05, p = 0.72), density of gastroenterologists (rho -0.03, p = 0.79), PCPs (rho -0.05, p = 0.71) or surgeons separately did not correlate with county-level all-stage CRC incidence. However, a significant difference was observed on stratifying by stage at diagnosis. Incidence of early-stage CRC did not show a significant correlation with all physicians, PCP or gastroenterologist density. This was in contrast to late-stage CRC incidence which was inversely associated with both PCP (rho -0.25, p = 0.05) and gastroenterologist density (rho -0.25, p = 0.04). This remained even after adjusting for SES. Excluding the most urban counties (i.e. a rural/urban continuum score of 1) further strengthened this association (rho -0.38, p = 0.007).
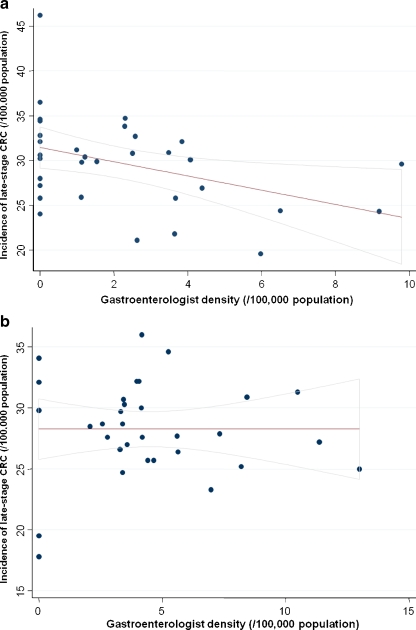
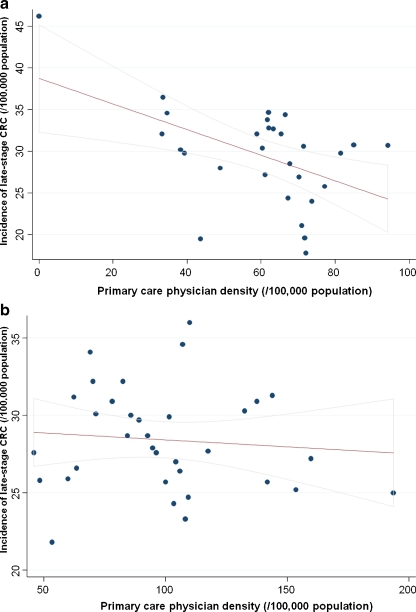
The association between density of PCP or gastroenterologists and late-stage CRC incidence was seen predominantly in non-metropolitan counties or those with low population density (Table 3). On multivariate linear regression, non-metropolitan counties with a high gastroenterologist density had significantly lower incidence of late-stage CRC (- 4/100,000, a reduction of 14%); this difference was not seen in metropolitan counties. Similarly, low population density counties had lower incidence of late-stage CRC by 5/100,000 (a reduction of 17%) when they had at least 3.3 gastroenterologists/100,000 population compared to low population density counties with a lower gastroenterologist-per-population ratio. Figures 1 and 2 demonstrate the above interactions graphically. Low population density counties differed from high population density counties in that they had a lower proportion of patients of black race, Hispanic ethnicity, a lower median per capita income and a higher proportion of patients residing in poverty or without health insurance (data not shown).
Table 3.
Relationship Between Primary Care Physicians or Gastroenterology Density and Incidence of Late-stage Colorectal Cancer, Stratified by Population Density, Metropolitan Status or Socioeconomic Deprivation
| Primary care physicians | Gastroenterologists | |
|---|---|---|
| Adjusted regression coefficient+ for high vs. low density counties | Adjusted regression coefficient+ for high vs. low density counties | |
| Population density | ||
| <130 / square mile | -4.48 (-8.96 to -0.01) * | -4.71 (-9.55 to 0.00) * |
| ≥130 / square mile | 1.38 (-1.40 to 4.16) | 0.76 (-2.14 to 3.67) |
| Metropolitan status | ||
| Non-metropolitan | -2.76 (-6.68 to 1.16) | -3.77 (-7.54 to 0.00) * |
| Metropolitan | 0.22 (-3.01 to 3.45) | 2.26 (-0.69 to 5.21) |
| Socioeconomic deprivation index | ||
| Low | -0.19 (-4.27 to 3.88) | 1.92 (-1.86 to 5.70) |
| Moderate | 1.49 (-2.61 to 5.59) | 1.1 (-2.82 to 5.02) |
| High | -4.06 (-8.51 to 0.39) + | -5.81 (-10.14 to -1.47)* |
The regression coefficient represents the absolute reduction in the number of late stage CRC cases per 100,000 for high density compared to low density counties.
*p < 0.05
+p = 0.07
Figure 1.
Correlation between gastroenterologist density and late-stage CRC incidence in Pennsylvania, by county (rural / urban) status. (a) Rural counties. Pearson’s correlation coefficient:-0.41, p = 0.02. CRC—colorectal cancer. (b) Urban counties. Pearson’s correlation coefficient: 0.00, p = 0.99. CRC—colorectal cancer.
Figure 2.
Correlation between primary care physician density and late-stage CRC incidence in Pennsylvania, by county population density. (a) Population density less than 130 persons/square mile. Pearson’s correlation coefficient: -0.45, p = 0.01. CRC—colorectal cancer. (b) Population density greater than 130 persons/square mile. Pearson’s correlation coefficient: -0.11, p = 0.52. CRC—colorectal cancer.
For the time period 1997-1999, gastroenterologist density was not associated with all CRC, early or late-stage CRC incidence. In contrast to the significant correlation between gastroenterologist supply and late-stage CRC incidence in non-metropolitan counties during 2004-06 (after Medicare coverage), there was no similar correlation prior to Medicare coverage of colonoscopy for routine screening (rho -0.22, p = 0.21) (Table 4). However, the relationship between PCP density and late-stage CRC incidence in non-metropolitan counties remained borderline significant during 1997-99 (rho -0.31, p = 0.07), suggesting that gastroenterologist density has become a more significant factor after approval of Medicare coverage of colonoscopy for routine screening.
Table 4.
Correlation Between Primary Care Physician / Gastroenterologist Density and Incidence of Late-stage Colorectal Cancer (CRC) Before (1997-1999) and After (2004-2006) Medicare Coverage of Colonoscopy for Routine CRC Screening
| Primary care physicians | Gastroenterologists | |||
|---|---|---|---|---|
| Correlation coefficient (p-value) | Correlation coefficient (p-value) | |||
| 1997–1999 | 2004–2006 | 1997–1999 | 2004–2006 | |
| Metropolitan status | ||||
| Non-metropolitan | -0.31 (p = 0.07) | -0.39 (p = 0.03) | -0.22 (p = 0.21) | -0.41 (p = 0.02) |
| Metropolitan | 0.19 (p = 0.29) | -0.05 (p = 0.79) | 0.13 (p = 0.49) | 0.00 (p = 0.99) |
Compared to counties with low SES deprivation, those with moderate (2.8, 95% CI 0.09–5.52) and high deprivation (3.05, 95% CI 0.45–5.65) had higher incidence of late-stage CRC, but were no different in overall or early-stage CRC incidence. Neither gastroenterologist (-1.39, p = 0.2), PCP (-0.90, p = 0.4), nor oncologist density (-1.43, p = 0.2) were predictive of lower CRC mortality rates. This remained true on subgroup analyses. There was also no correlation between county racial distribution and overall or stage-specific incidence of CRC.
Sensitivity Analysis
We found no correlation between physician density and incidence of all cancers overall or by stage. Gastroenterologist density did not correlate with all-cancer incidence suggesting that the association between PCP / gastroenterologist density and late-stage CRC incidence was specific for CRC. Inclusion of obstetrics and gynecology physician density within the category PCP did not significantly change the correlation coefficients of overall or stage-specific CRC incidence.
DISCUSSION
Access to physicians may be an important determinant of health outcomes36. In this ecologic analysis using data from the state of Pennsylvania, we demonstrate that high density of PCP or gastroenterologists (but not overall physician density) is associated with 14–17% lower incidence of late-stage CRC. However, this relationship appears to hold true predominantly for rural counties or those with low population density.
Several authors have examined the relationship between physician supply and health outcomes. Higher physician supply was associated with lower mortality rates in some, though not all studies37. Three prior studies have examined the relationship between physician supply and CRC32-34. Shipp et al. found that an increase in the number of physicians/1000 population was associated with a modestly higher rate of CRC (relative risk 1.14)34. In contrast Roetzheim et al. showed that higher PCP density negatively correlated with both CRC incidence and mortality32. The same authors also found that for each 10-percentile increase in PCP supply, the odds of late-stage CRC diagnosis decreased by 5%33. However, the converse was true for specialists, with an increase in late-stage CRC diagnosis with each 10-percentile increase in specialty physician-per-population ratio. Density of gastroenterologists did not correlate with stage at diagnosis in their study. Our findings of the association between PCP density and late-stage CRC incidence is consistent with the findings of these two latter studies32,33. The novel finding of the association between gastroenterologist supply and late-stage CRC incidence in our study compared to prior studies33 may be due to Medicare coverage of colonoscopy for average-risk CRC screening beginning in July 2001. Since this coverage, there has been increasing use of endoscopic modalities for CRC screening8,10. In a prior study, Ananthakrishnan et al. found that 3.8% of all eligible Medicare patients received a screening colonoscopy in one calendar year in 2002-038 compared to 1.4% reported by Ko et al. for a period prior to the expansion of Medicare coverage9. Correspondingly, colonoscopy comprised 42% of all screening tests in the latter period8 compared to 35% in 19989. While a reduction in CRC mortality has been demonstrated with FOBT38, this reduction may be attributable to patients with positive tests undergoing colonoscopy with removal of adenomatous polyps39. A reduction in CRC mortality with use of screening colonoscopy has also been demonstrated40. It is possible that with this present trend, gastroenterologist availability also becomes important in determining CRC incidence and outcomes.
The reduction in late-stage CRC incidence in counties with higher physician supply could have a few explanations. The specificity of our finding a negative association between late-stage CRC incidence and PCPs/gastroenterologists density, but not overall physician density suggests that availability of these two physician groups most likely to be involved in screening or early detection of CRC is an important factor. This is further supported by the fact that after the initiation of Medicare coverage of colonoscopy for average-risk CRC, gastroenterologist density became more strongly associated with reduced late-stage CRC incidence than prior to such coverage. Physician recommendation is an important determinant of CRC screening24-26 with individuals with more frequent physician contact being more likely to undergo screening27. Inadequate physician time with the patient is another barrier to screening25. As county-level rates of CRC screening are not available for each county in Pennsylvania, we were unable to examine the impact of differential screening rates. Given the generally lower rates of CRC screening compared to other preventive health services, differences in screening are unlikely to be the sole factor. In addition, given the known timeline for development of CRC, changes in screening practices after 2001 are unlikely to be the sole determinants of changes in late-stage CRC diagnosis in 2004–2006. However, increase in screening has been associated with a higher proportion of early-stage CRC diagnosis even short-term7. Higher physician supply may also result in earlier care care-seeking behavior for patients with symptoms suggestive of CRC, resulting in early diagnosis.
It is interesting that the relationship between gastroenterologists/PCP supply and late-stage CRC differed by metropolitan status and population density. Studies examining the availability of PCPs in urban areas identified weak correlations with health outcomes41 while the link appeared to be stronger in some, but not all studies, examining rural health care. Pathman et al. found that higher number of persons per physician in each county was associated with longer travel times but no other significant barriers to care41 with no difference in the utilization of preventive health services. However, among patients who were covered under Medicaid or were uninsured, lower physician-per-population ratio was associated with lower satisfaction with care and difficulty in contacting medical personnel. Intuitively, it stands to reason that in rural areas with a scattered population, there may be longer travel times to physician offices in counties with a low physician-per-population ratio resulting in longer waiting times. Reduced access to PCP or gastroenterologists in these counties may also delay CRC screening, surveillance, or diagnostic evaluations in those with symptoms and consequently a higher incidence of late-stage cancer. In urban counties, physician density may be above the threshold for such relationships to hold true and no longer acts as a rate-limiting step. It is also interesting that the relationship with physician supply did not hold true for overall CRC incidence or mortality. There a few potential explanations for this finding. In prior studies, having had a screening endoscopy was associated with a lower risk of only late-stage diagnosis (odds ratio (OR) 0.46, 95% CI 0.22–0.98)6. Gross et al. demonstrated an association between increasing colonoscopy use with earlier stage at diagnosis for proximal but not distal colon lesions7.
Our study has a few limitations. Ecologic analysis assigns the same characteristics to each individual residing within the county. County-level socioeconomic characteristics may not represent the SES of the individual. However, we believe that measures such as availability of or access to physicians, our primary variable of interest, are more meaningful when measured over a wider geographic area. Performing such analysis at the level of zip code or census tract may be fallacious as individuals are unlikely to restrict their care to physicians within their zip codes of residence. We also did not have information on location of CRC or county-level CRC screening rates. While the Behavioral Risk Factor Surveillance System and the National Health Interview Survey track overall screening rates in the US and in select population areas, there is currently limited mechanisms for obtaining county-level screening rates for each county for any state in the US. We believe that it is important to develop such county-level or other small-geographic area level databases to track various health behaviors in order to identify high-risk populations. Another limitation of our analysis is the inability to adjust for some known individual risk factors for CRC including obesity and smoking status though adjusting for county level proportion of smokers did not influence our estimates. It is possible though unlikely that the above variables vary systematically enough with physician density to influence our results.
There are several implications to our study. Recent concerns have been raised about the potential shortage of physicians42,43. The Lewin group projected that by the year 2020, there might be a shortage of between 1000–1500 gastroenterologists nationwide in the US44. By demonstrating a relationship between county-level physician supply and late-stage CRC incidence, our study supports these concerns. It is important to recognize that the increase in physician supply may need to be targeted to non-metropolitan counties (comprising 12.6% of the state population) or those with low population densities (comprising approximately 12.7% of the state population). In urban counties, physician supply was not an important determinant of CRC incidence; increasing the physician-per-population supply in such counties may consequently have a limited impact. In rural counties with a population that is sparse and more spread out, availability of PCP and/or gastroenterologists may be important in reducing CRC incidence. However, it is almost important to remember that physician density represents only availability of healthcare but does not take into account affordability or acceptability of CRC screening practices45. Physician density may have a limited impact on CRC incidence and outcomes if the other barriers to healthcare are more dominant factors.
In conclusion, we demonstrate that higher density of PCP and gastroenterologists is associated with a 14-17% decrease in the incidence of late-stage CRC with this association being seen predominantly in non-metropolitan counties or those with low population density. This suggests that measures aimed at increasing physician supply to decrease disparity in CRC incidence and outcomes should target such underserved areas.
Acknowledgments
There was no funding for this study.
Conflicts of interest None disclosed.
References
- 1.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 2.Smith RA, Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, Brooks D, Creasman W, Cohen C, Runowicz C, Saslow D, Cokkinides V, Eyre H. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001--testing for early lung cancer detection. CA Cancer J Clin. 2001;51:38–75. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- 3.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 4.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627-37. [DOI] [PubMed]
- 5.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 6.Fazio L, Cotterchio M, Manno M, McLaughlin J, Gallinger S. Association between colonic screening, subject characteristics, and stage of colorectal cancer. Am J Gastroenterol. 2005;100:2531–9. doi: 10.1111/j.1572-0241.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 7.Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. Jama. 2006;296:2815–22. doi: 10.1001/jama.296.23.2815. [DOI] [PubMed] [Google Scholar]
- 8.Ananthakrishnan AN, Schellhase KG, Sparapani RA, Laud PW, Neuner JM. Disparities in colon cancer screening in the Medicare population. Arch Intern Med. 2007;167:258–64. doi: 10.1001/archinte.167.3.258. [DOI] [PubMed] [Google Scholar]
- 9.Ko CW, Kreuter W, Baldwin LM. Effect of Medicare coverage on use of invasive colorectal cancer screening tests. Arch Intern Med. 2002;162:2581–6. doi: 10.1001/archinte.162.22.2581. [DOI] [PubMed] [Google Scholar]
- 10.Ko CW, Kreuter W, Baldwin LM. Persistent demographic differences in colorectal cancer screening utilization despite Medicare reimbursement. BMC Gastroenterol. 2005;5:10. doi: 10.1186/1471-230X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadel MR, Blackman DK, Shapiro JA, Seeff LC. Are people being screened for colorectal cancer as recommended? Results from the National Health Interview Survey. Prev Med. 2002;35:199–206. doi: 10.1006/pmed.2002.1070. [DOI] [PubMed] [Google Scholar]
- 12.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, Coates RJ. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093–103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 13.Seeff LC, Shapiro JA, Nadel MR. Are we doing enough to screen for colorectal cancer? Findings from the 1999 Behavioral Risk Factor Surveillance System. J Fam Pract. 2002;51:761–6. [PubMed] [Google Scholar]
- 14.Baldwin LM, Dobie SA, Billingsley K, Cai Y, Wright GE, Dominitz JA, Barlow W, Warren JL, Taplin SH. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97:1211–20. doi: 10.1093/jnci/dji241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005;104:629–39. doi: 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- 16.Coughlin SS, Richards TB, Thompson T, Miller BA, VanEenwyk J, Goodman MT, Sherman RL. Rural/Nonrural differences in colorectal cancer incidence in the United States, 1998-2001. Cancer. 2006;107:1181–8. doi: 10.1002/cncr.22015. [DOI] [PubMed] [Google Scholar]
- 17.Gomez SL, O'Malley CD, Stroup A, Shema SJ, Satariano WA. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:193. doi: 10.1186/1471-2407-7-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorey KM, Vena JE. The association of near poverty status with cancer incidence among black and white adults. J Community Health. 1995;20:359–66. doi: 10.1007/BF02283060. [DOI] [PubMed] [Google Scholar]
- 19.Gorey KM, Vena JE. Cancer differentials among US blacks and whites: quantitative estimates of socioeconomic-related risks. J Natl Med Assoc. 1994;86:209–15. [PMC free article] [PubMed] [Google Scholar]
- 20.Henry KA, Niu X, Boscoe FP. Geographic disparities in colorectal cancer survival. Int J Health Geogr. 2009;8:48. doi: 10.1186/1476-072X-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paquette I, Finlayson SR. Rural versus urban colorectal and lung cancer patients: differences in stage at presentation. J Am Coll Surg. 2007;205:636–41. doi: 10.1016/j.jamcollsurg.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 22.Roetzheim RG, Pal N, Gonzalez EC, Ferrante JM, Durme DJ, Krischer JP. Effects of health insurance and race on colorectal cancer treatments and outcomes. Am J Public Health. 2000;90:1746–54. doi: 10.2105/AJPH.90.11.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trivers KF, Shaw KM, Sabatino SA, Shapiro JA, Coates RJ. Trends in colorectal cancer screening disparities in people aged 50-64 years, 2000-2005. Am J Prev Med. 2008;35:185–93. doi: 10.1016/j.amepre.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008;19:339–59. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 25.Guerra CE, Schwartz JS, Armstrong K, Brown JS, Halbert CH, Shea JA. Barriers of and facilitators to physician recommendation of colorectal cancer screening. J Gen Intern Med. 2007;22:1681–8. doi: 10.1007/s11606-007-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy BT, Nordin T, Sinift S, Rosenbaum M, James PA. Why hasn't this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007;20:458–68. doi: 10.3122/jabfm.2007.05.070058. [DOI] [PubMed] [Google Scholar]
- 27.Zarychanski R, Chen Y, Bernstein CN, Hebert PC. Frequency of colorectal cancer screening and the impact of family physicians on screening behaviour. Cmaj. 2007;177:593–7. doi: 10.1503/cmaj.070558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roetzheim RG, Pal N, Durme DJ, Wathington D, Ferrante JM, Gonzalez EC, Krischer JP. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. J Am Acad Dermatol. 2000;43:211–8. doi: 10.1067/mjd.2000.106242. [DOI] [PubMed] [Google Scholar]
- 29.Campbell RJ, Ramirez AM, Perez K, Roetzheim RG. Cervical cancer rates and the supply of primary care physicians in Florida. Fam Med. 2003;35:60–4. [PubMed] [Google Scholar]
- 30.Ferrante JM, Gonzalez EC, Pal N, Roetzheim RG. Effects of physician supply on early detection of breast cancer. J Am Board Fam Pract. 2000;13:408–14. doi: 10.3122/15572625-13-6-408. [DOI] [PubMed] [Google Scholar]
- 31.Fleisher JM, Lou JQ, Farrell M. Relationship between physician supply and breast cancer survival: a geographic approach. J Community Health. 2008;33:179–82. doi: 10.1007/s10900-008-9090-z. [DOI] [PubMed] [Google Scholar]
- 32.Roetzheim RG, Gonzalez EC, Ramirez A, Campbell R, Durme DJ. Primary care physician supply and colorectal cancer. J Fam Pract. 2001;50:1027–31. [PubMed] [Google Scholar]
- 33.Roetzheim RG, Pal N, Gonzalez EC, Ferrante JM, Durme DJ, Ayanian JZ, Krischer JP. The effects of physician supply on the early detection of colorectal cancer. J Fam Pract. 1999;48:850–8. [PubMed] [Google Scholar]
- 34.Shipp MP, Desmond R, Accortt N, Wilson RJ, Fouad M, Eloubeidi MA. Population-based study of the geographic variation in colon cancer incidence in Alabama: relationship to socioeconomic status indicators and physician density. South Med J. 2005;98:1076–82. doi: 10.1097/01.smj.0000184844.01148.10. [DOI] [PubMed] [Google Scholar]
- 35.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) J Epidemiol Community Health. 2003;57:186–99. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrin JM, Valvona J. Does increased physician supply affect quality of care? Health Aff (Millwood) 1986;5:63–72. doi: 10.1377/hlthaff.5.4.63. [DOI] [PubMed] [Google Scholar]
- 37.Gulliford MC, Jack RH, Adams G, Ukoumunne OC. Availability and structure of primary medical care services and population health and health care indicators in England. BMC Health Serv Res. 2004;4:12. doi: 10.1186/1472-6963-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 39.Lang CA, Ransohoff DF. Fecal occult blood screening for colorectal cancer. Is mortality reduced by chance selection for screening colonoscopy? Jama. 1994;271:1011–3. doi: 10.1001/jama.271.13.1011. [DOI] [PubMed] [Google Scholar]
- 40.Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770–5. doi: 10.1016/j.cgh.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 41.Pathman DE, Ricketts TC, 3rd, Konrad TR. How adults' access to outpatient physician services relates to the local supply of primary care physicians in the rural southeast. Health Serv Res. 2006;41:79–102. doi: 10.1111/j.1475-6773.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.AAMC. AAMC Statement on the Physician Workforce. http://www.aamc.org/workforce/workforceposition.pdf. Accessed May, 2010.
- 43.Mitka M. Looming shortage of physicians raises concerns about access to care. JAMA. 2007;297:1045–6. doi: 10.1001/jama.297.10.1045. [DOI] [PubMed] [Google Scholar]
- 44.The Lewin Group I. The impact of improved colorectal cancer screening rates on adequacy of future supply of gastroenterologists. http://www.olympusamerica.com/CRCadvocacy/docs/Lewin-Gastroenterologist-Report.pdf. Accessed May, 2010.
- 45.Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care. 1981;19(2):127–40. doi: 10.1097/00005650-198102000-00001. [DOI] [PubMed] [Google Scholar]