Abstract
The P2X7 receptor (P2X7R) has been implicated in the process of multinucleation and cell fusion. We have previously demonstrated that blockade of P2X7Rs on osteoclast precursors using a blocking antibody inhibited multinucleated osteoclast formation in vitro, but that P2X7R KO mice maintain the ability to form multinucleated osteoclasts. This apparent contradiction of the role the P2X7R plays in multinucleation has prompted us to examine the effect of the most commonly used and recently available P2X7R antagonists on osteoclast formation and function. When added to recombinant RANKL and M-CSF human blood monocytes cultures, all but one compound, decreased the formation and function of multinucleated TRAP-positive osteoclasts in a concentration-dependent manner. These data provide further evidence for the role of the P2X7R in the formation of functional human multinucleated osteoclasts and highlight the importance of selection of antagonists for use in long-term experiments.
Keywords: P2X7, ATP, Osteoclast, P2 receptor, Resorption, Formation, Fusion
Introduction
The P2X7R is a 595 amino acid plasma membrane receptor with approximately 40% sequence identity to other members of the P2X purinergic family [1]. However, this receptor subtype possesses properties that distinguish it from other P2X receptors. Under conditions of transient nucleotide agonist stimulation, the P2X7R functions like other P2X receptors in that it is selectively permeable to small cations. However, when activated by repeated or prolonged exposure of nucleotide agonists, it has the unique ability amongst the P2 receptor family to form non-selective pores in the plasma membrane that are permeable to molecules of up to 900 Da [2, 3]. The last 177 amino acids in the intracellular C-terminal end of the receptor are crucial for induction of this non-selective pore [4].
The physiological significance of the activation of the P2X7R remains an area of much interest. Several possible roles for this receptor have been suggested including the maturation and release of interleukin 1β (IL-1β) and ATP-induced apoptosis [5, 6]. In addition, it has been demonstrated that macrophage cell clones expressing high levels of P2X7R spontaneously fuse in vitro, whereas clones lacking the P2X7R do not. Furthermore, multinucleated giant cell formation from macrophage fusion could be blocked with a monoclonal antibody directed against the extracellular domain of the P2X7R [7]. These data strongly implicate P2X7R-induced pore formation in the development of multinucleated giant cells and, more specifically, in their fusion.
Osteoclasts are multinucleated, terminally differentiated cells formed by the fusion of mononuclear osteoclast precursors found in the monocyte fraction of blood. The activity of bone-resorbing osteoclasts contributes to the highly coordinated process of bone remodelling, where old tissue is replaced with newly synthesised bone in the continuous process of bone regeneration [8]. Therefore, the regulation of osteoclast formation and resorption is important in maintaining skeletal homeostasis.
The factors responsible for the commitment of mononuclear cells to the osteoclast lineage have now been characterised—namely macrophage colony stimulating factor (M-CSF) and receptor activator for nuclear factor kappa beta (NFκB) ligand (RANKL) [9–11]. The isolation of these factors, and the subsequent availability of soluble recombinant RANKL, has presented researchers with the means to generate and study isolated human osteoclasts in vitro. Whilst the conditions that promote osteoclastogenesis in vitro have been defined, the exact molecular mechanisms via which mononuclear cells fuse to form multinucleated osteoclasts are not fully understood. We and others have shown that osteoclasts and their precursors express P2X7R [12–15], and we have recently demonstrated that addition of an antibody raised against the external domain of the P2X7R that specifically blocks P2X7R function [16] inhibits osteoclast fusion in vitro [17]. In contrast, P2X7R deficient mice were shown to maintain the ability to form multinucleated osteoclasts, both in vivo and in vitro [18]. The P2X7R deficient mice displayed significant reduction in total and cortical bone content and periosteal circumference in femurs, and reduced periosteal bone formation and increased trabecular bone resorption in tibias [19]. These mice have more recently been found to have reduced sensitivity to mechanical loading as shown by lower bone formation rate per unit of mechanical strain in vivo, and that prostaglandin (PG) E2 release from P2X7R null osteoblasts is unaffected by fluid shear stress in vitro [20]. Mechanically induced intercellular calcium signalling among osteoclasts has also been shown to be inhibited by P2X7R blockade [21]. These data suggest that the P2X7R is fundamental to normal bone development and adaptive remodelling.
In this study, in order to better clarify the role of the P2X7R in osteoclast physiology, rather than activating the receptor using exogenous agonists which ultimately results in cell death [3], we have examined the effect of the most commonly used commercially available P2X7R antagonists (Fig. 1a–c) on the formation of functional multinucleated human osteoclasts. In addition, we have included a small-molecule, from here on in called AZ15d, from a distinct chemical series which is based on the cyclic imide compounds (Fig. 1d). These compounds have been demonstrated to be potent, pIC50 = 7.3 for plasma membrane pore formation in THP-1 cells [22], and selective for P2X7R [23]. These results provide further evidence for the important role of the P2X7R in the formation of functional multinucleated human osteoclasts.
Fig. 1.
Chemical structure of P2X7R antagonists. a oATP, b KN62, c A-438079 and d AZ15d
Material and methods
Materials
α-MEM, FCS L-glutamine, penicillin/streptomycin and Superscript™ II reverse transcriptase were purchased from GIBCO Life Technologies (Paisley, UK). Dentine discs were provided by Ultrabone (Liverpool, UK) and 6 mm glass coverslips were from Richardsons of Leicester (Leicester, UK). Recombinant human RANKL was purchased from Insight Biotechnology (Wembley, UK) and recombinant human M-CSF was kindly provided by Genetics Institute (Cambridge, MA, USA). DNaseI was from Roche Diagnostics (Lewes, UK). Histopaque®-1077, nucleotides, TRI® reagent, tartrate-resistant acid phosphatase (TRAP) staining kit, periodate-oxidised ATP (oATP) and KN62 were from Sigma (Poole, UK). PCR primers were synthesised by Vh-Bio (Newcastle, UK). A-438079 was purchased from Tocris (Bristol, UK) and compounds AZ15d and AZ408 were synthesised in-house by Medicinal Chemistry, AstraZeneca R&D Charnwood [22].
Recombinant RANKL-induced generation of human osteoclasts from blood monocytes
Osteoclasts were generated from human peripheral blood monocytes as previously described [24]. Sterilisation of 6-mm coverslips was performed by baking at 180° for 2 h. Dentine discs were sterilised by sonication, washing in 70% ethanol, followed by U.V. light irradiation. Following ethical committee approval, venous blood was obtained from healthy volunteers and separated using Histopaque®-1077. The monocyte fraction was collected and washed in α-MEM then re-suspended in α-MEM. An appropriate volume of cell suspension containing 5 × 105 cells was then added to pre-wetted coverslips or dentine slices in a 96 well plate. Cells were incubated for a minimum of 1 h to allow adherence to the dentine or glass surface. Non-adherent cells were subsequently washed away with α-MEM. Adherent cells were incubated in 100 μl α-MEM containing 10% FCS, 100 IU/ml penicillin, 100 μg/ml streptomycin and 2 mM l-glutamine (referred to as complete α-MEM) and supplemented with 25 ng/ml M-CSF, 30 ng/ml recombinant RANKL plus vehicle (0.1% DMSO for AZ15d, AZ408 and KN62) or the appropriate concentration of the antagonist, at 37°C in a humidified atmosphere of 93% air and 7% CO2 for 3 weeks. The complete α-MEM containing 25 ng/ml M-CSF, 30 ng/ml RANKL and all drug treatments was replaced every 2–3 days.
TRAP staining of osteoclasts
Cells were grown on 6-mm coverslips then fixed in acetone and stained for TRAP using a commercially available kit used according to the manufacturer’s protocol or fixed in formalin and stained as previously described [25].
Fixation of dentine discs and quantification of resorption
After 3 weeks in culture, dentine discs were washed in PBS, fixed in 4% glutaraldehyde in 0.2 M sodium cacodylate, and stained for 5 min in 1% (w/v) toluidine blue in 0.5% disodium tetraborate. Alternatively, dentine wafers were fixed in formalin and stained for TRAP as previously described [25]. Excess stain was removed by washing in 70% ethanol for 1 min. Dentine discs were then washed in distilled water and air-dried. Resorption lacunae were identified using reflective light microscopy and plan area of resorption was determined by point counting [26].
RNA isolation and complementary DNA synthesis
Total RNA was isolated using the TRI® reagent according to the manufacturer’s protocol. Before first-strand complementary DNA (cDNA) synthesis, RNA was DNAse-treated with RNAse-free DNAse1 (35 U/ml) for 60 min at 37°C. DNase was inactivated by heating at 70°C for 15 min, and the RNA precipitated for at least 1 h at −20°C in 3 volumes 100% ethanol and 0.1 volumes sodium acetate, pH 5.2. 5 μg of DNAse-treated total RNA was used as a template for first-strand cDNA synthesis in a 20 μl reaction containing 0.5 μg oligo (dT), 0.5 mM dNTPs, 20 U of RNAse inhibitor, 10 mM of dithiothreitol, 6 mM MgCl2, 40 mM KCl, 50 mM Tris–HCl, pH 8.3 and 200 U of Superscript II RT. The reaction mix was incubated at 42°C for 50 min, and the reaction stopped by heating at 70°C for 15 min. cDNA was stored at −20°C until required.
Polymerase chain reaction
Twenty-five microlitre reactions were performed on a MJ Research PTC-200 Peltier Thermal cycler. Reactions contained 1 μl of cDNA, 0.5 units of thermostable Taq DNA polymerase, 50 pmol each of sense and antisense primer, 0.2 mM each of dATP, dCTP, dGTP and dTTP and 1.5 mM MgCl2 in 1× (final) reaction buffer. The PCR reactions involved an initial 3-min denaturation step at 94°C, followed by the amplification step (94°C for 10 s, annealing Tm for 30 s, extension for 30 s at 72°C) for 35 cycles, plus a final 5-min extension step at 72°C. For analysis of DNA, PCR products were loaded onto 1% agarose gels containing 0.3 μg/ml ethidium bromide, gels were run at 90–100 mA and the position of DNA in the gel was visualised by exposure to UV light. PCR primers and Tms were: GAPDH: 5′-ggt gaa ggt cgg agt caa cgg-3′ sense, 5′-ggt cat gag tcc ttc cac gat-3′ antisense, Tm = 58°C; P2X7R: 5′-tga agg gga tag cag agg tga-3′ sense, 5′-tgg gat ggc agt gat gga-3′ antisense, Tm = 56°C.
Statistical analysis
Statistical analysis was by ANOVA using GraphPad Prism®. The significance between groups was determined using Dunnett’s Multiple Comparison Post-Test. Logistic curve fitting was performed using the least squares (ordinary fit) method and a standard Hill slope.
Results
P2X7R mRNA is expressed during osteoclastogenesis in vitro
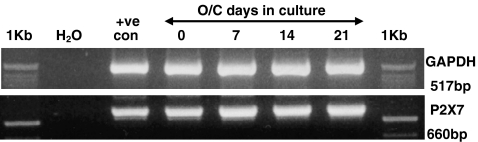
The identification of M-SCF and RANKL as factors requisite for osteoclastogenesis has enabled studies to be performed on non-pathological human osteoclasts in vitro. Human blood monocyte cultures grown in the presence of recombinant RANKL and M-CSF develop osteoclast marker expression that accurately reflects the differentiation of osteoclast precursors into mature osteoclast cells in a similar manner to the processes occurring at bone-resorbing sites in vivo. The isolated fraction of human peripheral blood monocytes on day 0 is positive for TRAP and includes both monocytes and macrophages. By day 7, the population of cells are largely mono- or bi-nucleate committed osteoclast precursors and start to express the osteoclast marker calcitonin receptor but do not yet resorb dentine. By day 14, the majority of cells have fused to become multinucleated, express the mature osteoclast marker cathepsin K and start to resorb the dentine. No further increase in osteoclast number is seen between days 14 and 21 during which time the osteoclasts become fully differentiated and actively resorb dentine [27–29]. In order to see if P2X7R expression was restricted to one specific stage of osteoclast development, RNA was isolated from blood monocytes grown in recombinant RANKL and M-CSF supplemented medium after 0, 7, 14 and 21 days in culture. PCR of cDNA generated from this RNA using primers specific to P2X7R mRNA revealed that P2X7R was expressed at every stage of the culture period (Fig. 2). PCR for GAPDH was performed on the same cDNA samples to confirm the presence of equal amounts of cDNA between samples (Fig. 2).
Fig. 2.
P2X7R mRNA is expressed during osteoclastogenesis in vitro. Osteoclasts were generated from monocytes cultured in the presence of recombinant M-CSF and RANKL. RNA was isolated from these cells after 0, 7, 14 and 21 days in culture. cDNA was generated and subsequently used as a template for PCR with primers designed specifically to the P2X7R. Resulting PCR products were separated by agarose gel electrophoresis and visualised under UV transillumination. +ve con = positive control, 1 Kb = 1 Kb molecular weight marker
Effect of P2X7R antagonists on human osteoclast formation
To determine the effects of P2X7R specific antagonists on osteoclast formation in recombinant RANKL and M-CSF supplemented cultures of blood monocytes, antagonists were introduced at various concentrations by inclusion in the medium at each medium change. At the end of the culture period cells were fixed and stained for TRAP, and the number of osteoclasts per coverslip were determined by counting all TRAP-positive cells with three or more nuclei.
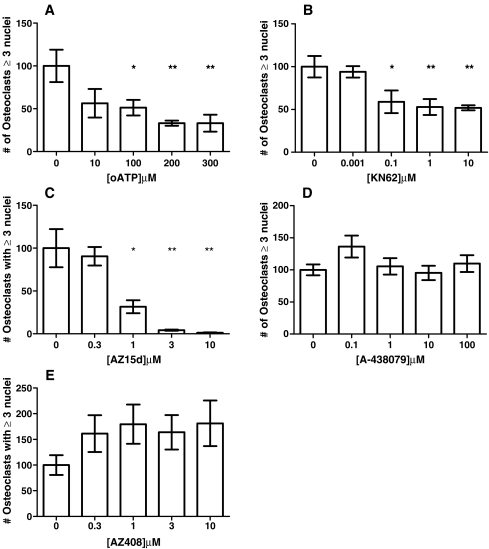
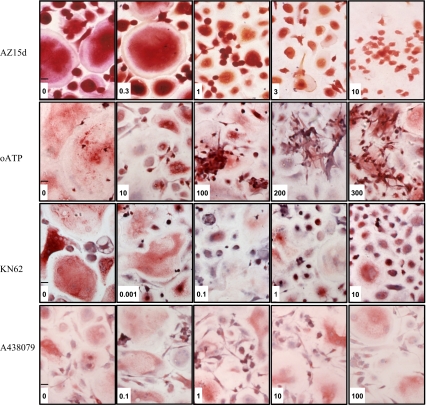
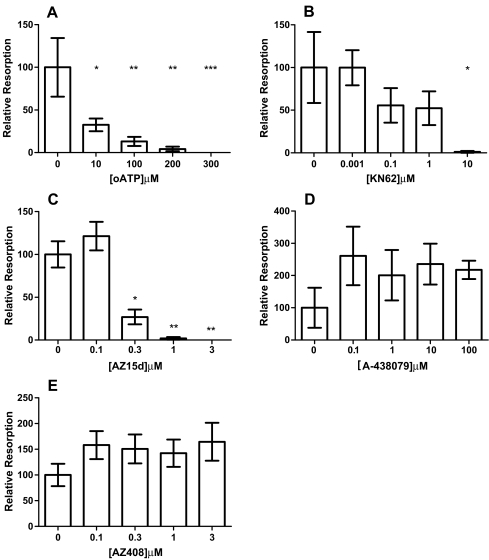
Treatment with AZ15d, KN62 and oATP significantly and dose-dependently inhibited osteoclast formation (Fig. 3a–c) in the cultures. These compounds only appeared to inhibit fusion of cells; as the concentration of the compounds increased, the size and multinucleation of cells decreased, but other smaller, mononuclear, cells remained (Fig. 4). Clusters of mononuclear cells were observed in oATP and AZ15d treated cultures, indicating that migration of cells towards each other had not been inhibited, but fusion of the cells was prevented. The clusters were not as apparent in the KN62 treated cultures. IC50 values were calculated and AZ15d was the most potent at 0.7 μM, followed by KN62 at 2 μM and with oATP being the least potent at 91 μM. A-438079 did not have any significant effect on osteoclast number (Fig. 3d) when used between 100nM to 100 μM (reported IC50 = 0.125 μM) nor did AZ408, a compound that is structurally similar to AZ15d but ineffective at the P2X7R, when used between 100nM to 10 μM (Fig 3e).
Fig. 3.
Effect of P2X7R antagonists on human osteoclast formation. Osteoclasts were generated from human blood monocytes cultured on 6-mm coverslips in recombinant M-CSF and RANKL-supplemented medium. Antagonists were introduced throughout the culture by inclusion in the medium at each medium change at the concentrations shown on the x axis. Vehicle was complete α-MEM plus recombinant RANKL (30 ng/ml) and M-CSF (25 ng/ml) and 0.1% DMSO for KN62, AZ15d and AZ408. The number of osteoclasts was determined by counting all TRAP-positive cells with three or more nuclei. Data show means ± SEM, *p < 0.05, **p < 0.01 ***p < 0.0001 Graph representative of four repeat experiments, n = 7. a oATP, b KN62, c AZ15d, d A-438079 and e AZ408
Fig. 4.
P2X7R antagonists inhibit human osteoclast formation but are not toxic. Osteoclasts were generated from human blood monocytes cultured on 6-mm coverslips in recombinant M-CSF and RANKL-supplemented medium. Antagonists were introduced throughout the culture by inclusion in the medium at each medium change at the concentrations shown. Vehicle was complete α-MEM plus recombinant RANKL (30 ng/ml) and M-CSF (25 ng/ml) and 0.1% DMSO for KN62, and AZ15d. The coverslips were fixed in acetone and stained for tartrate-resistant acid phosphatase (TRAP) using a commercially available kit used according to the manufacturer’s protocol or fixed in formalin and stained as previously described [25]. Typical fields of view of cells following treatment in μM as indicated. Scale bar 30 μ
Effect of P2X7R antagonists on human osteoclast resorption
Whilst the criteria of TRAP-positive cells with three or more nuclei to identify osteoclasts cultured in vitro on coverslips is widely accepted, the only definitive marker for osteoclasts remains the ability to form resorption lacunae on calcified substrates. Therefore, to determine whether the P2X7R antagonists could also inhibit the formation of fully functional human osteoclasts, blood monocytes were cultured on dentine discs in the presence of recombinant RANKL and M-CSF. The antagonists were introduced throughout a 3-week culture period by inclusion in the medium at each medium change at various concentrations. At the end of the culture, the area of resorption excavated by active osteoclasts was determined. Treatment with AZ15d, KN62 and oATP significantly decreased the area of resorption produced on each disc in a dose-dependent manner (Fig. 5a-c). Again, A-438079 and AZ408 did not have any significant effect on resorption or osteoclast number (Fig. 5d-e). IC50 values were similar to those for formation, with AZ15d being the most potent at 0.2 μM, followed by KN62 at 1 μM and with oATP being the least potent at 29 μM.
Fig. 5.
Effect of P2X7R antagonists on human osteoclast resorption. Osteoclasts were grown on dentine discs from blood monocytes in the presence of recombinant M-CSF and RANKL. Antagonists were introduced throughout the culture by inclusion in the medium at each medium change at the concentrations shown on the x axis. Vehicle was complete α-MEM plus recombinant RANKL (30 ng/ml) and M-CSF (25 ng/ml) and 0.1% DMSO for KN62, AZ15d and AZ408. After 3 weeks in culture, discs were fixed and stained with toluidine blue or TRAP. The area of resorption excavated by these cells was determined by point counting [26]. Data show means ± SEM, *p < 0.05, **p < 0.01 ***p < 0.0001. Graphs representative of four repeat experiments, n = 7. a oATP, b KN62, c AZ15d, d A-438079 and e AZ408
Discussion
In this study, we have further investigated the role of P2X7R in the formation of functional human osteoclasts from their monocytic precursors found in peripheral blood. We have shown that P2X7R mRNA expression was present at all stages investigated of a 21-day culture of blood monocytes cultured in the presence of recombinant RANKL and M-CSF (Fig. 2). This is consistent with our previous reports that both P2X7R mRNA and protein were expressed throughout the 3-week culture period [17]. The exact physiological function of the P2X7R in osteoclasts is still contended. A recent study provided evidence that P2X7R activation on rabbit osteoclasts causes a Ca2+ influx that could possibly lead to the inhibition of resorption [15], and we have previously shown, using human giant cell tumour-derived osteoclast-like cells, that P2X7R activation potently inhibited bone resorption in vitro by inducing apoptosis of osteoclasts [30]. We have also previously demonstrated that inhibition using the blocking monoclonal antibody for the P2X7R significantly inhibits the fusion of osteoclast precursors to form multinucleated osteoclasts. A recent report demonstrated that when RAW 264.7 cells were exposed to high levels of ATP overnight, surface expression of P2X7Rs was down-regulated and this prevented cell fusion [31]. The apparent contradiction that both activation and inhibition of P2X7R signalling reduces osteoclast numbers and inhibits resorption reflects the complex nature of the P2X7R. In addition, a role for this receptor in cell fusion has long been speculated due to the observation that macrophage cell clones expressing high levels of P2X7R spontaneously fuse in vitro and that the P2X7R is preferentially localised at sites of cell-to-cell contact [32]. Therefore, we have examined the effect of the most commonly used commercially available P2X7R antagonists, as well as a small-molecule compound AZ15d on formation of functional multinucleated human osteoclasts.
Introduction of the P2X7R antagonists AZ15d, KN62 and oATP to blood monocytes cultured in the presence of recombinant RANKL and M-CSF dose-dependently decreased the formation of multinucleated TRAP-positive osteoclasts (Fig. 3) and the overall area of resorption excavated on dentine discs by these cells (Fig. 5). Addition of a compound with similar chemical structure to AZ15d but that is inactive at the P2X7R had no effect on any of the parameters measured. Interestingly, A-438079 had no significant effect on osteoclast formation or resorption. This was somewhat surprising given its reported potency and specificity at the P2X7R [33, 34]. One possible explanation for the lack of effect with A-438079 is that it is a competitive and reversible antagonist [34]. Oxidised ATP is an irreversible P2X7R inhibitor [35, 36], whilst KN62 and the cyclic imide group of antagonists are non-competitive allosteric inhibitors [37, 38]. Given that these cultures were performed over a three week period and we have measured a long-term response, it could be possible that A-438079 has been competed off the receptor during this time, thus reducing its efficacy.
These data demonstrate that P2X7R antagonists acted to inhibit the formation of osteoclasts from their precursors, which manifested itself functionally in a decrease in resorption pits excavated on dentine by these cultures. Despite the decreasing number of osteoclasts generated as the concentration of antagonists increased, the viability of the mononuclear cells remaining in the culture was unaffected, demonstrating that the antagonists were not causing cell toxicity. As can be seen in Fig. 4, the mononuclear cells appeared to form cell aggregates indicating that they were following a pathway of activity leading to fusion, but were unable to carry out this final step when higher concentrations of P2X7R antagonists were present. This is consistent with a report describing how P2X7Rs preferentially localise to sites of cell-to-cell contact [32] and a previous observation that the formation of cell aggregates in culture was a prerequisite to cell fusion [7].
The hypothesis for the involvement of the P2X7R in the actual fusion process is somewhat contradicted by the fact that two different models of P2X7R KO mice both maintained the ability to produce multinucleated osteoclasts in vitro and in vivo [18, 19]. However, the data presented in this manuscript using two of the most commonly used commercially available P2X7R antagonists and AZ15d, part of a series of highly selective and potent small-molecule P2X7R antagonists, supports the argument that the P2X7R is indeed directly involved in osteoclast fusion but that in its absence it can be replaced by some other machinery such as a common pore-structure that is activated by amongst other things maitotoxin [39]. Within the bone microenvironment osteoblasts, osteoclast precursors and osteoclasts express the majority of P2X and P2Y receptor sub-types [40–42] and it is feasible that P2 receptors other than the P2X7R may play a role in the formation of functional human osteoclasts. Indeed, we have previously shown that ATP in co-cultures of osteoblasts and osteoclasts stimulated osteoclast resorption at low micromolar concentration, however, when added to cultures of osteoclasts alone no effect was observed. The effect of low micromolar concentration of ATP (i.e., via P2Y or P2X receptors other than P2X7R) on osteoclasts appears to be an indirect effect mediated via the upregulation of RANKL expression by osteoblasts [28]. It is also possible that other P2X and P2Y receptors may be upregulated in the absence or blockade of the P2X7R as a compensatory mechanism.
In summary, these data provide further evidence for the involvement of the P2X7R in the complex chain of events leading to the formation of human osteoclasts. In skeletal homeostasis, a balance between osteoclastic resorption and osteoblastic bone formation is essential to a functional skeleton. The availability of these potent and selective P2X7R antagonists may prove to be an important advancement in the management of bone diseases with an increase in osteoclast number, such as Paget’s disease of bone, or in disorders of remodelling where there is a reduced bone mass, such as osteoporosis.
Acknowledgments
The authors thank the Arthritis Research Campaign for funding (KAB, ref G0566). AA, AG and JAG were kindly supported by the European Commission under the 7th Framework Programme (proposal #202231) performed as a collaborative project among the members of the ATPBone Consortium (Copenhagen University, University College London, University of Maastricht, University of Ferrara, University of Liverpool, University of Sheffield, and Université Libre de Bruxelles), and is a sub study under the main study “Fighting osteoporosis by blocking nucleotides: purinergic signalling in bone formation and homeostasis”. We would also like to thank Dr. W.B. Bowler for his support of this project.
References
- 1.Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- 2.Chessell IP, Michel AD, Humphrey PP. Effects of antagonists at the human recombinant P2X7 receptor. Br J Pharmacol. 1998;124:1314–1320. doi: 10.1038/sj.bjp.0701958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 4.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 6.Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991;112:279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virgilio F, Falzoni S, Chiozzi P, Sanz JM, Ferrari D, Buell GN. ATP receptors and giant cell formation. J Leukoc Biol. 1999;66:723–726. doi: 10.1002/jlb.66.5.723. [DOI] [PubMed] [Google Scholar]
- 8.Marks S, Jr, Gartland A, Odgren P. Skeletal development. In: Martini L, editor. Encyclopaedia of endocrinology and endocrine diseases. San Diego: Academic; 2004. pp. 261–272. [Google Scholar]
- 9.Biskobing DM, Fan X, Rubin J. Characterization of MCSF-induced proliferation and subsequent osteoclast formation in murine marrow culture. J Bone Miner Res. 1995;10:1025–1032. doi: 10.1002/jbmr.5650100706. [DOI] [PubMed] [Google Scholar]
- 10.Fujikawa Y, Quinn J, Sabokbar A, McGee J, Athanasou N (1996) Human osteoclasts differentiate from a sub-population of circulating monocytes. J Bone Miner Res 11:M399
- 11.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 12.Gartland A, Gallagher J, Bowler W.Expression of the pore-forming P2X7 receptor by human giant cell tumour, osteoclasts and human RANKL-generated osteoclasts J Bone Miner Res 200015361–369.10703939 [Google Scholar]
- 13.Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR. Expression of P2 receptors in bone and cultured bone cells. Bone. 2000;27:503–510. doi: 10.1016/S8756-3282(00)00351-3. [DOI] [PubMed] [Google Scholar]
- 14.Modderman WE, Weidema AF, Vrijheid-Lammers T, Wassenaar AM, Nijweide PJ. Permeabilization of cells of hemopoietic origin by extracellular ATP4-: elimination of osteoclasts, macrophages, and their precursors from isolated bone cell populations and fetal bone rudiments. Calcif Tissue Int. 1994;55:141–150. doi: 10.1007/BF00297190. [DOI] [PubMed] [Google Scholar]
- 15.Naemsch LN, Dixon SJ, Sims SM. Activity-dependent development of P2X7 current and Ca2+ entry in rabbit osteoclasts. J Biol Chem. 2001;276:39107–39114. doi: 10.1074/jbc.M105881200. [DOI] [PubMed] [Google Scholar]
- 16.Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S, Gretener D, Grahames C, Kaur R, Kosco-Vilbois MH, Humphrey PP. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521–3528. [PubMed] [Google Scholar]
- 17.Gartland A, Buckley KA, Bowler WB, Gallagher JA. Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro. Calcif Tissue Int. 2003;73:361–369. doi: 10.1007/s00223-002-2098-y. [DOI] [PubMed] [Google Scholar]
- 18.Gartland A, Buckley KA, Hipskind RA, Perry MJ, Tobias JH, Buell G, Chessell I, Bowler WB, Gallagher JA. Multinucleated osteoclast formation in vivo and in vitro by P2X7 receptor-deficient mice. Crit Rev Eukaryot Gene Expr. 2003;13:243–253. doi: 10.1615/CritRevEukaryotGeneExpr.v13.i24.150. [DOI] [PubMed] [Google Scholar]
- 19.Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Liu D, Ke HZ, Duncan RL, Turner CH (2005) The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem 280:42952-42959 [DOI] [PubMed]
- 21.Jorgensen NR, Henriksen Z, Sorensen OH, Eriksen EF, Civitelli R, Steinberg TH. Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J Biol Chem. 2002;277:7574–7580. doi: 10.1074/jbc.M104608200. [DOI] [PubMed] [Google Scholar]
- 22.Alcaraz L, Baxter A, Bent J, Bowers K, Braddock M, Cladingboel D, Donald D, Fagura M, Furber M, Laurent C, Lawson M, Mortimore M, McCormick M, Roberts N, Robertson M. Novel P2X7 receptor antagonists. Bioorg Med Chem Lett. 2003;13:4043–4046. doi: 10.1016/j.bmcl.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Stokes L, Jiang LH, Alcaraz L, Bent J, Bowers K, Fagura M, Furber M, Mortimore M, Lawson M, Theaker J, Laurent C, Braddock M, Surprenant A. Characterization of a selective and potent antagonist of human P2X(7) receptors, AZ11645373. Br J Pharmacol. 2006;149:880–887. doi: 10.1038/sj.bjp.0706933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzaki K, Udagawa N, Takahashi N, Yamaguchi K, Yasuda H, Shima N, Morinaga T, Toyama Y, Yabe Y, Higashio K, Suda T. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem Biophys Res Commun. 1998;246:199–204. doi: 10.1006/bbrc.1998.8586. [DOI] [PubMed] [Google Scholar]
- 25.van’t Hof RJ, Tuinenburg-Bol Raap AC, Nijweide PJ. Induction of osteoclast characteristics in cultured avian blood monocytes; modulation by osteoblasts and 1, 25-(OH)2 vitamin D3. Int J Exp Pathol. 1995;76:205–214. [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh CA, Beresford JN, Birch MA, Boothroyd B, Gallagher JA. Application of reflected light microscopy to identify and quantitate resorption by isolated osteoclasts. J Bone Miner Res. 1991;6:661–671. doi: 10.1002/jbmr.5650060703. [DOI] [PubMed] [Google Scholar]
- 27.Buckley KA, Chan BY, Fraser WD, Gallagher JA. Human osteoclast culture from peripheral blood monocytes: phenotypic characterization and quantitation of resorption. Methods Mol Med. 2005;107:55–68. doi: 10.1385/1-59259-861-7:055. [DOI] [PubMed] [Google Scholar]
- 28.Buckley KA, Hipskind RA, Gartland A, Bowler WB, Gallagher JA. Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-kappa B ligand. Bone. 2002;31:582–590. doi: 10.1016/S8756-3282(02)00877-3. [DOI] [PubMed] [Google Scholar]
- 29.Chan BY, Gartland A, Wilson PJ, Buckley KA, Dillon JP, Fraser WD, Gallagher JA. PPAR agonists modulate human osteoclast formation and activity in vitro. Bone. 2007;40:149–159. doi: 10.1016/j.bone.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Gartland A, Ginty A, Gallagher J, Bowler W. Activation of P2X7 receptors expressed by human osteoclastoma modulates bone resorption. Calcif Tissue Int. 1999;64:S56. [Google Scholar]
- 31.Steinberg TH, Heiken JF. P2 receptors in macrophage fusion and osteoclast formation. Purinergic Signalling. 2007;3:53–57. doi: 10.1007/s11302-006-9036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falzoni S, Chiozzi P, Ferrari D, Buell G, Virgilio F. P2X(7) receptor and polykarion formation. Mol Biol Cell. 2000;11:3169–3176. doi: 10.1091/mbc.11.9.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT, Donnelly-Roberts DL, Niforatos W, Honore P, Jarvis MF, Faltynek CR, Carroll WA. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem. 2006;49:3659–3666. doi: 10.1021/jm051202e. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:573–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murgia M, Hanau S, Pizzo P, Rippa M, Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- 37.Gargett CE, Wiley JS. The isoquinoline derivative KN-62 a potent antagonist of the P2Z-receptor of human lymphocytes. Br J Pharmacol. 1997;120:1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michel AD, Ng SW, Roman S, Clay WC, Dean DK, Walter DS. Mechanism of action of species-selective P2X(7) receptor antagonists. Br J Pharmacol. 2009;156:1312–1325. doi: 10.1111/j.1476-5381.2009.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schilling WP, Wasylyna T, Dubyak GR, Humphreys BD, Sinkins WG. Maitotoxin and P2Z/P2X(7) purinergic receptor stimulation activate a common cytolytic pore. Am J Physiol. 1999;277:C766–C776. doi: 10.1152/ajpcell.1999.277.4.C766. [DOI] [PubMed] [Google Scholar]
- 40.Gallagher JA, Buckley KA. Expression and function of P2 receptors in bone. J Musculoskelet Neuronal Interact. 2002;2:432–439. [PubMed] [Google Scholar]
- 41.Gartland A, Buckley KA, Hipskind RA, Bowler WB, Gallagher JA. P2 receptors in bone—modulation of osteoclast formation and activity via P2X7 activation. Crit Rev Eukaryot Gene Expr. 2003;13:237–242. doi: 10.1615/CritRevEukaryotGeneExpr.v13.i24.140. [DOI] [PubMed] [Google Scholar]
- 42.Orriss IR, Knight GE, Ranasinghe S, Burnstock G, Arnett TR. Osteoblast responses to nucleotides increase during differentiation. Bone. 2006;39:300–309. doi: 10.1016/j.bone.2006.02.063. [DOI] [PubMed] [Google Scholar]