Abstract
Background
Hyaline articular cartilage has limited repair and regeneration capacity. Intraarticular administration of glucocorticoid and local anesthetic injections play an important role in the therapy of osteoarthritis. Glucocorticoids and anesthetics reportedly enhance apoptosis in chondrocytes, but effects of the combined use of glucocorticoids and local anesthetics are unknown.
Questions/purposes
We asked whether glucocorticoid and local anesthetic agents combined had any synergistic effects on chondrocyte apoptosis.
Methods
Cell viability and apoptosis/necrosis assessment of human articular chondrocytes were performed in vitro (chondrocyte cell cultures) and ex vivo (osteochondral specimens) using flow cytometry and TUNEL analysis, respectively.
Results
Glucocorticoids and local anesthetics induce apoptosis in chondrocytes at various rates. When used in combination, the percentage of dead chondrocytes was increased in in vitro chondrocyte cell cultures and osteochondral ex vivo specimens.
Conclusions
We observed a time-dependent decrease in chondrocyte viability after concurrent steroid and local anesthetic exposure.
Clinical Relevance
The combination of glucocorticoids and local anesthetics has an adverse effect on articular chondrocytes, and it raises a question regarding whether concomitant administration should be used in treating osteoarthritis.
Introduction
Osteoarthritis is the most common form of joint disease and represents the most notable basis of disability afflicting greater than 5% of the world’s population [11, 29]. Osteoarthritis is characterized by progressive erosion, degradation, and degeneration of the articular cartilage, osteophyte formation, and subchondral changes. If articular cartilage damage is not extensive, nonoperative treatment is preferable.
According to the European League Against Rheumatism (EULAR) recommendation, the optimal treatment of osteoarthritis constitutes a combination of nonpharmacologic and pharmacologic therapeutic modalities [1, 19, 22]. The nonoperative pharmacologic treatments include putative oral chondroprotective drugs (dietary supplements), oral or topical nonsteroidal antiinflammatory drugs, and intraarticular injections such as corticosteroids, local anesthetics, or viscosupplementing agents.
Intraarticular corticosteroid therapy was first used by Hollander in 1951 to treat rheumatoid arthritis [20]. In 1956, Leveaux and Quin were possibly the first who investigated hydrocortisone and procaine combined injections for osteoarthritis [31]. Since then, many studies have been published that focus on the overall effects of corticosteroid and local anesthetic drugs for pain relief, improvement in ROM, or functional ability [12, 14, 27, 32, 33, 40, 41, 47, 52, 54]. Numerous studies report short-term intraarticular corticosteroid injection for the treatment of osteoarthritis [1, 2, 4, 8, 16, 21, 42, 44]. The possible long-term effects and the unpredictable duration are controversial and matters of debate [13, 48]. Repeated use of corticosteroids especially could facilitate tissue atrophy, joint destruction, or cartilage degeneration [9, 15, 35].
Recent studies suggest intraarticular anesthetics have a chondrotoxic action on articular chondrocytes [7, 23, 39], and the different glucocorticoids also are known to induce apoptosis in various cell types, because they are used as therapeutic solutions in some cases [34]. Nonetheless, it is universally accepted practice to use glucocorticoid injections along with local anesthetics to treat osteoarthritis [45]. The simultaneous use of intraarticular local anesthetic and corticosteroid may be rational and effective in reducing pain and inflammatory response because of its fast and prolonged effect. Nevertheless, the combined effect of steroids and local anesthetics on the chondrocytes is predominantly unknown, although the survival of articular chondrocytes is essential to maintain cartilage function.
Our aim was to determine whether (1) different types of glucocorticoids and local anesthetics alone and (2) different glucocorticoids in combination with various local anesthetics would produce cell death in chondrocytes.
Materials and Methods
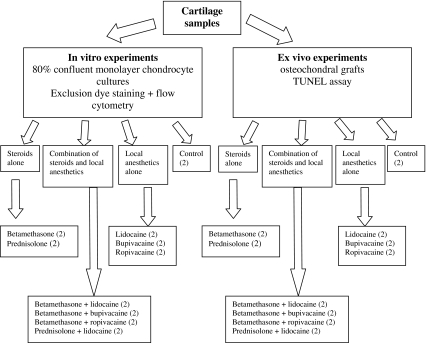
To determine and evaluate chondrocyte apoptosis and necrosis under in vitro and ex vivo circumstances, we used chondrocyte cultures (after enzymatic digestion) and osteochondral specimens from human femoral condyles. These two kinds of specimens (using material from two specimens in each group) were treated with 10 different agents, glucocorticoids and local anesthetics alone or in various combinations. Each experiment was repeated three times. The cell death in chondrocyte cultures was evaluated by flow cytometry after a dye exclusion test. Apoptosis and necrosis appearing in osteochondral tissues were investigated by TUNEL assay (Fig. 1). The study was approved by the Regional Research Ethics Committee.
Fig. 1.
A diagram of the experimental design is shown. All experiments were repeated three times.
We prepared articular cartilage biopsy specimens and series of osteochondral explants from human femoral condyles obtained from the removed specimens in TKAs. Only lateral femoral condyles that would appear unaffected by osteoarthritis were selected based on the Outerbridge score, which is the most frequently used grading system for the description of cartilage lesions [6, 49]. For chondrocyte isolation purposes, we meticulously dissected away all bony and soft tissues. Human osteochondral explants were cut into 1 × 1-cm pieces and preserved at 4°C until further processing (not longer than 24 hours).
For chondrocyte culture experiments, we transferred cartilage specimens to the laboratory in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Gaithersburg, MD) containing penicillin and streptomycin 10 U/mL. Cartilage samples were cleaned and carefully cut into 1 × 1-mm pieces. Serial enzymatic digestion was started in a CO2 incubator with continuous agitation in DMEM containing collagenase Type II 250U/mL (Biochrom, Berlin, Germany) and hyaluronidase 37.5 U/mL (Sigma, St Louis, MO). After the 24-hour incubation, cells were filtered through a cell strainer (pore size of 100 μm) (BD Falcon, Franklin Lakes, NJ), washed in phosphate-buffered saline, and plated onto six-well culture plates using special chondrocyte culture medium (Chondrocyte Basal Medium; Lonza, Basel, Switzerland). Once primary cultures had become 80% confluent (typically on Days 7–10), cell cultures were trypsinized (0.25% trypsin/EDTA; Sigma) and expanded.
To confirm chondral phenotype of the cells grown in culture, we obtained total RNA samples from the cells. After the second cell passage, at 80% cell confluence, mRNA samples were obtained from the chondrocyte cell cultures and the levels of chondrocyte-specific collagen Type II and aggrecan-producing activity were measured by reverse transcriptase-PCR. Total RNA was isolated using TriReagent (Sigma-Aldrich, Hamburg, Germany). We performed complementary DNA synthesis from 2 μg total RNA reverse transcribed with Superscript III RT (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s protocol. Presence of cDNA encoding for the Type II collagen and aggrecan was tested by PCR amplification with the following primers: Coll II: sense 5′-CCGGGCAGAGGGCAATAGCAGGTT-3′, antisense 5′-GTTACTACCCCTCCGCACTC-3′; Agg: sense 5′-TCCGAGGGTGCCGTGAG-3′, antisense 5′-AGGCTCCCACGGCACTC-3′. The PCR contained 300 μmol/L of each dNTP, 1.5 mmol/L MgSO4, 1 μmol/L of each primer, 2 μL cDNA, and 2.5 units of ProofStart DNA polymerase (Qiagen, Hilden, Germany) in a 50-μL final volume. Thermal cycling was performed with the following profile: 95°C for 5 minutes, 35 cycles of 95°C for 1 minute, 57°C for 30 seconds, 72°C for 1 minute, and a final extension at 72°C for 10 minutes. The collagen Type II and aggrecan pattern showed acceptable chondrocyte characteristics in all cases (data not shown).
After the second cell passage, cartilage cell cultures were allocated randomly into 10 groups. The cells in each group were exposed to different solutions alone (betamethasone, prednisolone, lidocaine, bupivacaine, and ropivacaine) or in combinations (betamethasone with lidocaine, betamethasone with bupivacaine, betamethasone with ropivacaine, prednisolone with lidocaine) or phosphate-buffered saline (control) for 2, 6, and 24 hours (Table 1). Chondrocytes in Groups I and II were exposed to two different types of glucocorticoid solution: 1 mL betamethasone (2 mg/mL betamethasone-sodium-phosphate plus 5 mg/mL betamethasone-dipropionate compound; Diprophos®; Schering-Plough, Kenilworth, NJ) or 1 mL prednisolone (25 mg/mL prednisolone-sodium-succinate; Diadreson F Aquosum®; NV Organon, Oss, Holland) in culture flasks for 2, 6, and 24 hours. Groups III through V were treated with 1 mL of the following local anesthetics: 1 mL lidocaine (10 mg/mL xylocaine 1% injection; AstraZeneca, London, UK), 1 mL bupivacaine (5 mg/mL Marcaine 0.5% injection; AstraZeneca), or 1 mL ropivacaine (7.5 mg/mL Naropin; AstraZeneca) alone. Groups VI through IX received 2 mL of different glucocorticoid–local anesthetic combinations such as betamethasone-lidocaine (Group VI), betamethasone-bupivacaine (Group VII), betamethasone-ropivacaine (Group VIII), or prednisolone-lidocaine (Group IX), respectively. Exposure to phosphate-buffered saline served as a control (Group X). Experiments were repeated three times using chondrocyte cells of different donors. All concentrations were calculated to represent the intraarticular environment after one intraarticular knee injection based on the average amount of synovial lining in the knee: 3 to 4 mL [24, 45, 50].
Table 1.
Solutions and combinations used in the experiments*
| Agent | Concentration | Group number | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | ||
| Betamethasone | 7 mg/mL | X | X | X | X | |||||
| Prednisolone | 25 mg/mL | X | X | |||||||
| Lidocaine | 10 mg/mL | X | X | X | ||||||
| Bupivacaine | 5 mg/mL | X | X | |||||||
| Ropivacaine | 7.5 mg/mL | X | X | |||||||
* Different steroids and local anesthetics were used alone or in combinations.
Apoptotic chondrocytes were identified by examining phosphatidylserine expression using flow cytometry. Chondrocytes were stained with FITC-labeled annexin-V and propidium-iodide (PI) as an accepted method for detecting apoptosis and necrosis in cultured cells [7, 10, 53]. The assay identifies living cells labeled with neither stain, apoptotic cells labeled only with FITC-annexin-V stain, and necrotic cells, which have FITC-annexin-V and PI staining.
After exposure to different solutions (Table 1), chondrocyte cell cultures were removed from plates using a mixture of 0.25% Trypsin and 0.2% ethylene diamine tetra-acetate and centrifuged and resuspended in ice-cold phosphate-buffered saline. A total of 5 μL of labeled Annexin V (BD Biosciences, San Jose, CA) and 10 μL of 20 μg/mL PI (BD Biosciences) in 400 μL of annexin binding buffer (ABB; 10 mmol/L HEPES [pH 7.4], 140 mmol/L NaCl, 2.5 mmol/L CaCl2) were added to the cells followed by incubation in the dark for 15 minutes. After that, cells were washed twice in ABB and resuspended in 400 mL ABB before flow cytometric measurement. We performed quantitative analysis of the chondrocytes using a BD FacsCalibur flow cytometer (Becton Dickinson, Mountain View, CA), and the apoptotic/necrotic percentage of the cells was determined by Cellquest software (BD Biosciences). Quadrant dot plot was introduced to identify living, apoptotic, and necrotic cells. Cell viability and the rate of apoptotic and necrotic cells were measured at three times (2, 6, and 24 hours).
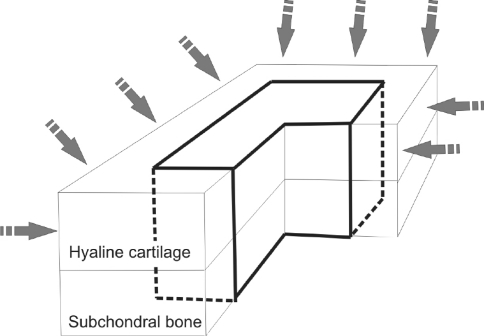
Cartilage samples of osteochondral explants were allocated randomly and were treated with different solutions (Table 1) for 24 hours. To avoid false results attributable to diffusion from cartilage side cuts, the peripheral 2 mm of the osteochondral blocks was removed before evaluation; thus, the diffusion would reach the chondrocytes only from the superficial layer mimicking in vivo intraarticular conditions (Fig. 2).
Fig. 2.
An illustration of the osteochondral explants used for the ex vivo experiments is shown. The peripheral 2 mm from each side of the specimen was removed after the treatment because diffusion through the side cuts of the cartilage may bias the results. Only the remaining central portion of the cartilage was used for assessment.
To assess the appearance and rate of the apoptotic and necrotic cells in the cartilage tissue, the treated osteochondral explant specimens were analyzed with the help of TdT-mediated dUTP nick-end labeling (TUNEL) assay using the DeadEndTM Fluorimetric TUNEL System (Promega, Madison, WI). This method allowed us to examine cartilage specimens histologically for identification and quantification of TUNEL-positive cells in situ, which represent apoptotic cell death in the tissue [10, 30]. After a 24-hour treatment with various solutions (Table 1), sections were stained following the manufacturer’s instructions. Briefly, cartilage specimens were cut into 6-μm sections and fixed with buffered 4% paraformaldehyde for 10 minutes and then washed with phosphate-buffered saline and permeabilized on ice with 0.1% Triton X-100 in 0.1% sodium citrate. Samples were incubated with a reaction mixture (TdT enzyme) and digoxigenin-labeled dNTPs at 37°C in a humidified chamber. After 1 hour, the labeled nucleotides were detected by peroxidase conjugated antidigoxigenin antibodies in a humidified chamber. The sections also were counterstained with 4′,6-diamidine-2′-phenylindole-dihydrochloride. Quantitative examination of the chondrocyte cell death was performed by microscopically visually scanning through the entire cartilage section. Viability of cells was assessed and expressed as the percentage of TUNEL-stained cells to the number of all cells.
All experiments reported in this study were repeated three times, and the results were presented as the mean of the total number of trials performed to obtain more objective data. All values are expressed as mean ± SD. We determined differences in apoptosis and the necrosis rate between the effects of various agents and the control phosphate-buffered saline using one-way ANOVA followed by Dunnett’s post hoc test. We performed all analyses using GraphPad Prism 5® (GraphPad Software, Inc, La Jolla, CA).
Results
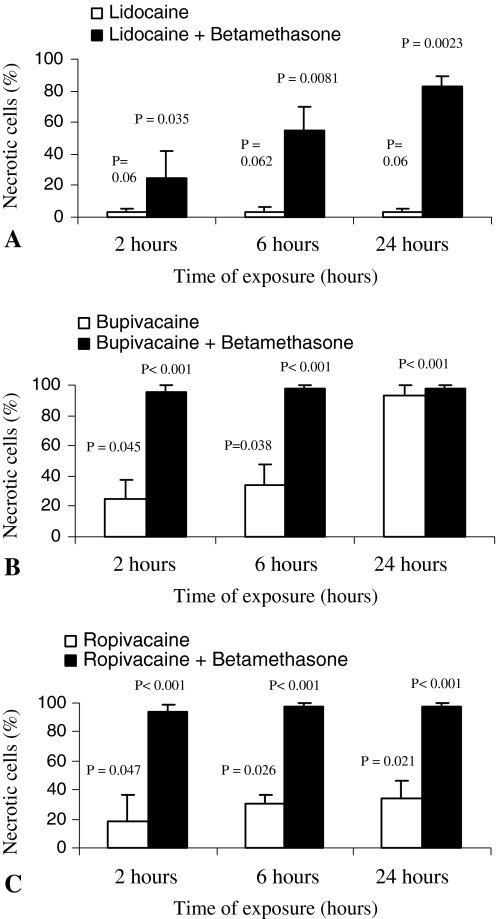
We observed different types of glucocorticoids and local anesthetics alone produced differences on chondrocyte cell cultures when comparing apoptosis and the necrosis rate with the control. When chondrocytes were cultured in phosphate-buffered saline (control group; Group X), almost no apoptosis occurred (Fig. 3). Cells exposed to lidocaine (Group III) showed increased apoptosis compared with the control group reaching almost 20% by 24 hours (Fig. 3B). Ropivacaine treatment (Group V) alone showed approximately 40% of cell necrosis by 24 hours (Fig. 4). Bupivacaine (Group VI) was the most cytotoxic among the three anesthetics, because it induced almost 100% cell necrosis after 24 hours of exposure (Fig. 4B).The two distinct steroid preparations, betamethasone (Group I) and prednisolone (Group II), showed similar characteristics in inducing cell death, reaching 20% of cell death by 24 hours (Fig. 3C–D).
Fig. 3A–F.
The results of flow cytometry analysis of chondrocyte cell cultures treated with (A) control phosphate-buffered saline, (B) lidocaine, (C) betamethasone, (D) prednisolone, (E) betamethasone-lidocaine combination, and (F) prednisolone-lidocaine combination are shown. The time course changes of apoptotic and necrotic cells up to 24 hours of exposure with respect to the three detection points of 2, 6, and 24 hours are indicated in the X axis. The ratio of the apoptotic or necrotic cells is shown in the Y axis in percent. The bars represent the mean of three independent experiments with standard error as error bars. Results from the various experimental groups were compared with those of the PBS control group. Exact probability values also are shown as p values.
Fig. 4A–C.
The ratios of necrotic chondrocytes treated with different local anesthetics alone or in combination with betamethasone are shown. Substantial differences are detectable between administration of (A) lidocaine or lidocaine + betamethasone, (B) bupivacaine or bupivacaine + betamethasone, and (C) ropivacaine or ropivacaine + betamethasone combinations. The X axis represents the exposure time in hours. The Y axis shows the ratio of necrotic chondrocytes in percent. The bars represent the mean of the three independent experiments with standard error as error bars. Results from the various experimental groups were compared with those of the PBS control group. Exact probability values are shown as p values.
Different glucocorticoids in combination with various local anesthetics on chondrocyte cell cultures were associated with greater apoptosis and necrosis rates in some experiments. The combination of betamethasone and lidocaine (Group VI) increased chondrocyte apoptosis even after 6 hours when compared with betamethasone or lidocaine administration alone (Fig. 3E). This combination also increased the number of necrotic cells after 24 hours of exposure, because 83% of the cells were necrotic by that time (Figs. 3E, 4A). The 2- or 6-hour exposure of chondrocytes to prednisolone with lidocaine (Group IX) did not cause a major increase in apoptosis or in necrosis rate. However, the percentage of apoptotic cells detected in chondrocyte cell cultures at 24 hours of exposure reached 65% with an additional 25% of necrotic cells (Fig. 3F). At each detection point, in cases of combined use of betamethasone-bupivacaine (Group VII) and betamethasone-ropivacaine (Group VIII), the necrotic rate was almost 100% (Fig. 4B–C).
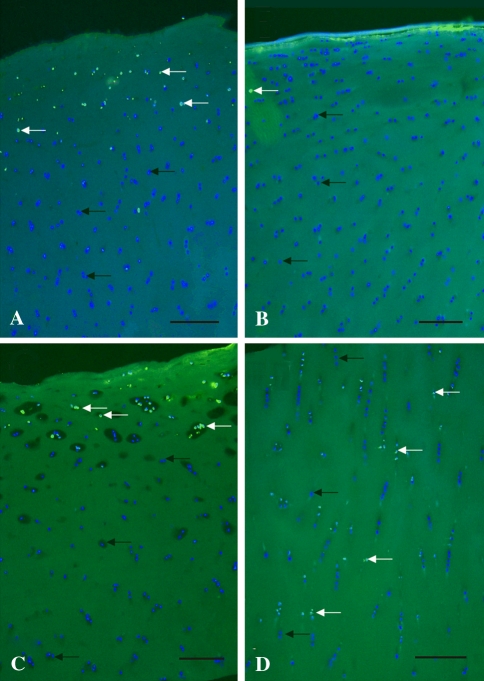
In the osteochondral specimens, administration of glucocorticoids and local anesthetics alone affected only the superficial layer of the specimens. TUNEL assay of osteochondral specimens treated with lidocaine for 24 hours showed the presence of several dead cells (16% of all cells) in the superficial zone, whereas chondrocytes situated in the deeper zones showed consistently high viability (> 98% of all cells) (Fig. 5A). Betamethasone treatment led to minor cytotoxic activity (3%) because only a few TUNEL-positive dead cells were detectable in the osteochondral specimens (Fig. 5B). After prednisolone exposure, TUNEL-positive cells (22% of all cells) were situated predominantly in the superficial 1/3 of the hyaline cartilage but not in the deeper cartilage zones (Fig. 5C).
Fig. 5A–D.
An histologic assessment of the distribution of chondrocytes in articular cartilage explants was performed. The TUNEL assay (FITC-Annexin-V) with DAPI counterstaining shows apoptotic cells after 24 hours of treatment in osteochondral ex vivo specimens. The results of (A) lidocaine, (B) betamethasone, (C) prednisolone, and (D) prednisolone-lidocaine combinations are shown. The blue staining shows all nuclei of living cells (black arrows), illuminating green (TUNEL-positive) cells are dead (white arrows) (Original magnification, ×50). Scale bar = 150 μm.
The glucocorticoid-local anesthetic combination showed a higher cell death ratio in the osteochondral specimens. Prednisolone-lidocaine combined exposure resulted in several randomly scattered dead cells (36% of all cells across the whole depth of the cartilage sample) (Fig. 5D). The ratio of nonviable chondrocytes after betamethasone-lidocaine combination was similar (32% of all cells) to that after prednisolone-lidocaine combined treatment (picture not shown); however, cells were affected mostly in the superficial zone.
Discussion
Traditionally, in addition to others, the pharmacologic treatment of osteoarthritis includes intraarticular corticosteroid and/or local anesthetic injections. Recent studies have proved local anesthetics and glucocorticoids have cytotoxic effects on various cell types [7, 23, 34, 39]. The link between the articular degeneration in osteoarthritis and apoptosis has been investigated in previous studies [5, 25, 51, 55]. This connection can be explained by the specific structure of articular cartilage as in the absence of macrophages, the apoptotic bodies release their content (proteases) into the extracellular space causing irreversible damage to extracellular matrix [5, 18]. If the chondrocytes die of apoptosis as a result of their limited replication ability, this results in a serious loss of the cells that are in charge of ECM maintenance. As a consequence, any intraarticular medication causing apoptosis of chondrocytes eventually would accelerate osteoarthritis. The aim of our study was to investigate the effects of local anesthetics and corticosteroids alone or in combinations on chondrocyte viability.
Some limitations of this study must be considered. First, we investigated only limited numbers of glucocorticoids and local anesthetics and other agents could show different results. We intended to select the most commonly used agents and combinations. Second, the responses of monolayer chondrocyte cultures may not reflect those of complex and highly differentiated cartilage tissue. To reduce this limitation, we also investigated chondrocyte viability in osteochondral specimens. However, the cartilage tissue specimens are stored in the media in a static manner and diffusion characteristics likely differ in a moving knee. Nevertheless, the passive diffusion characteristics (which are basic processes in a moving knee as well) were represented accordingly under these experimental circumstances.
Intraarticular corticosteroid injections are recommended by the EULAR and the American College of Rheumatology guidelines to be used in rheumatology and orthopaedics to alleviate the symptoms of osteoarthritis by suppressing inflammation and inflammatory flares for patients with signs of local synovitis and joint effusion and who do not respond to other forms of treatment [1, 3, 8, 17, 22]. Intraarticular injection of corticosteroid is an effective and common treatment for osteoarthritis, although clinical evidence suggests it provides a relatively short-lived benefit in pain relief [16, 21, 26, 27, 37, 42, 44, 47]. One study suggested certain dosages of corticosteroids used clinically as antiinflammatory drugs for osteoarthritis are harmful to articular cartilage [15], whereas lower dosages are reportedly chondroprotective and delay progression of cartilage lesions [8, 28, 36, 38, 43]. In vitro we found betamethasone and prednisolone had no effect in inducing chondrocyte cell death when compared with the control group. In the ex vivo experiments, betamethasone had no substantial cytotoxic effect, whereas prednisolone induced more extensive cell death, especially in the upper 1/3 of the hyaline cartilage. We attempted to compare our observations with those from other studies (Table 2). However, it is difficult to synthesize these data, because the origin of the investigated chondrocytes, the culture conditions, and the exposure times are distinct. Nevertheless, our data are consistent with that of Seshadri et al. [46] who also reported greater cytotoxicity after combined exposure of agents.
Table 2.
Summary of findings from different studies
| Study | Cell type | Agent and concentration used for exposure | Time of assessment (time of exposure if different) | Cytotoxicity |
|---|---|---|---|---|
| Nakazawa et al. [34] | Human articular chondrocyte monolayer culture | Triamcinolone 10−4 mol/L | 72 hours | 10.4% |
| Fubini et al. [15] | Equine articular chondrocyte monolayer culture | Methylprednisolone 1 × 109 pg/mL (2 mmol/L) | 72 hours | 96% |
| Chu et al. [7] | Bovine articular chondrocytes cultured in alginate beads | Bupivacaine 0.5% | 1 hour (15-30-60 minutes) | 99% |
| Piper & Kim [39] | Human articular chondrocyte monolayer culture | Bupivacaine 0.5% Ropivacaine 0.5% |
24 hours (30 minutes) 24 hours (30 minutes) |
62.6% 36.1% |
| Seshadri et al. [46] | Bovine articular chondrocytes cultured in alginate beads | Methylprednisolone 8 mg/mL Methylprednisolone 8 mg/mL + lidocaine 1% |
24 hours (60 minutes) 24 hours (60 minutes) |
62.4% 99% |
| Current study | Human articular chondrocyte monolayer culture | Betamethasone 7 mg/mL Lidocaine 10 mg/mL Ropivacaine 7.5 mg/mL Betamethasone 7 mg/mL + lidocaine 10 mg/mL Betamethasone 7 mg/mL + ropivacaine 7.5 mg/mL |
24 hours 24 hours 24 hours 24 hours 24 hours |
20% 20% 39% 83% 98% |
Other widely used agents for intraarticular osteoarthritis treatment are local anesthetics alleviating pain effectively, yet they may have a cytotoxic action on chondrocytes [7, 23, 39]. The most widely used local anesthetic, lidocaine, acts more rapidly and is more stable than others, whereas bupivacaine has a slow onset and prolonged duration of action [45]. In our study, lidocaine showed only a slight necrotic effect on chondrocytes and affected only the superficial zone of the osteochondral specimens, whereas ropivacaine and especially bupivacaine had a much stronger toxic effect on chondrocytes under in vitro and ex vivo circumstances.
Local anesthetic medication is recommended and often added to corticosteroids for intraarticular injections [45]. The rationale would be that the local anesthetic component acts quickly after administration, it provides fast alleviation of pain, and it may last for up to the point when the steroid component starts to exert its effect. Although it has been reported that steroids and local anesthetics have a strong cytotoxic effect on chondrocytes, concomitant administration of corticosteroids and local anesthetics in osteoarthritis is still a widely used and investigated method [12, 27, 32, 45–47]. Similar to our findings, a recent study revealed a methylprednisolone-lidocaine combination showed a cytotoxic effect on bovine chondrocytes cultured in alginate beads [46]. Our in vitro results revealed the combination of certain local anesthetics and steroids has an unexpectedly high cytotoxic effect. The combination of betamethasone and bupivacaine induced almost 100% cell death in cell cultures. When investigated in osteochondral explants, betamethasone-lidocaine and especially prednisolone-lidocaine combinations exerted a high percentage of cell death among chondrocytes throughout the entire cartilage specimens (possibly attributable to a greater diffusion rate and strong apoptosis-inducing capacity). The combined forms induce a considerably greater necrosis rate in chondrocytes compared with the effects of anesthetics or steroids alone.
These observations imply an actual synergistic rather than just an additive effect of the glucocorticoid and local anesthetic on cell death. The safety of intraarticularly administered corticosteroids and anesthetics is controversial, because their effects on the cartilage structure and metabolism are not completely elucidated and studies related to this topic are divergent. The data suggest the combinations of certain types of steroids and local anesthetics have a deleterious effect on articular chondrocytes; therefore, it raises the question regarding whether concomitant administration of these two agents is justified in the treatment of osteoarthritis. Additional studies must be performed to analyze the effect of these agents in vivo and to gain insight into the involvement of different intracellular cascades.
Footnotes
One or more of the authors (TB) have received funding from the Mutual Foundation of the Ministry of Economy and European Union (GVOP Project 3.1.1-2004-05-0219/3.0), ETT National Scientific Committee for Health-related Researches, and the János Bolyai Research Fellowship and Research Foundation (OTKA 53065).
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and informed consent for participation in the study was obtained.
Contributor Information
Boglárka Farkas, Email: boglarka_farkas@hotmail.com.
Tamás Bárdos, Email: tbardos@hotmail.com.
References
- 1.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869. doi: 10.1136/bmj.38039.573970.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;CD005328. [DOI] [PubMed]
- 4.Bjordal JM, Klovning A, Ljunggren AE, Slordal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: a meta-analysis of randomised placebo-controlled trials. Eur J Pain. 2007;11:125–138. doi: 10.1016/j.ejpain.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Blanco FJ, Guitian R, Vazquez-Martul E, Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis: a possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 6.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85(suppl 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 7.Chu CR, Izzo NJ, Papas NE, Fu FH. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693–699. doi: 10.1016/j.arthro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Creamer P. Intra-articular corticosteroid injections in osteoarthritis: do they work and if so, how? Ann Rheum Dis. 1997;56:634–636. doi: 10.1136/ard.56.11.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creamer P. Intra-articular corticosteroid treatment in osteoarthritis. Curr Opin Rheumatol. 1999;11:417–421. doi: 10.1097/00002281-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 10.D’Lima DD, Kuhn K, Lotz MK. Detection of apoptosis in cartilage in situ and in isolated chondrocytes. Methods Mol Med. 2004;100:275–290. doi: 10.1385/1-59259-810-2:275. [DOI] [PubMed] [Google Scholar]
- 11.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan J, Casale FF, Thomas TL, Desai KB. Intra-articular injection for pain relief in patients awaiting hip replacement. Ann R Coll Surg Engl. 1988;70:156–157. [PMC free article] [PubMed] [Google Scholar]
- 13.Frean SP, Cambridge H, Lees P. Effects of anti-arthritic drugs on proteoglycan synthesis by equine cartilage. J Vet Pharmacol Ther. 2002;25:289–298. doi: 10.1046/j.1365-2885.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 14.Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7:850–856. [PubMed] [Google Scholar]
- 15.Fubini SL, Todhunter RJ, Burton-Wurster N, Vernier-Singer M, MacLeod JN. Corticosteroids alter the differentiated phenotype of articular chondrocytes. J Orthop Res. 2001;19:688–695. doi: 10.1016/S0736-0266(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 16.Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis. 1995;54:379–381. doi: 10.1136/ard.54.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray RG, Gottlieb NL. Intra-articular corticosteroids: an updated assessment. Clin Orthop Relat Res. 1983;177:235–263. [PubMed] [Google Scholar]
- 18.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995;38:1541–1546. doi: 10.1002/art.1780381104. [DOI] [PubMed] [Google Scholar]
- 20.Hollander JL. Intra-articular hydrocortisone in arthritis and allied conditions; a summary of two years’ clinical experience. J Bone Joint Surg Am. 1953;35:983–990. [PubMed] [Google Scholar]
- 21.Jones A, Doherty M. Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis. 1996;55:829–832. doi: 10.1136/ard.55.11.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, Gunther K, Hauselmann H, Herrero-Beaumont G, Kaklamanis P, Lohmander S, Leeb B, Lequesne M, Mazieres B, Martin-Mola E, Pavelka K, Pendleton A, Punzi L, Serni U, Swoboda B, Verbruggen G, Zimmerman-Gorska I, Dougados M. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621–1627. doi: 10.1177/0363546507304719. [DOI] [PubMed] [Google Scholar]
- 24.Kraus VB, Stabler TV, Kong SY, Varju G, McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007;15:1217–1220. doi: 10.1016/j.joca.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn K, D’Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis Cartilage. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31:2265–2268. [PubMed] [Google Scholar]
- 27.Lambert RG, Hutchings EJ, Grace MG, Jhangri GS, Conner-Spady B, Maksymowych WP. Steroid injection for osteoarthritis of the hip: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2007;56:2278–2287. doi: 10.1002/art.22739. [DOI] [PubMed] [Google Scholar]
- 28.Larsson E, Erlandsson HH, Larsson A, Mansson B, Saxne T, Klareskog L. Corticosteroid treatment of experimental arthritis retards cartilage destruction as determined by histology and serum COMP. Rheumatology (Oxford) 2004;43:428–434. doi: 10.1093/rheumatology/keh073. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Prakash KV, Pengatteeri YH, Park SE, Koh HS, Han CW. Chondrocyte apoptosis in the regenerated articular cartilage after allogenic chondrocyte transplantation in the rabbit knee. J Bone Joint Surg Br. 2007;89:977–983. doi: 10.1302/0301-620X.89B7.18983. [DOI] [PubMed] [Google Scholar]
- 31.Leveaux VM, Quin CE. Local injection of hydrocortisone and procaine in osteo-arthritis of the hip joint. Ann Rheum Dis. 1956;15:330–337. doi: 10.1136/ard.15.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lockman LE. Practice tips. Knee joint injections and aspirations: the triangle technique. Can Fam Physician. 2006;52:1403–1404. [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JH, White J, Norton TH. The value of intra-articular injections in osteoarthritis of the knee. J Bone Joint Surg Br. 1958;40:636–643. doi: 10.1302/0301-620X.40B4.636. [DOI] [PubMed] [Google Scholar]
- 34.Nakazawa F, Matsuno H, Yudoh K, Watanabe Y, Katayama R, Kimura T. Corticosteroid treatment induces chondrocyte apoptosis in an experimental arthritis model and in chondrocyte cultures. Clin Exp Rheumatol. 2002;20:773–781. [PubMed] [Google Scholar]
- 35.Papacrhistou G, Anagnostou S, Katsorhis T. The effect of intraarticular hydrocortisone injection on the articular cartilage of rabbits. Acta Orthop Scand Suppl. 1997;275:132–134. doi: 10.1080/17453674.1997.11744766. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier JP, DiBattista JA, Raynauld JP, Wilhelm S, Martel-Pelletier J. The in vivo effects of intraarticular corticosteroid injections on cartilage lesions, stromelysin, interleukin-1, and oncogene protein synthesis in experimental osteoarthritis. Lab Invest. 1995;72:578–586. [PubMed] [Google Scholar]
- 37.Pelletier JP, Martel-Pelletier J, Raynauld JP. Most recent developments in strategies to reduce the progression of structural changes in osteoarthritis: today and tomorrow. Arthritis Res Ther. 2006;8:206. doi: 10.1186/ar1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelletier JP, Mineau F, Raynauld JP, Woessner JF, Jr, Gunja-Smith Z, Martel-Pelletier J. Intraarticular injections with methylprednisolone acetate reduce osteoarthritic lesions in parallel with chondrocyte stromelysin synthesis in experimental osteoarthritis. Arthritis Rheum. 1994;37:414–423. doi: 10.1002/art.1780370316. [DOI] [PubMed] [Google Scholar]
- 39.Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986–991. doi: 10.2106/JBJS.G.01033. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen S, Kehlet H. Intraarticular glucocorticoid, morphine and bupivacaine reduces pain and convalescence after arthroscopic ankle surgery: a randomized study of 36 patients. Acta Orthop Scand. 2000;71:301–304. doi: 10.1080/000164700317411924. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen S, Larsen AS, Thomsen ST, Kehlet H. Intra-articular glucocorticoid, bupivacaine and morphine reduces pain, inflammatory response and convalescence after arthroscopic meniscectomy. Pain. 1998;78:131–134. doi: 10.1016/S0304-3959(98)00123-7. [DOI] [PubMed] [Google Scholar]
- 42.Ravaud P, Moulinier L, Giraudeau B, Ayral X, Guerin C, Noel E, Thomas P, Fautrel B, Mazieres B, Dougados M. Effects of joint lavage and steroid injection in patients with osteoarthritis of the knee: results of a multicenter, randomized, controlled trial. Arthritis Rheum. 1999;42:475–482. doi: 10.1002/1529-0131(199904)42:3<475::AID-ANR12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 43.Raynauld JP. Clinical trials: impact of intraarticular steroid injections on the progression of knee osteoarthritis. Osteoarthritis Cartilage. 1999;7:348–349. doi: 10.1053/joca.1998.0193. [DOI] [PubMed] [Google Scholar]
- 44.Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, Uthman I, Khy V, Tremblay JL, Bertrand C, Pelletier JP. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–377. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 45.Saunders S, Longworth S. Injection Techniques in Orthopaedic and Sports Medicine: A Practical Manual for Doctors and Physiotherapists. 3. London, England: Churchill-Livingstone/Elsevier; 2006. [Google Scholar]
- 46.Seshadri V, Coyle CH, Chu CR. Lidocaine potentiates the chondrotoxicity of methylprednisolone. Arthroscopy. 2009;25:337–347. doi: 10.1016/j.arthro.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shrader MW, Draganich LF, Pottenger LA, Piotrowski GA. Effects of knee pain relief in osteoarthritis on gait and stair-stepping. Clin Orthop Relat Res. 2004;421:188–193. doi: 10.1097/01.blo.0000119248.70353.a5. [DOI] [PubMed] [Google Scholar]
- 48.Smith MD, Wetherall M, Darby T, Esterman A, Slavotinek J, Roberts-Thomson P, Coleman M, Ahern MJ. A randomized placebo-controlled trial of arthroscopic lavage versus lavage plus intra-articular corticosteroids in the management of symptomatic osteoarthritis of the knee. Rheumatology. 2003;42:1477–1485. doi: 10.1093/rheumatology/keg398. [DOI] [PubMed] [Google Scholar]
- 49.Spahn G, Klinger HM, Hofmann GO. How valid is the arthroscopic diagnosis of cartilage lesions? Results of an opinion survey among highly experienced arthroscopic surgeons. Arch Orthop Trauma Surg. 2009;129:1117–1121. doi: 10.1007/s00402-009-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Standring S. Gray’s Anatomy. 40. New York, NY: Churchill Livingstone; 2008. [Google Scholar]
- 51.Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Noth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vecchio PC, Hazleman BL, King RH. A double-blind trial comparing subacromial methylprednisolone and lignocaine in acute rotator cuff tendinitis. Br J Rheumatol. 1993;32:743–745. doi: 10.1093/rheumatology/32.8.743. [DOI] [PubMed] [Google Scholar]
- 53.Vermes I, Haanen C, Reutelingsperger C. Flow cytometry of apoptotic cell death. J Immunol Methods. 2000;243:167–190. doi: 10.1016/S0022-1759(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 54.Wada J, Koshino T, Morii T, Sugimoto K. Natural course of osteoarthritis of the knee treated with or without intraarticular corticosteroid injections. Bull Hosp Jt Dis. 1993;53:45–48. [PubMed] [Google Scholar]
- 55.Wei L, Sun XJ, Wang Z, Chen Q. CD95-induced osteoarthritic chondrocyte apoptosis and necrosis: dependency on p38 mitogen-activated protein kinase. Arthritis Res Ther. 2006;8:R37. doi: 10.1186/ar1891. [DOI] [PMC free article] [PubMed] [Google Scholar]