Abstract
Background
Computer-assisted navigation was recently introduced to aid the resection of musculoskeletal tumors. However, it has not always been possible to directly navigate the osteotomy with real-time manipulation of available surgical tools. Registration techniques vary, although most existing systems use some form of surface matching.
Questions/purposes
We developed and evaluated a workflow model of computer-assisted bone tumor surgery and evaluated (1) the applicability of currently available software to different bones; (2) the accuracy of the navigated excision; and (3) the accuracy of a new registration technique of fluoro-CT matching.
Methods
Our workflow involved detailed preoperative planning with CT-MRI image fusion, three-dimensional mapping of the tumor, and planning of the resection plane. Using the workflow model, we reviewed 15 navigation procedures in 12 patients, including four with joint-saving resections and three with custom implant reconstructions. Intraoperatively, registration was performed with either paired points and surface matching (Group 1, n = 10) or a new technique of fluoro-CT image matching (Group 2, n = 5). All osteotomies were performed under direct computer navigation. Postoperatively, each case was evaluated for histologic margin and gross measurement of the achieved surgical margin.
Results
The margins were free from tumor in all resected specimens. In the Group 1 procedures, the correlation between preoperative planned margins and actual achieved margins was 0.631, whereas in Group 2 procedures (fluoro-CT matching), the correlation was 0.985.
Conclusions
Our findings suggest computer-assisted navigation is accurate and useful for bone tumor surgery. The new registration technique using fluoro-CT matching may allow more accurate resection of margins.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-010-1465-7) contains supplementary material, which is available to authorized users.
Introduction
With enhanced diagnostic techniques and increasing availability of limb-saving approaches and implants, surgeons are undertaking increasingly more challenging cases. For example, intraepiphyseal resection close to the joint requires a high level of precision, especially when a good fit is essential for custom implant reconstruction. Likewise, resection in difficult anatomic regions such as the pelvis and sacrum is demanding and requires careful preoperative planning and intraoperative precision. Computer-assisted navigation can increase the precision of the osteotomies required to resect tumors in such situations [2, 3, 5, 6, 9].
Navigation provides the surgeon with three-dimensional (3D) assessment and visualization of the tumor bulk and the ability to plan the resection on a computer graphic platform. This data set can then be used to carry out the bone cuts during the actual operation. The gap between the 3D navigation plan and the actual bone cut is bridged by surgical tools integrated within the system and navigated in real-time.
Although computer-assisted navigation has been used in craniofacial surgery, joint arthroplasty, trauma, and spine surgery [1, 4, 7, 8] for some time, its application in orthopaedic bone tumor surgery is more recent [2, 3, 5, 6, 9]. To date, there is no software designed specifically for surgery on long bone and pelvic tumors. Much of what has been reported was adapted from other areas such as spine software. Hufner et al. [5] used navigated surgical tools to intraoperatively identify tumor margins in three patients with sacral tumors. Krettek et al. [6] performed computer-aided tumor resection on two patients with pelvic tumor using intraoperative navigated Kirschner wires and chisels. In both studies, there was no description of detailed preoperative planning of resection planes. Wong et al. [9] performed six bone tumor resections on five patients using CT and MRI fusion images for preoperative planning. The planned landmarks were identified and marked with diathermy, but the osteotomy was performed free-handed in the conventional manner.
We therefore investigated the capabilities and limitations of computer-assisted navigation in bone tumor surgery. A seamless workflow model was applied to a wide variety of clinical situations. We specifically evaluated (1) the applicability of currently available software to different bones, eg, pelvis and extremity long bones; (2) the accuracy of the navigated excision compared with preoperative planning; and (3) the accuracy of a new registration technique of fluoro-CT matching compared with the conventional paired points and surface matching technique.
Patients and Methods
Fourteen patients were recruited with planned tumor resection between January 2008 and January 2009. There were eight male and six female patients ranging in age from 10 to 56 years (average, 26.9 years). There were 18 navigation procedures in total as a result of more than one bone being involved in some of the patients. The bones navigated were the femur (six), tibia (five), fibula (one), pelvis (two), sacrum (one), humerus (one), and radius (one). Pathologic diagnosis included osteosarcoma (six), chondrosarcoma (four), adamantinoma (one), chordoma (one), malignant giant cell tumor (one), and giant cell tumor (one). The surgical procedures performed were wide excision plus endoprosthesis (nine), wide excision alone (four), wide excision plus vascularized fibular graft (one), and wide excision plus iliac graft (one). Of these, four cases were joint-saving excisions and three involved custom implant reconstruction. Of the 18 navigation procedures, three were excluded from the study for the following reasons: one procedure on the tibia (resection of the tibial plateau for endoprosthesis reconstruction) in a case of distal femur osteosarcoma was excluded because there was no tumor in the tibia to measure from; one procedure in an osteosarcoma of the femur was excluded because there was a substantial discrepancy between MRI T1- and T2-weighted marrow signals, making real assessment of the tumor level difficult; and a third procedure was excluded because margin status could not be assessed accurately. This was a case of sacral chordoma in which additional bone was removed after the navigated resection from the anterior sacral foramen to free and save one more sacral nerve root. For the remaining 15 procedures available for final analysis in 12 patients, 10 were in Group 1 using paired points and surface matching for registration and five were in Group 2 using fluoro-CT matching for registration (Table 1). In Group 1, there were two failures of surface registration (Patients 4 and 7) despite successful paired points registration, both occurring in the proximal tibia. The minimum followup was 6 months (mean, 16 months; range, 6-21 months).
Table 1.
Details of cases performed under computer-assisted navigation
| Navigation procedure | Patient number | Age (years) | Gender | Diagnosis | Region | Operation | Planned margin (mm) | Achieved margin (mm) | Followup (months) | Oncologic outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1* | ||||||||||
| 1 | 1 | 30 | Female | Clear cell chondrosarcoma | Femoral head and neck | Excision + endoprosthesis | 10 | 8 | 21 | DF |
| 2 | 2 | 54 | Female | Chondrosarcoma | Pelvis (ischium) | Joint-sparing excision | 10 | 20 | 21 | DF |
| 3 | 3 | 10 | Female | Osteosarcoma | Distal Femur | Excision + custom growing prosthesis | 30 | 20 | 6 | Died as a result of metastasis |
| 4 | Tibial plateau | 5 | 13 | |||||||
| 5 | 4 | 47 | Male | Malignant giant cell tumor | Tibia | Excision + endoprosthesis | 30 | 35 | 21 | Alive with metastasis |
| 6 | 5 | 32 | Male | Giant cell tumor | Radius | Excision + fibular graft | 20 | 14 | 20 | DF |
| 7 | 6 | 33 | Female | Chondrosarcoma | Humerus | Excision + allograft-prosthesis composite | 20 | 23 | 19 | Soft tissue recurrence, died as a result of metastasis |
| 8 | 7 | 46 | Female | Adamantinoma | Tibia | Excision + custom segmental implant | 8 | 15 | 18 | DF |
| 9 | 30 | 20 | ||||||||
| 10 | 8 | 15 | Male | Osteosarcoma | Distal Femur | Excision + endoprosthesis | 30 | 20 | 18 | DF |
| Group 2† | ||||||||||
| 1 | 9 | 12 | Male | Osteosarcoma | Distal femur | Excision + custom segmental implant | 15 | 16 | 14 | DF |
| 2 | 10 | 53 | Male | Chondrosarcoma | Pelvis (pubis) | Excision | 10 (left side) | 17 | 14 | DF |
| 3 | 6 (right side) | 7 | ||||||||
| 4 | 11 | 13 | Female | Osteosarcoma | Fibula | Excision | 37 | 37 | 10 | DF |
| 5 | 12 | 24 | Male | Osteosarcoma | Distal femur | Excision + endoprosthesis | 45 | 50 | 9 | DF |
* Group 1 = registration using paired points and surface matching; †Group 2 = registration using fluoro-CT matching; DF = disease-free.
The objective of our study was to test the null hypothesis that the margins of the two population means were equal. Using a one-tailed test, we established an alpha of 0.050. With a proposed sample size of 10 and five for the two groups, the study had a power of 79.7% to yield a significant result assuming the mean difference in the margins was 4.3 mm and the within-group standard deviation was 3.0 mm. (Supplemental materials are available with the online version of CORR.)
We used BrainLAB VectorVision® Spine navigation software (BrainLAB Ltd, Hong Kong) in all patients. All went through the same workflow with the exception of one variation to the registration procedure as described subsequently.
First, for preoperative planning, CT-MRI image fusion was achieved followed by 3D localization of the tumor on the MR images using the “object creation” function. The highlighted “virtual tumor” was “expanded” to the extent of the intended surgical margin and the resection plane was then planned with the creation of multiple “trajectories” (Fig. 1). These data were transferred to the navigation platform before surgery.
Fig. 1.
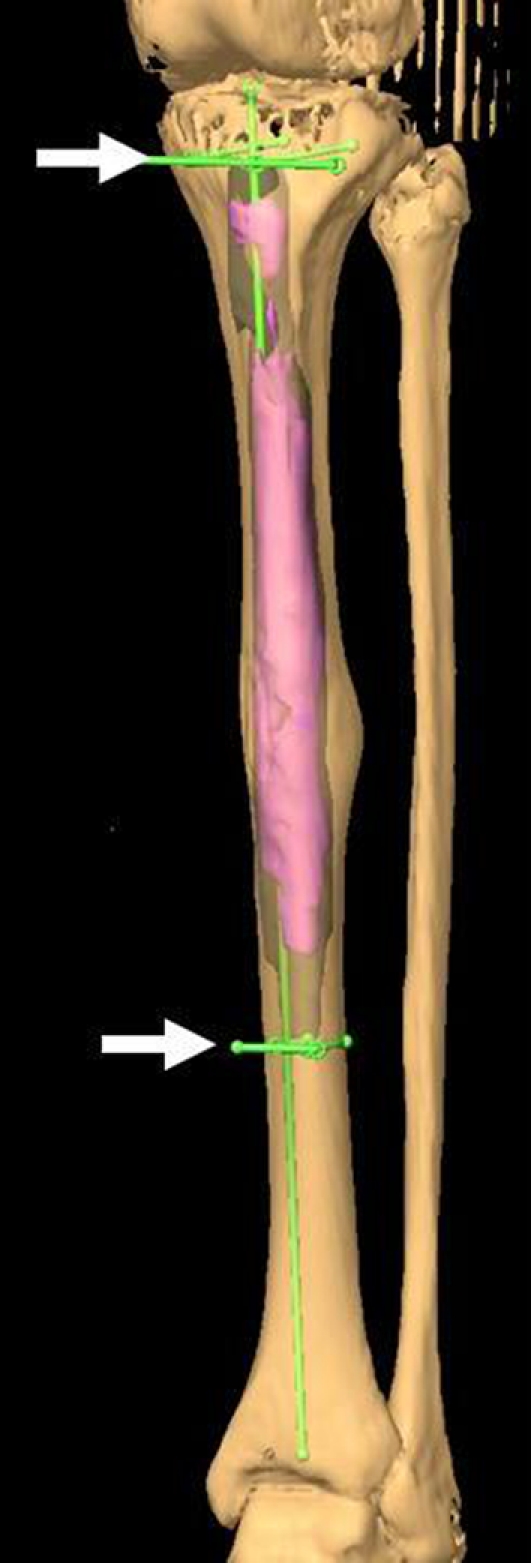
An image shows preoperative planning for Patient 7 with adamantinoma of the tibia. This three-dimensional model shows the highlighted tumor extent, which was expanded for the desired margins. The two arrows indicate the “trajectories” created to form the proximal and distal resection planes, respectively. The longitudinal line indicates the anatomic axis of the tibia. The defect was reconstructed with a custom segmental implant. The plane of the proximal osteotomy must be perfectly perpendicular to the anatomic axis of the bone to ensure precise fit and maximize bone support for the custom implant.
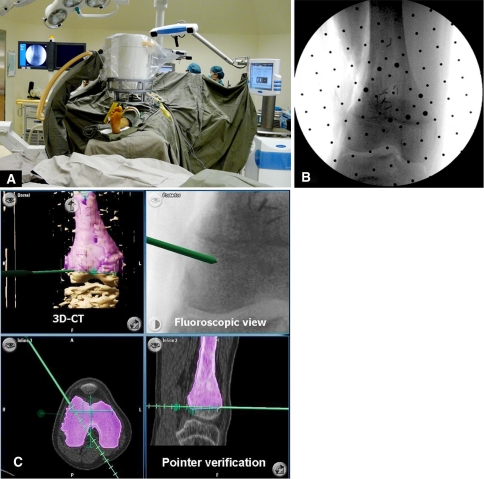
Second, for intraoperative navigation, registration of bony anatomy was carried out after insertion of a tracker assembly using either one of two methods. In the first 13 procedures, paired points and surface matching were used (Group 1). A surface pointer was used to match four bony landmarks (paired points) and 20 points on the exposed surface of the bone were used (surface matching) to increase registration accuracy. In the last five procedures, fluoro-CT matching was used (Group 2). A fluoroscopic registration kit (“phantom”) with multiple tungsten markers was attached to the C-arm (Fig. 2). Reflective discs on the “phantom” allowed recognition of its spatial position by the infrared camera. Two-plane images were taken and the images acquired were transferred by an analog/digital cable to the navigation platform. CT-fluoro matching was then performed using the navigation software. Accurate registration was ensured by holding the pointer to identifiable anatomic landmarks and verifying the position shown on the CT images on the navigation screen. In the paired points and surface matching technique, the accuracy was also calculated by the software; an accuracy value of 1.9 mm or less was considered acceptable. After registration accuracy was verified, specific surgical tools (osteotome and/or oscillating saw) were calibrated and then navigated in real-time to execute the planned bone cuts (Fig. 3).
Fig. 2A–C.
Images illustrate the fluoro-CT matching technique for registration. (A) The operating room setup for fluoro-CT matching for Patient 12 is shown. Although two-plane fluoroscopic images are being taken, the data are instantly exported to the navigation platform (right side of picture) for fluoro-CT matching automatically. (B) The fluoroscopic image as seen on the C-arm monitor is shown. The dots are the reference points on the attached “phantom.” (C) An integrated view of fluoro-CT images as seen on the navigation monitor is shown. The preoperative plan, three-dimensional (3D) CT images, fluoroscopic views, two-dimensional CT images, and intraoperative surgical tools can be visualized in real-time simultaneously.
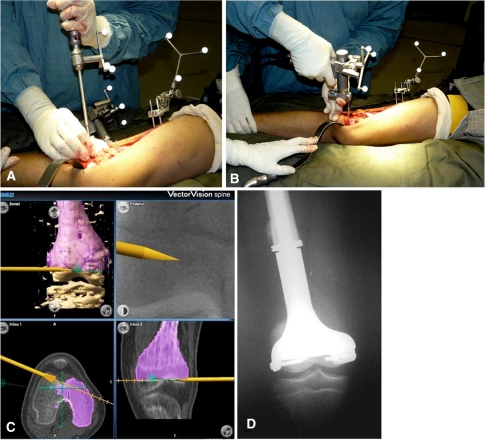
Fig. 3A–D.
Images illustrate navigated joint-saving excision in Patient 12 with osteosarcoma of the distal femur. (A) The navigated osteotome for initialing the bone cut is shown. (B) The navigated saw for the distal osteotomy is shown. (C) The saw can be visualized in real-time on the navigation monitor and directed toward the planned trajectory along the resection plane. (D) A custom-made minimally invasive growing implant was used for reconstruction in this patient.
For postoperative evaluation, the actual margin achieved in all cases was measured on the surgical specimen by the pathologist in a blinded fashion. The gross margins from tumor to the resection surface were measured on the sectioned specimen. Microscopic examination of the margins then followed to confirm clearance of the tumor. In situations in which the gross margin was considered distant from the tumor, a shaved margin was taken. When the gross margins were deemed close, multiple blocks were then obtained perpendicular to the resection plane and the microscopic margins were measured for further correlation with the gross measurement. The closest microscopic margin was then recorded. The number of blocks was not standardized because this is dependent on the size of the specimen. Typically, one section was prepared from each block; additional deeper sections from the same block would be prepared if the original section indicated inadequate exposure of the block.
The key dependent variable was the deviation of the actual closest margin from the planned margin. Group 1 (paired points and surface matching) and Group 2 (fluoro-CT matching) were first independently analyzed using Pearson correlation coefficient. We then determined the difference in the actual closest margin between these two groups using the nonparametric Mann-Whitney test. We analyzed our data using SPSS® Version 15.0 software (SPSS Inc, Chicago, IL).
Results
Direct navigation of a surgical osteotome or saw to achieve the desired osteotomy was possible in all cases.
All resection margins were free from tumor. The correlation between the margins preoperatively planned and the closest margin achieved was higher in Group 2 than in Group 1 (0.985 versus 0.631, respectively). The margins were more accurate (by 4.3 mm) in Group 2 (p = 0.013) than in Group 1.
One patient with osteosarcoma of the femur died from rapidly progressive systemic metastasis at 6 months. One patient with chondrosarcoma of the proximal humerus developed soft tissue recurrence in the distal biceps muscle remnant together with pulmonary metastasis and finally died at 19 months postoperatively. One patient with malignant giant cell tumor of tibia developed pulmonary metastasis 13 months postoperatively and is still living with disease. The other nine patients are disease-free.
Discussion
Although computer-assisted surgery has been used for a number of years, it has only recently been introduced for resecting tumors [2, 3, 5, 6, 9]. In previously published studies, most authors did not directly navigate the osteotomy with real-time manipulation of surgical tools. We therefore created a workflow to integrate an osteotomy with planned resection margins. We specifically performed computer-assisted bone tumor surgery and evaluated (1) the applicability of currently available software to different bones, eg, pelvis and extremity long bones; (2) the accuracy of the excision as compared with preoperative planning; and (3) the accuracy of a new registration technique of fluoro-CT matching as compared with the conventional paired points and surface matching technique.
We acknowledge several limitations to this study. First, the number of patients was small and we intended this to be exploratory work to judge the value of the concept. Second, with this approach, there could be discrepancies between the preoperative MRI signal and the actual tumor margin. Initially, we had a tendency to stay slightly outside of the planned margin. These two factors could explain under- and overresection, respectively. Third, the fluoro-CT matching cases were performed after the first group (using the paired points and surface matching technique), so the improved outcome could be influenced by increasing experience. Fourth, we did not compare the extra time and cost needed for the navigation process. We believed the time involved was related to the complexity of the particular case so it was difficult to quantify time investment in general. One previous study reported a mean additional time of 28 minutes for navigation procedures [9]. However, they believed defining the resection plane preoperatively might reduce the overall operative time because they no longer had to establish the resection margins during surgery. They also expected the navigation time would lessen as surgeons become more familiar with the procedure. The highest cost incurred was the one-time investment in the navigation system, which could be partly shared with other types of surgical applications.
Despite these limitations, our study has made some unique contributions to the application of computer-assisted navigation in orthopaedic bone tumor surgery. First, we applied the technique to almost every major long bone in the extremities and the pelvis. In the literature, applications have centered around the pelvis and femur [3, 6, 9] mostly because they were based on spine software, which may not be readily applied to the long bones. We believe there is a place for the application of navigation surgery to long bone tumors in certain situations, eg, custom prostheses and extraarticular resections in which a higher degree of precision is desirable.
Second, in some commercially available systems, the “registration accuracy” was actually an average value of accuracy of several points, whereas, in other systems, including the one used in this study, it referred to the maximum value of accuracy out of 20 points, making direct comparison of registration accuracy between systems impossible. Cho et al. [2, 3] used preoperatively placed Kirschner wires for registration and reported registration error was less than 1 mm (average), but there was no mention of final accuracy of the bone cut. Wong et al. [9] reported a registration accuracy of 0.36 to 0.44 mm (referring to the mismatch between the computer-generated images and the patient’s anatomy as calculated by the navigation software), but there was no documentation of the actual margin. In fact, in most published literature, “accuracy” only represents the accuracy of the registration process as recorded by the navigation machine itself. In our opinion, registration accuracy cannot be directly translated to bone cut accuracy, especially when the actual osteotomy is not navigated. We attempted to assess accuracy of the resection by measuring the actual margin achieved on the resected specimen by the pathologist. We believe this is a better way of measuring accuracy of the navigation outcome. The only other study using a direct measurement of the resection margin was that by Cho et al. [3]. They reported three cases with perfect correlation of the resection margin with the preoperative plan. However, no details were provided concerning their methodology of measuring the resection margins.
Third, with regard to registration technique, we had two failures of surface registration in Group 1, despite successful paired points registration, with both failures occurring in the proximal tibia. The CT images were suboptimal as a result of a deformed upper tibia and thinned bony cortices caused by the tumor. We were hesitant to place the sharp registration pointer over areas where there could be a tumor underneath thin bone. These factors might be the reasons for registration failure. In view of this, we switched to the fluoro-CT matching technique in the latter part of the study. When fluoro-CT matching was used, we did not have to register bony landmarks by hand and there was no risk of penetrating thin bone with tumor. Some extra equipment in terms of a fluoroscopic “phantom” is necessary and one has to ensure good AP and lateral views during the process. Normally, only one or two exposures of each view were good enough to achieve the purpose so the radiation exposure was minimal.
Our observations suggest performing navigation under fluoro-CT matching is more accurate than under paired points and surface matching. Part of this could be the result of surface registration failures as described. With surface registration technique, specific bony landmarks are necessary and the paired points should be close enough. Both factors are working to the disadvantage of diaphyseal long bones where direct application of software originally designed for the spine might not be ideal. Considering these factors, we recommend the use of fluoro-CT matching for situations when paired points and surface matching might not work too well, eg, (diaphyseal) long bones or when the registration points are close to the tumor surface. The accuracy of fluoro-CT matching technique in our hands was high with a correlation coefficient of 0.985, which is comparable to the 100% correlation in the study by Cho et al. [3] in which preoperatively placed Kirschner wires were used as fiducials. We are continuing the study with inclusion of more cases to further explore the potential of these techniques.
Our observations suggest computer-assisted bone tumor resection is useful in a wide variety of clinical situations. In our workflow model, there was no gap between preoperative planning and intraoperative performance of the bone cut. The new registration technique of fluoro-CT matching seems promising.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr Alex Chan and colleagues in the Department of Pathology, Queen Elizabeth Hospital, Hong Kong, for measuring the actual margins; Mr Anthony Leung and his team from BrainLAB Hong Kong for technical support both before and during the operations; and Dr Paul Unwin of Stanmore Implants Worldwide for reviewing the final manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Chauhan SK, Scott RG, Breidahl W, Beaver RJ. Computer-assisted knee arthroplasty versus a conventional jig-based technique: a randomized prospective trial. J. Bone Joint Surg. Br. 2004;86:372–377. doi: 10.1302/0301-620X.86B3.14643. [DOI] [PubMed] [Google Scholar]
- 2.Cho HS, Kang HG, Kim HS, Han I. Computer-assisted sacral tumor resection. J. Bone Joint Surg. Am. 2008;90:1561–1566. doi: 10.2106/JBJS.G.00928. [DOI] [PubMed] [Google Scholar]
- 3.Cho HS, Oh JH, Han I, Kim HS. Joint-preserving limb salvage surgery under navigation guidance. J. Surg. Oncol. 2009;100:227–232. doi: 10.1002/jso.21267. [DOI] [PubMed] [Google Scholar]
- 4.Grutzner PA, Suhm N. Computer aided long bone fracture treatment. Injury. 2004;35(Suppl 1):S-A57–S-A64. doi: 10.1016/j.injury.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Hufner T, Kfuri M, Jr, Galalski M, Bastian L, Loss M, Pohlemann T, Krettek C. New indications for computer-assisted surgery tumor resection in the pelvis. Clin Orthop Relat Res. 2004;426:219–225. doi: 10.1097/01.blo.0000138958.11939.94. [DOI] [PubMed] [Google Scholar]
- 6.Krettek C, Geerling J, Bastian L, Citak M, Rucker F, Kendoff D, Hufner T. Computer aided tumor resection in the pelvis. Injury. 2004;35(Suppl 1):S-A79–S-A83. doi: 10.1016/j.injury.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Richter M, Cakir B, Schmidt R. Cervical pedicle screws: conventional versus computer-assisted placement of cannulated screws. Spine.(Phila Pa 1976). 2005;30:2280–2287. doi: 10.1097/01.brs.0000182275.31425.cd. [DOI] [PubMed] [Google Scholar]
- 8.Schramm A, Suarez-Cunqueiro MM, Barth EL, Essig H, Bormann KH, Kokemueller H, Rücker M, Gellrich NC. Computer-assisted navigation in craniomaxillofacial tumors. J Craniofac Surg. 2008;19:1067–1074. doi: 10.1097/SCS.0b013e3181760fc0. [DOI] [PubMed] [Google Scholar]
- 9.Wong KC, Kumta SM, Chiu KH, Antonio GE, Unwin P, Leung KS. Precision bone tumor resection and reconstruction using image-guided computer navigation. J. Bone Joint Surg. Br. 2007;89:943–947. doi: 10.1302/0301-620X.89B7.19067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.