Abstract
This Classic Article is a reprint of the original work by Otto Heinrich Warburg, The Chemical Constitution of Respiration Ferment. An accompanying biographical sketch of Otto Heinrich Warburg, PhD, MD, is available at DOI 10.1007/s11999-010-1533-z. The Classic Article is from Warburg O. The chemical constitution of respiration ferment. Science. 1928;68:437–443. Reprinted with permission from AAAS.
I
Ferments are substances which effect chemical reaction in living matter. It is one of their properties that they occur in living matter in extremely small concentration. It has not been possible thus far to measure the amount of a ferment present anywhere. It is also one of their properties that they are unstable. In all attempts to isolate the ferments they are usually destroyed, and we have not yet succeeded in the preparation of a pure ferment. Because of this it is not known how the ferments are chemically constituted, and hence it is not understood how chemical reactions occur in living matter.
The ferment which I shall discuss is the respiration ferment, of which it has been said that it rules the organic world, for in everything that happens in living matter respiration furnishes the driving force. Chemically speaking, respiration is an oxidation by the oxygen of the air, and hence the respiration ferment is a substance which takes up the atmospheric oxygen and transfers it to the organic substances. This ferment is, as we have found, in its chemical constitution related to the red pigment of the blood, hemoglobin. I shall at first describe the significant properties of the red blood pigment and of its related substances and will also show that these properties are as well the properties of the respiration ferment.
Hemoglobin, the red blood pigment, consists of a colorless component, the basic protein globin, and of a colored component, hemin. These components can be separated. When, however, they are brought together under certain conditions they unite, as Robert Hill, of Cambridge, has recently shown, to form hemoglobin again. In this reaction the globin can be replaced by other and simpler bases, for example, by pyridin or nicotin. Then instead of the hemoglobin we obtain hemopyridin or hemonicotin. These compounds, of which many can be prepared, are indeed different in their chemical properties, but their specific and biologically important reactions are similar, and all of them depend upon the hemin, which therefore must be regarded as the reactive nucleus of the hemin compounds.
Hemin was discovered in 1853 by Teichmann by the acid hydrolysis of hemoglobin and obtained in a crystalline form. Hemin is a complex iron compound, more exactly a tetrapyrrole iron compound, in which the iron is bound to the pyrrole nitrogen. Its structure is relatively simple, and its molecular weight is 650. Its constitution has been worked out by Nencki, Küster, Willstätter and Hans Fischer.
Just as hemin is the reactive nucleus of hemin compounds, so is iron the reactive nucleus of hemin. The specific and biologically important reactions of hemin are reactions of complex iron compounds.
II
Of all the hemin compounds the one most clearly worked out is hemoglobin. The iron of hemoglobin reacts in a reversible manner with molecular oxygen according to the equation
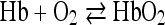 |
1 |
in which one atom of iron combines with one molecule of oxygen.
It is on this reaction that the function of the hemoglobin in the body is based. In the capillaries of the lungs hemoglobin takes up oxygen, transports it via the blood stream throughout the body and gives it up by dissociation in the capillaries of the tissues in which the oxygen pressure is lower. The oxygen then diffuses through the capillary walls into the tissue cells, where it is utilized for respiration. Thus hemoglobin is a transport agency for oxygen and not a catalyzer or a ferment. It does not transfer oxygen to organic molecules, but it merely transports the oxygen from one place in the body to another place. A catalytic action of hemoglobin would even be at variance with its physiologic function. For it only delivers to the tissues the oxygen which it has taken up in the lungs, but does not utilize it on its way in any chemical reaction.
III
The iron of the hemoglobin reacts not only with oxygen but also carbon monoxide, reversibly, according to the equation
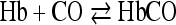 |
2 |
in which one atom of iron takes up one molecule of CO.
If oxygen and CO are permitted to react simultaneously with hemoglobin, then the two gases compete for the iron atom and according to the partial pressures the CO suppresses the oxygen or vice versa:
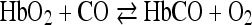 |
3 |
Finally, equilibrium is obtained in the following manner:
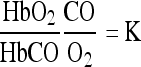 |
4 |
which means that the distribution of hemoglobin between oxygen and CO is completely determined by the ratio 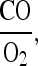 i.e., the ratio of the partial pressures of the two gases. It is on the replacement of oxygen by carbon monoxide as in equation (3) that the poisonous effect of carbon monoxide depends. When the pressure of CO in the respired air is overwhelmingly great the carbon monoxide replaces the oxygen on the hemoglobin. It is then that the hemoglobin can no longer transport any oxygen, and the tissues of the body are asphyxiated.
i.e., the ratio of the partial pressures of the two gases. It is on the replacement of oxygen by carbon monoxide as in equation (3) that the poisonous effect of carbon monoxide depends. When the pressure of CO in the respired air is overwhelmingly great the carbon monoxide replaces the oxygen on the hemoglobin. It is then that the hemoglobin can no longer transport any oxygen, and the tissues of the body are asphyxiated.
CO-hemoglobin has the remarkable property, discovered in 1897 by John Haldane, that it is split into CO and hemoglobin upon being exposed to light.1
On the other hand, oxyhemoglobin is quite stable in light. Hence the distribution of hemoglobin between CO and O2 is altered on exposure to light. If hemoglobin is brought into equilibrium with CO and O2 in the dark and is subsequently exposed to light the CO compound diminishes in concentration and the O2 compound increases until a new stationary equilibrium is reached, which depends upon the intensity of the illumination.
In principle the free reduced hemin and the compounds of hemin with other bases behave like hemoglobin, only the velocity of the reaction and the affinity of the iron atom towards O2 and CO are altered. This explains the fact that one hemin compound is capable of catalytic action, as the hemonicotin, while another hemin compound, as hemoglobin, has no such capacity.
IV
Hemoglobin predominates in the blood of the higher animals. Other hemin compounds occur, as discovered by MacMunn in 1886, in cells and indeed not only in the cells of hemoglobin carrying animals, but also in all cells. Keilin demonstrated in 1925 the presence of cell hemins in plants, bacteria and yeasts. In the course of evolution hemin must have appeared in nature earlier than hemoglobin, as MacMunn had already surmised.
The work of MacMunn was neglected and partly doubted, and it was only recently that Hans Fischer and Keilin brought it the credit that it deserves. Hans Fischer isolated porphyrin, i.e., hemin minus the iron, from yeast and showed that yeast can synthesize hemin from porphyrin and iron. Keilin confirmed and extended the discovery of MacMunn in an excellent spectroscopic investigation and called the cellular hemin cytochrome.
Such are the fundamental facts of the chemistry and physiology of hemin compounds, of which we shall make use in the following discussion. I repeat them: The universal distribution of hemin in nature; the reversible reaction of its iron atom with oxygen; reversible reaction of its iron atom with CO; the distribution between O2 and CO according to the equation of distribution, and finally the sensitivity to light of the CO compound.
V
Since Claude Bernard discovered in the middle of the preceding century carboxy-hemoglobin it has been believed that CO combines in the organism exclusively with hemoglobin and not with cellular substances. Particularly illuminating was an investigation of Haldane in 1895. When he placed mice in air containing CO, the CO displaced the oxygen in the hemoglobin, and the mice died for lack of oxygen. But when he raised the oxygen pressure to two atmospheres, then the total hemoglobin remained indeed combined with the CO, but the oxygen which was in a state of physical solution in the blood increased tenfold. In this case the mice did not die, because the physically dissolved oxygen sufficed to supply the tissues.
This experiment indicates clearly that the CO even at pressures at which it does not affect the respiration of the cells still combines with the hemoglobin, but it does not indicate that the respiration ferment is incapable of reacting with CO at any CO pressure.
In fact it developed that in this case only quantitative differences are involved. If the pressure of CO is permitted to rise to about one atmosphere then the respiration ferment combines with the CO. In this case the respiration of the cells ceases because the CO compound of the ferment can not transfer catalytically any oxygen. The catalytically active iron atom of the respiration ferment is blocked, as it were, by the carbon monoxide. If the CO pressure is permitted again to drop, normal respiration reappears. The respiration ferment (Fe) therefore reacts, just as the hemoglobin, reversibly with carbon monoxide:
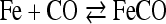 |
5 |
We have discovered this reaction in our investigations on yeast and later we recognized it as a general cellular reaction.
If the CO pressure is kept constant and the O2 pressure is permitted to rise then the effect of the CO upon the respiration decreases, to disappear completely at very high oxygen pressures. It is possible, therefore, to suppress the effect of CO upon the respiration ferment by means of oxygen.
From our measurements of respiration at various CO and O2, pressures we deduced the law according to which the distribution of the respiration ferment between CO and O2 occurs. We found that
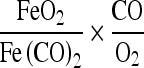 |
6 |
is constant. Hence the respiration ferment is subject to the same distribution equation as hemoglobin. Only the numerical values of the constants are different, the constant for the hemoglobin equation being 0.01, the constant for the ferment equation about 10.
Furthermore: If the respiration of the cells is inhibited in the dark by means of CO, the cells being then exposed to light, the inhibition of the respiration diminishes at once and disappears completely on excessively intense illumination. Thus we see that light displaces the distribution of the respiration ferment between CO and O2 in favor of the oxygen, exactly in the same manner in which light effects the distribution of hemoglobin between the two gases. Also in this case the differences between the hemoglobin and the respiration ferment are only quantitative. In order to effect photochemically the distribution of hemoglobin very great intensity is required; on the other hand, the intensity of light required to effect the distribution of the respiration ferment is one ten thousandth of the intensity of sunlight.
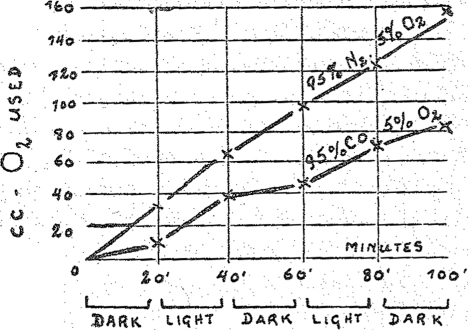
The course of an experiment with respiring cells is graphically shown in Fig. 1. The abscissas show the time t, and the ordinates the amounts of oxygen respired. The slope of the line against the abscissa represents the rate of respiration. The experiment began in the dark, and exposure to light began after twenty minutes. After another twenty-minute period it was placed in the dark again, etc. The lower line shows the behavior in carbon monoxide: at each change from light to darkness and vice versa there is seen a sudden bend; and also it is ‘seen that the respiration is less in the dark and greater in light. The upper line shows the relation of respiration under exactly the same conditions but without carbon monoxide. This line does not show any effect of illumination.
Fig. 1.
Effect of carbon monoxide upon the respiration of living cells (dark and light).
Summarizing, we have found that the respiration ferment has three characteristic properties of hemoglobin: It reacts reversibly with CO and O2; it is distributed between CO and O2 according to the definite equation of distribution; in combination with CO it is sensitive to light.
VI
The differences between hemoglobin and the respiration ferment are: hemoglobin, in contrast to the respiration ferment, does not act as a catalyst. Hemoglobin binds CO more firmly than the respiration ferment, and this combination is decomposed by light with much greater difficulty than the CO respiration ferment compound.
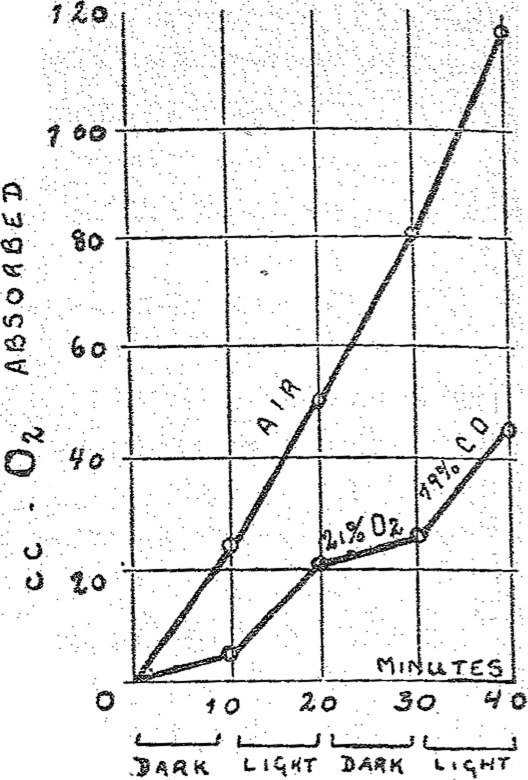
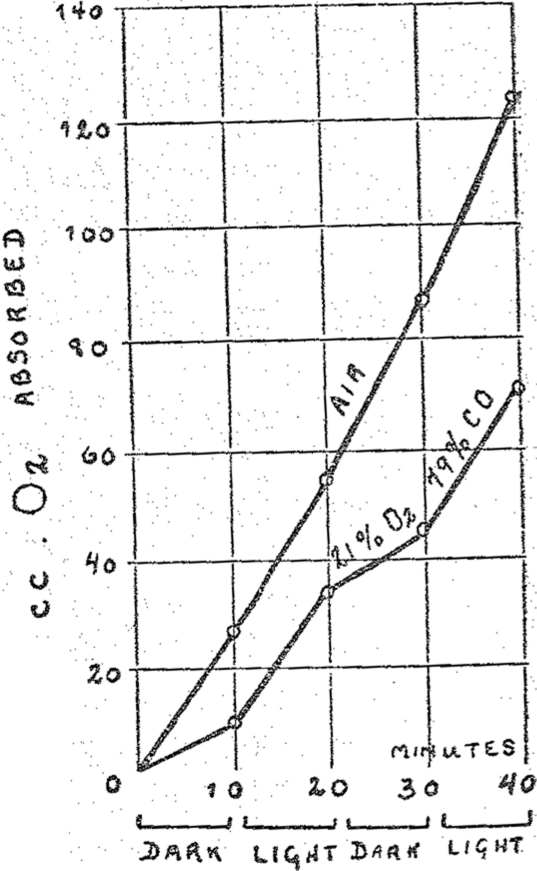
The properties of the respiration ferment are approached more closely if the globin is removed from the iron-carrying component of hemoglobin. Free hemin behaves as a catalyst and, as was shown by D. C. Harrison, of Cambridge, it is capable, for example, of oxidizing catalytically cystein in aqueous solution to cystin. This catalysis, according to the investigation of H. A. Krebs, is inhibited by the same pressure of CO as is respiration. Still the sensitivity to light of CO-hemin is only one ten thousandth of the sensitivity to light of the CO-respiration ferment compound. Even this discrepancy can be overcome, If hemin is combined with pyridin or nicotin, we obtain powerfully active catalysts whose Fe is capable of transferring two thousand molecules of oxygen to cystein. The CO compounds of these hemin derivatives were decomposed by light of one ten thousandth of the intensity of sunlight, according to H. A. Krebs. They are therefore just as sensitive to light as the CO compound of the respiration ferment. The above described experiments with living cells could be repeated by us without living cells in simple solutions. In Figs. 2 and 3 I am showing two illumination experiments in simple solutions in which hemopyridin and hemonicotin were used as the catalysts. Both these figures are very similar to the first figure. Thus if one chooses appropriate hemin compounds for catalysts one can obtain a system which bears the same relation towards CO and light as the respiring living substance.
Fig. 2.
Effect of carbon monoxide upon the catalytic action of hemopyridin (dark and light).
Fig. 3.
Effect of carbon monoxide upon the catalytic action of hemonicotin (dark and light).
VII
If the respiration ferment is a hemin compound, and indeed one finds spectroscopically a hemin compound in all cells (Keilin’s cytochrome) then we might believe that the respiration ferment and cytochrome are identical. That this is not correct is shown by the following experiments:
Cytochrome-hemin occurs in considerable concentration in yeast. If, however, one attempts to determine quantitatively the ferment-hemin (for which we have a method at our disposal which is supposed to indicate the presence of one millionth of one per cent.) then, as was found by Mr. F. Kubowitz, the amount of ferment present is zero. This means that only a minimal fraction of the cell hemin is ferment hemin.
While hemoglobin, the respiration ferment, and many other hemin compounds which we have investigated react in the reduced state with O2 and CO, cytochrome on the other hand does not combine either with oxygen or with CO, at least not at a pressure of one atmosphere. Therefore, cytochrome represents a hemin compound which has lost the characteristic properties of hemin. Just as hemoglobin is a denatured respiration ferment, because its Fe atom no longer behaves catalytically, so is also cytochrome a denatured ferment whose Fe atom is no longer capable of reacting with oxygen. Thus, the respiration ferment and cytochrome are different substances which differ by their respective concentrations in the cells and by their behavior toward O2 and CO. Incidentally, this again confirms the old experience that substances which occur in the cells in any considerable concentration are not ferments.
VIII
It follows from the above that there are two conditions which make it difficult to identify the respiration ferment as hemin: the minute concentration of the ferment and the great concentration of the cell hemin which is not ferment hemin. The inactive cell hemin obscures the ferment hemin in all analytic procedures, whether chemical or spectroscopical. It must, therefore, suffice to state that the respiration ferment behaves as a hemin and is, therefore, a hemin, or a method must be found which can differentiate the ferment hemin from the other hemins of the cell. Such a method is the following:
We inhibit the respiration of living cells by CO and then expose to light of different wave-lengths. On illumination the respiration rises, as we have seen above. To this the new observation can be added that the respiration rises to different levels according to the wave-length which we employ. Thus, if we render the intensities of the different wave-lengths equal, i.e., we expose the cells to light of different colors but of equal intensities, then we find that the effect upon the respiration in the ultra-violet at 366 μμ is very small, great in blue, again small in green, etc. If we represent the effect of the light in relation to its wave-length graphically we obtain a curve which we designate as the action curve. The varying effects of the wave-length may have various reasons, but by all means the most probable cause lies in the fact that the respiration ferment absorbs light of different wave-lengths to a different extent. In such a ease our action curve is nothing else but an absorption spectrum of the respiration ferment. If this is accepted then the absorption spectrum of the respiration ferment can be determined by means of exposing cells in carbon monoxide to lights of different colors but of equal intensity and by comparing the results; or by varying the intensities of the different colors in such a way that equal results are obtained.
It is clear that with such an arrangement the catalytically inactive cell hemins, such as the cytochrome, do not interfere. It is true that the inactive hemins absorb light just as the ferment hemin does, but the light absorbed by the inactive hemin has no effect upon respiration. Only that light which is absorbed by the respiration ferment affects respiration. It is of equally little account in the above procedure that the concentration of the ferment is infinitely small, since we measure infinitely great effects of the ferment in relation to the amount of ferment present.
IX
Since the principle of determining the absorption spectrum of a catalyst from the photochemical effect upon the catalysis is new, it was desirable to test it out under simple conditions before applying it to a living system. We sought to prove first that it is the difference of absorption only which determines the different effects of the colors.
Particularly adaptable for this purpose is the hemonicotin model which is demonstrated by a great sensitivity to light. With this model the absorption spectrum of the catalyst can be taken photometrically or bolometrically, to begin with from a clear aqueous solution in which only the catalyst itself—hemonicotin—absorbs light. Then the action curve can be photographed in which the catalysis is being inhibited by CO and stimulated by different colored lights, and the effect of the light is represented as a function of the wave-length. If our assumption is correct, the directly measured absorption curve and the action curve must have the same form.
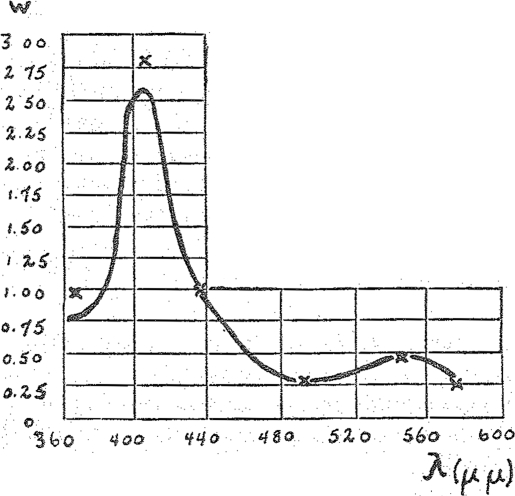
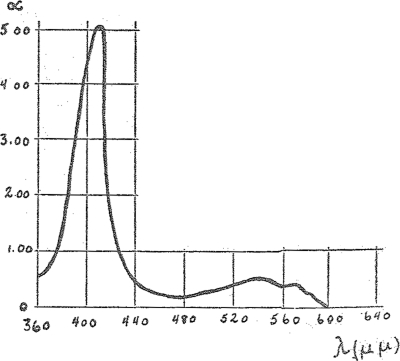
In our experiment we have employed six regions of the spectrum, i.e., six lines of the mercury vapor lamp: ultra-violet—366 μμ, violet—408 μμ, blue—436 μμ, blue green—492 μμ, green—546 μμ, and yellow of 578 μμ. The results are shown in the curves of Figs. 4 and 5. The drawn-out line shows the bolometrically measured absorption spectrum. The crosses indicate the actions observed with light of different colors but of equal intensity, referred to the blue mercury line of 436 μμ. The light intensities were equal either in calories (Fig. 4) or in quanta (Fig. 5). In both cases the crosses fall very closely upon the absorption curves. Thus no matter whether one calculates in calories or quanta intensities the observed catalytic action yields in effect very closely the absorption spectrum. This shows that the assumption agrees with our method. The spectrum of a catalytically active hemin complex can be obtained from the photo-chemical effect upon the catalysis.
Fig. 4.
Spectrum of CO-hemonicotin. Crosses (×)—catalytic action at equal caloric intensity.
Fig. 5.
Spectrum of CO-hemonicotin. Crosses (×)—catalytic action at equal quantum intensity.
X
In applying this method to living material we used yeast cells. Their respiration was inhibited by means of CO. Then they were exposed to different lights of equal (quanta) intensity and the action upon respiration was measured. The spectral regions used were again the above six mercury lines and in addition two regions in the red which were isolated from the radiation of a metal filament lamp.
Before showing you the results I shall throw upon the screen the spectrum of CO-hemin in order to orient ourselves in the general form of the hemin spectrum (Fig. 6). The characteristic features are the high and the relatively sharp band in the blue, the minimum in the blue green, a flat band in the green yellow, and a very slight absorption in the red. It is because of this that all these pigments in thick layers appear red as blood.
Fig. 6.
Spectrum of CO-compound of (reduced) hemin (taken bolometrically and photometrically).
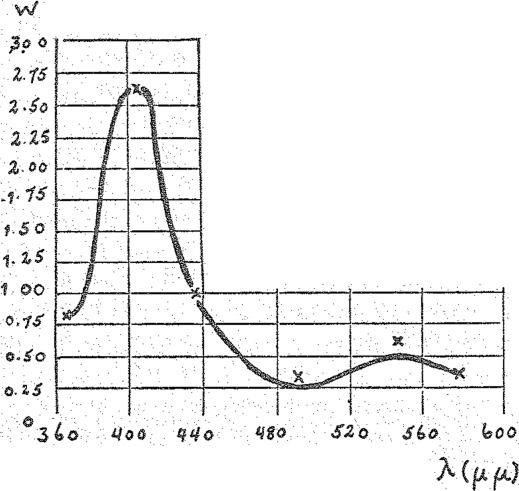
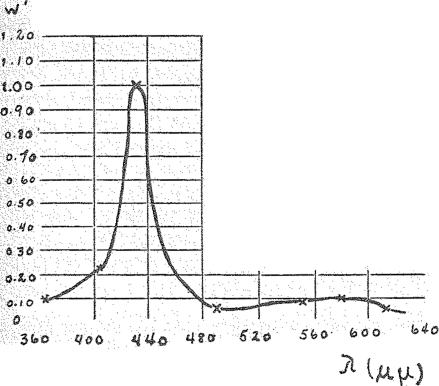
Now I shall show you the absorption spectrum of the respiration ferment (Fig. 7). You will see here the typical hemin spectrum: the high and relatively sharp band in the blue, the minimum in the blue green, the flat band in the green yellow and the very slight absorption in the red.
Fig. 7.
Spectrum of the respiration ferment. (Crosses, ×,—catalytic action on illumination with lights of equal quantum intensity.)
XI
On closer examination of the spectra of the respiration ferment and of the known hemin compounds a difference is revealed. The maximum of the blue band for the respiration ferment lies at 436 μμ and at 408 μμ for CO-hemin. The spectra of none of the known hemin compounds coincide completely with that of the respiration ferment, the latter being displaced towards the red in respect to the former.
It must be recalled here that the respiration ferment is not in solution in the cell but is embedded in the solid cellular components of the surfaces. The spectrum of the respiration ferment which I have shown is not of the dissolved but of the solid ferment, whereas the spectra of the other hemin compounds were taken from solutions. It is known that a displacement of the spectrum of a pigment would be associated with a fixation of the pigment upon the cellular substance. Thus the spectrum of chlorophyll bound to the (solid) cellular phase is displaced to 20 μμ towards the red in relation to the spectrum of chlorophyll in solution. This corresponds to the direction and order of magnitude of the displacement shown by the ferment-hemin in relation to the dissolved hemin. This does not mean that the state of aggregation alone determines the displacement, for the effect may also be due to chemical differences.
In concluding, I wish to acknowledge the credit due for a great part of the above described investigation to my coworkers, Mr. Negelein and Mr. Krebs.
Footnotes
The first observation concerning the sensitivity to light of an iron-carbonyl compound was made by L. Mond and C. Langer (Jour. Chem. Soc., 59, 1090, 1891). They found that Fe(CO)5 splits off CO on exposure to light.
Address before the Kaiser Wilhelm Gesellsehaft on February 22, 1928. Published in Die Naturwissenschaften, Vol. 16, p. 345, May 18, 1928. Translated by Dr. W. A. Perlzweig, the Johns Hopkins Hospital.
Richard A. Brand MD (✉) Clinical Orthopaedics and Related Research, 1600 Spruce Street, Philadelphia, PA 19103, USA e-mail: dick.brand@clinorthop.org