Abstract
Background
The choices of treatment for patients with extensive tumors of the femur include total femur megaprosthesis or large allograft-prosthetic composites. Previous reports suggest variable survival ranging from 60–70% at 1 to 2 years. However, these studies described earlier prostheses and techniques.
Questions/purposes
To confirm previous reports we determined (1) risk of local recurrence; (2) overall survivorship; and (3) function in patients with total femur reconstructions for tumors.
Methods
We retrospectively reviewed 23 patients with total femur megaprostheses implanted between 1987 and 2006 after resection of bone tumors. Two patients lost at followup were excluded; the remaining 21 included 15 males and six females with a mean age of 21 years. The mean followup was 48 months (range, 1 month 17 years). Function was assessed according to the MSTS System II.
Results
No patient developed a local recurrence during followup. At last followup, six patients were continuously disease-free at a mean of 148 months, one patient had no evidence of disease after treatment of a recurrence, one patient was alive with disease, and 13 patients died of their disease at a mean time of 17 months. In 15 patients evaluated with the MSTS score, the mean score was 66%; four patients had over 75%, eight from 51% to 75%, three from 26% to 50%. Four patients (19%) had complications requiring further surgery in absence of trauma. A fifth patient had a posttraumatic periprosthetic fracture.
Conclusions
A total femur prosthesis allows a limb-preserving procedure in tumors with extensive femoral involvement or in the presence of a skip lesion along the femur. The prognosis of these tumors is poor, but this reconstruction provides function with a relatively low rate of major complications.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The femur is one of the most frequent sites for bone sarcomas [4, 27]. In the past, amputation, hip disarticulation, and hemipelvectomy were the treatments of choice for these patients depending on the location and extent of the tumor. In the last 30 years, advances in diagnostic imaging, adjuvant and neoadjuvant chemotherapy, and surgical techniques helped establish limb salvage as an accepted method of treatment for malignant bone tumors of the femur [3, 14, 26, 28]. The goals of the procedure are to achieve local control of the tumor by wide excision and reconstruction of the extremity while retaining function [5, 6, 12, 19, 23].
When tumor involves a large segment of the femur, a less conventional approach is required to eradicate the tumor [20]. Total femur resection has been proposed for diaphyseal lesions that extend proximally to the lesser trochanter and distally to the distal diaphysometaphyseal junction and cause extensive bone destruction [22]. This procedure is technically demanding and needs detailed preoperative planning and careful reconstruction. Reconstructive options after total resection include a total femur allograft or a total femur prosthesis [12, 19, 20].
Early reports of megaprostheses suggest a short-term implant survival ranging from 60% to 70% at 1 to 2 years [7, 17, 21]. The introduction of modular prostheses improved prosthetic reconstruction of the total femur. In recent years, new designs of prostheses were developed that provided functional scores according to the MSTS system [8] ranging from 72% to 80% [10, 11, 13]. The long-term function and durability of the alternative reconstruction techniques are important since most of these patients are young and many now survive their disease. The use of total femur prostheses has been extended to selected nontumor patients such as those with failed hip and knee arthroplasties with extensive bone loss. One study reporting total femur prosthesis in revision arthroplasty reported high complication rates. Thirty two percent of patients had one or more complications: 13% with deep infections, 6% with dislocations, 3% with material failure, 2% with patellar problems, 1% with peroneal nerve palsy [11]. None of the patients had aseptic loosening at a mean followup of 59 months. Contrasted to revision arthroplasty, tumor patients often undergo chemotherapy and the amount of major muscle resection increase the risk of complications. Nonetheless, we believe it useful compare the complications and function in total femur prostheses in challenging revisions to those in patients with tumors.
We therefore determined (1) risk of local recurrence; (2) overall survivorship; and (3) function in patients with total femur reconstructions for tumors.
Patients and Methods
We retrospectively reviewed 28 patients with femoral tumors treated with megaprostheses of the total femur treated between September 1987 and November 2006. Twenty-two of the 28 patients had primary total femur implants after resection of tumor involving a large segment of the entire femur; six had a previous partial reconstruction of the femur converted into a secondary total femur reconstruction because of failure of the previous primary implant. Of the six patients converted to a total femoral prosthesis, two had infections with massive allografts, three had mechanical failures of distal femur megaprostheses, and one had a skip metastasis requiring secondary surgery. For this study, we included only the 22 patients with primary resections of large tumors of the femur and the one patient with a one skip metastasis in a distal femur arthroplasty. Two patients were lost to followup and were excluded, leaving 21 patients: 15 males and six females with a mean age of 21 years. Histologic diagnoses included 11 high-grade osteosarcomas, six Ewing’s sarcomas, one angiosarcoma, one fibrosarcoma, and two chondrosarcomas. Four patients presented with a pathologic fracture of the femur (one osteoblastic osteosarcoma, two with Ewing’s sarcomas, and one with proximal femur skip metastasis of clear cell chondrosarcoma). One patient with angiosarcoma was treated elsewhere with intralesional surgery and consequent contamination. Of the 21 patients two patients died of their disease within 6 months after the operation and one had an early hip disarticulation owing to infection. With these five exclusions the minimum followup of the remaining 18 patients was 7 months (mean, 59.5 months; range, 7–210 months). All information was obtained from the charts or images; no patients were recalled specifically for this study.
All patients had preoperative staging and planning. Patients were staged according to the staging system of Enneking et al: the stage was IIB in 16 and III in five [9]. Three patients had lung metastases at presentation, one had lymph node metastasis, and one a skip metastasis in the bone (Table 1). The diagnosis was established with an incisional biopsy in 17 patients and with a trocar biopsy in four. Eighteen patients received chemotherapy according to the protocols used in our hospital at the time of treatment; 17 had both preoperative and postoperative chemotherapy. Local radiotherapy was used in metastatic cases. Histologic responses to preoperative chemotherapy were classified by the criteria of Rosen et al. [24]; Grade I and II were defined as poor response and Grade III and IV as good response. There were nine poor responders and eight good responders to preoperative chemotherapy.
Table 1.
Baseline characteristic and operative data on patients
| Patient number | Gender/age (years) | Diagnosis | Stage | Surgical margin | Chemotherapy | Tumor necrosis |
|---|---|---|---|---|---|---|
| 1 | Male/56 | Angiosarcoma Grade 3 | IIB | Wide | A | – |
| 2 | Male/35 | Fibrosarcoma Grade 3–4 | IIB | Wide | – | – |
| 3 | Female/14 | Osteoblastic osteosarcoma Grade 4 | IIB | Wide | N/A | 100% |
| 4 | Male/18 | Osteoblastic osteosarcoma Grade 3 | IIB | Wide | N/A | >90% |
| 5 | Female/11 | Chondroblastic osteosarcoma Grade 4 | IIB | Wide | N/A | < 60% |
| 6 | Male/7 | Osteoblastic osteosarcoma Grade 4 | IIB | Wide | N/A | 96,8% |
| 7 | Male/19 | Ewing’s sarcoma | IIB | Wide | N/A | 100% |
| 8 | Female/14 | Osteoblastic osteosarcoma Grade 4 | IIB | Wide | N/A | 89% |
| 9 | Male/29 | Osteoblastic osteosarcoma Grade 4 | IIB | Wide | N/A | 58,6% |
| 10 | Female/15 | Ewing’s sarcoma | IIB | Wide | N/A | No |
| 11 | Male/15 | Osteoblastic osteosarcoma Grade 4 | IIB | Wide | N/A | 97% |
| 12 | Female/12 | Osteoblastic osteosarcoma Grade 3–4 | IIIB | Wide | N/A | 78% |
| 13 | Male/16 | Ewing’s sarcoma | IIIB | Wide | N/A | < 90% |
| 14 | Male/17 | Ewing’s sarcoma | IIIB | Wide | N/A | 90% |
| 15 | Male/15 | Osteoblastic osteosarcoma Grade 3–4 | IIB | Wide | N/A | 86% |
| 16 | Female/24 | Clear cell chondrosarcoma | IIIB | Wide | – | – |
| 17 | Male/43 | Chondroblastic osteosarcoma Grade 4 | IIB | Wide | N/A | No |
| 18 | Male/13 | Osteoblastic osteosarcoma Grade 3–4 | IIB | Wide (contaminated) | N/A | 92% |
| 19 | Male/27 | Ewing’s sarcoma | IIB | Wide | N/A | 74% |
| 20 | Female/62 | Central chondrosarcoma Grade 2 | IIB | Wide | – | – |
| 21 | Male/19 | Ewing’s sarcoma | IIIB | Wide | N/A | 75% |
N = neoadjuvant chemotherapy; A = adjuvant chemotherapy; N/A = neoadjuvant and adjuvant chemotherapy.
The endoprosthetic reconstruction was achieved with modular total femur prostheses, including KMFTR® prostheses (Stryker-Howmedica Inc, Rutherford, NJ), HMRS® prostheses (fixed hinge and rotating hinge; Stryker-Howmedica Inc), and GMRS® prostheses (Stryker-Howmedica Inc). The first prosthesis used was the Kotz modular femur and tibia reconstruction system (KMFTR). This prosthesis was developed in 1982. Between 1987 and 1988, one KMFTR total femur arthroplasty was performed. In 1987, the HMRS prosthesis was introduced. From February 1989 to November 2006, 19 total femur HMRS prostheses were implanted, 17 with fixed hinge and two prototype HMRS rotating hinge. In one patient, the modular GMRS system was used for total femur arthroplasty. Two of 19 HMRS were used in growing patients; in one case, the implant was revised three times using a longer connection piece each time; in the other, an expandable type was implanted. The patient underwent 12 consecutive minimally invasive procedures to gradually increase the prosthetic body length; at the end of growth, the definitive HMRS was implanted.
We used a Watson-Jones approach to the hip with a long posterolateral incision that reached the anterolateral aspect of the patellar tendon and tibial tuberosity. The femoral insertion of the gluteus maximus muscle was reflected and further retracted in a posterior direction, exposing the retrogluteal area, external rotators, sciatic nerve, abductors, and posterior capsule. We retracted the abductors to expose the hip. Part or all of the quadriceps was excised en bloc with the tumor according to standard tumor surgical principles [21]. The vastus lateralis muscle was reflected distally from its origin when possible. The rotator muscles were detached en bloc 1 cm from their insertion on the proximal femur when this was sufficient to achieve wide margins. The capsule was opened longitudinally along its anterolateral aspect and detached circumferentially from the femoral neck and the femur was dislocated anterolaterally. At the knee through an anterolateral arthrotomy, the cruciate ligaments, collateral ligaments, and menisci as well as capsular and muscular attachments to the distal femur were resected. The femur was dislocated anterolaterally. The proximal tibia was osteotomized and reamed in the same manner as for a standard knee arthroplasty. Finally, the psoas and adductor muscles were resected. The entire femur was excised en bloc with the vastus intermedius muscle; the vastus lateralis, when possible, rectus femoris, patella, and patellar tendon were preserved. After resection of the proximal femur, the length of the femur, size of the femoral head, and diameter of the distal medullary canal were measured. The prosthetic components were then chosen and assembled, the joint capsule extended over the femoral head component, and a trial reduction performed to test stability, tension, and ROM of the hip. Pulses were checked distally; if diminished, the length of the prosthesis was shortened. Any remaining joint capsule was replaced over the femoral head component, and the ROM of the hip was tested; we ensured the prosthesis was stable in flexion, adduction, and internal rotation. If not stable, we either modified the modular femoral head or spacer to obtain the appropriate length of the reconstruction.
The modular prosthesis was then assembled. Limb length was evaluated by comparing the bilateral distance between the ASIS and the medial malleoli. The femoral pulse was again assessed. Special attention was given to establish hip and knee stability and provide adequate muscle coverage of the prosthesis. Stability and muscle coverage enhance the chance of better function and reduce the risk of deep periprosthetic infection should there be superficial wound infection or dehiscence. The remaining hip capsule was sutured and reinforced by rotating the external rotator muscles proximally and suturing them to the repaired capsule. For glutei reinsertion to the prosthesis, different techniques were used: polyethylene plate fixation to prosthesis in five cases, a special device named “enhanced tendon attachment system” (ETA®; Stryker-Howmedica Inc) in two cases, Dall Miles cables in one case, direct reinsertion to the prosthesis and/or suture to the fascia lata in 13 cases. A bipolar head was used with acetabular preservation in all patients. The wound was closed.
The surgical margins were judged macroscopically and microscopically according to the classification of Enneking et al. [9]. The surgical margins were wide in 20 cases and wide/contaminated in one case of osteosarcoma.
When swelling around the hip and thigh was resolved, an abduction brace was customized for the patient. Isometric exercises were started the day after surgery. We began progressive mobilization using walker or crutches within the first several postoperative days as tolerated. To prevent postoperative edema and prosthetic dislocation, the extremity was kept elevated and abducted in balanced suspension for at least 5 days when the patient was not walking. Usually postoperative immobilization in a cast was used for 3 to 4 weeks for soft tissue healing and a brace with progressive ROM for a further 2 months. At discharge from the hospital, all patients were instructed to continue physical therapy and assisted walking for 6 weeks supervised by a physical therapist.
Followup routine evaluation was performed every 3 months for the first 2 years, every 6 months for the next 3 years, and then annually. Each followup evaluation included a clinical examination, standard radiographs, and chest CT. Function was assessed according to the Musculoskeletal Tumor Society functional evaluation system, MSTS II [8]. We had MSTS II scores for the 18 patients with more than 6 months followup. We recorded complications noted in the charts.
Three of us (GB, EP, PR) independently reviewed all image studies. Current radiographs were evaluated for lucent lines around the stems, areas of cortical resorption or periosteal reactions indicating loosening and material failure.
Survival of patients with osteosarcoma was evaluated using Kaplan-Meier survival curves [16]. The starting point was defined as the date of implantation of the endoprosthesis and the end point as the patient’s death or first metastases.
Results
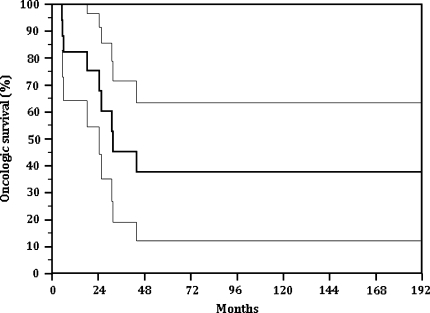
We identified no patients with local recurrence at last followup. The overall continuously disease free survival of patients (patients surviving without evidence of recurrence or metastases) was 38% at 16 years (Fig. 1).
Fig. 1.
The overall continuously disease free survival of patients (without evidence of recurrence or metastases) was 38% at 16 years.
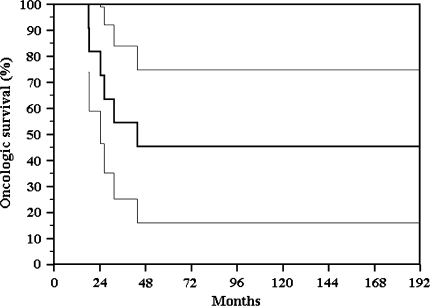
At last followup six of the 21 patients were continuously disease-free (CDF) at 148 months (all had preoperative chemotherapy), one patient had no evidence of disease (NED) after treatment of lung metastasis at 53 months, one patient was alive with disease (AWD), and 13 patients dead with disease (DWD) at a mean of 17 months (five osteosarcomas, six Ewing’s sarcomas, one angiosarcoma, and one fibrosarcoma); seven of these had preoperative chemotherapy with a poor response (Grade II). The continuously disease free survival (patients surviving without evidence of recurrence or metastases) of 11 patients with osteosarcoma was 45% at 10 years (Fig. 2). In this group, minimum followup was 16 months (average, 82.4 months; range, 16–210 months); the final survival status was CDF in five patients, NED after treatment of lung metastasis in one, and five are DWD at mean time of 24.7 months (Table 2). Two patients (50%) with pathologic fracture and the patient with nailing contamination died within 8 months after tumor resection.
Fig. 2.
The continuously disease free survival (without evidence of recurrence or metastases) of 11 patients with osteosarcoma was 45% at 10 years.
Table 2.
Oncologic outcome in patients with osteosarcoma
| Patient number | Stage | Surgical margin | Chemotherapy | Tumor necrosis | Pathologic fracture | Oncological status |
|---|---|---|---|---|---|---|
| 1 | IIB | Wide | N/A | 100% | – | CDF |
| 2 | IIB | Wide | N/A | > 90% | – | CDF |
| 3 | IIB | Wide | N/A | <60% | – | DWD |
| 4 | IIB | Wide | N/A | 96,8% | – | CDF |
| 5 | IIB | Wide | N/A | 89% | – | DWD |
| 6 | IIB | Wide | N/A | 58,6% | – | DWD |
| 7 | IIB | Wide | N/A | 97% | yes | CDF |
| 8 | IIIB | Wide | N/A | 78% | – | DWD |
| 9 | IIB | Wide | N/A | 86% | – | CDF |
| 10 | IIB | Wide | N/A | No | – | DWD |
| 11 | IIB | Wide (contaminated) | N/A | 92% | – | NED |
N/A = neoadjuvant and adjuvant chemotherapy; CDF = continuously disease-free; DWD = dead with disease; NED = no evidence of disease.
Of 15 patients evaluated with the MSTS scores, the mean score was 66%; four patients had over 75%, and eight from 51% to 75%, three from 26% to 50%. The average active range of knee motion was 60° (range, 0°–110°). Two patients required one cane or crutch. Four patients had a marked Trendelenburg gait. Three patients had mild, nondisabling pain; none had difficulties in standing or walking short distances.
Excluding growing patients, five patients required further operative procedures; one patient, with preexisting peripheral vascular disease, developed leg ischemia early postoperatively and subsequent deep infection requiring hip disarticulation; one patient underwent two plastic surgeries as a result of early wound infection, one case of partial prosthetic disconnection at 15 months, one trochanteric ETA® at 63 months, and one polyethylene wear requiring revision at 196 months. One patient had a posttraumatic periprosthetic tibial fracture treated with cast immobilization.
Discussion
In the past, most patients with extended sarcomas of the femur had amputation, hip disarticulation, or hemipelvectomy as treatment with poor function [19, 20]. The advent of new drugs and improved imaging techniques allowed conservative treatment of tumors of the femur with limb salvage procedures [3, 14, 26, 28]. Total femur replacement in tumors has not yet been widely reported in literature [20, 23, 28]. Although there have been recent advances in designs and materials, total femur prosthetic arthroplasty is still a demanding procedure reserved for expert surgeons [3, 20, 23] and the prognosis in large sarcomas of the femur is worse than in other sites of the lower limb [4, 27]. We reviewed our experience in total femoral prosthetic reconstruction in bone sarcomas to evaluate: (1) the local control of the tumor; (2) the patients’ survival; and (3) function.
Our study is subject to a number of limitations. First, although we report our experience over a 20 year span, the number of patients is small owing to the infrequent indications. Second, during this interval, three different megaprosthesis models have been implanted. The length of followup is different among the three and there are too few patients to draw conclusions about individual devices. More definitive conclusions would require assessing findings from multiple studies. Third, we included patients with several types of tumors, each with their own general prognoses, but we did this owing to the rare indications to this type of reconstruction. We are unable to determine whether tumor type related to survival. Fourth, careful statistical analysis cannot be applied to better interpret data in a series of small patients.
Large tumors with extensive femoral involvement or multiple femoral lesions are indications for total femur resection and replacement. Wide resection without intraoperative contamination is crucial for local control. In our series, surgical margins were contaminated in one case; in no patient was a local recurrence observed. In 1988, Nerubay et al. [23] reported four cases of local recurrence in 19 patients treated with total femur arthroplasty. Ward et al. [28] in 1995 reported on 21 total femur prosthetic reconstructions; the rate of local recurrence was 9.5% (two of 21). Mankin et al. [20] in 2005 reported three cases of local recurrence in 15 patients with total femur resection (13 tumors). In 2007, Jeon et al. [15] analyzed the clinical outcome of 13 patients after total femur resection for osteosarcoma; they observed four local recurrences in 13 patients (30%) (Table 3).
Table 3.
Oncologic outcome: comparison with previous studies
| Authors | Patients | Reconstruction method | Ratio of local recurrence | Number of patients with osteosarcoma | Surviving of patients with osteosarcomas |
|---|---|---|---|---|---|
| Nerubay et al. [23] | 19 | Prosthesis | 4/19 | 13 | 3/13 |
| Ward et al. [28] | 21 | Prosthesis | 2/21 | 7 | 3/7 |
| Mankin et al. [20] | 15* | Allograft-prosthesis composite (10 patients) Prosthesis (5 patients) |
3/13 | 4 | 1/4 |
| Jeon et al. [15] | 13 | Recycled bone-prosthesis composite | 4/13 | 13 | 4/13 |
| Ruggieri et al. [current study] | 21 | Prosthesis | –/21 | 11 | 6/11 |
* Thirteen tumors, two nononcologic patients.
Tumor control and patient survival are the primary aims of total femur resections [15, 18, 22]. In our series, patients with Ewing’s sarcoma had a poor prognosis with a survival of 35% at 30 months. Nerubay et al. [23] reported a rate of survival in 13 patients with osteosarcoma of 23%. Jeon et al. [15] reported an event-free survival rate of 33%. Our patients with osteosarcoma had a survival rate of 45% at 10 years (Table 3). All but one presented with Stage IIB with large extraosseous lesions; one patient had multiple pulmonary involvement. In no case were skip lesions detected. Previous studies report a correlation between survival and skip metastases independent from distant metastases [25, 29]. In 2006, Bacci et al. [1] described a correlation between response to chemotherapy and survival.
The aim of the prosthetic reconstruction is to restore the best possible function of the lower limb. Previous studies demonstrate a high rate of amputation or hip disarticulation resulting from complications of the procedure, ranging from 14% to 28% [18, 23, 28]. In 2000, Bickels et al. [2] reported on 18 total femur prostheses; they had two major complications requiring surgery (a deep infection and an aseptic loosening) with no consequent amputation. In a smaller series of five total femur prostheses, Mankin et al. [20] observed one major complication and no amputations. Our experience confirms this salvage procedure is challenging; we observed a 23% (five of 21) rate of complications requiring further surgery (three mechanical failures and two infections requiring hip disarticulation in one patient). Muscular reinsertion to the prosthesis is crucial to obtain a satisfactory function. The hip arthroplasty in total femur reconstructions is a key point. Hip dislocation is a common complication in proximal femur or total femur resections [2]. As reported by Bickels et al., acetabular preservation, capsular repair, and reconstruction of the abductor mechanism can decrease this complication rate [2]. They reported only one (1.7%) case of dislocation despite in early series dislocation ranging from 11% to 15% [15]. All cases reviewed in this study had a bipolar system for the hip with no acetabular cup implant; special care was given to capsule suturing and soft tissue reinsertion and the absence of dislocations confirms the value of these elements emphasized by Bickels et al. [2]. Bickels et al. had 66% satisfactory results in 18 total femur prostheses, whereas in three cases, patients required gait support [2]. Mankin described similar function with allograft-composite total femur reconstructions and total metallic prostheses, but the degree of Trendelenburg gait problems was greater for patients with metallic devices [20]. In our series, we had comparable functional score with a marked Trendelenburg gait in approximately 22% of patients.
Despite the rate of local recurrence previously reported [15, 20, 28], total femur resection has been useful to achieve local control in our series. The survival of patients with total femur resection is low, probably because of the behaviors of extensive sarcomas, but total femur prosthetic reconstruction maintained function in surviving patients.
Acknowledgments
We thank Teresa Calabrò, MD (orthopaedic surgeon), Giuseppe Ussia, MD (orthopaedic surgeon), and Marco Alberghini, MD (Chief of Surgical Pathology at Rizzoli Institute) for their help.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 2.Bickels J, Meller I, Henshaw RM, Malawer MM. Reconstruction of hip stability after proximal and total femur resections. Clin Orthop Relat Res. 2000;375:218–230. doi: 10.1097/00003086-200006000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factor in high-grade osteosarcoma of the extremities or trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 4.Campanacci M. Bone and Soft Tissue Tumors. NY, New York: Springer Verlag; 1999. [Google Scholar]
- 5.Eckhart JJ, Springfield DS, Malawer MM. Surgical management: hip and proximal femur. In: Simon MA, Springfield DS, eds. Surgery for Bone and Soft Tissue Tumors. Philadelphia, PA: Lippincott Raven; 1998:343–356.
- 6.Eckhart JJ, Springfield DS, Peabody TD. Surgical management: distal femur. In: Simon MA, Springfield DS, eds. Surgery for Bone and Soft Tissue Tumors. Philadelphia, PA: Lippincott Raven; 1998:357–374.
- 7.Engelbrecht E, Engelbrecht H. Total femur replacement using St George’s model of total hip and knee joint endoprosthesis. Chirurg. 1974;45:231–236. [PubMed] [Google Scholar]
- 8.Enneking WF, Dunham W, Gebhardt MC, Malawer M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–261. [PubMed] [Google Scholar]
- 9.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 10.Fabroni RH, Castagno A, Aguilera AL, Steverlynck AM, Zeballos J. Long-term results of limb salvage with the Fabroni custom made endoprosthesis. Clin Orthop Relat Res. 1999;358:41–52. doi: 10.1097/00003086-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Friesecke C, Plutat J, Block A. Revision arthroplasty with use of a total femur prosthesis. J Bone Joint Surg Am. 2005;87:2693–2701. doi: 10.2106/JBJS.D.02770. [DOI] [PubMed] [Google Scholar]
- 12.Gebhardt MC, Flugstad DI, Springfield DS, Mankin HJ. The use of bone allografts for limb salvage in high-grade extremity osteosarcoma. Clin Orthop Relat Res. 1991;270:181–196. [PubMed] [Google Scholar]
- 13.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–171. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 14.Gosheger G, Hillmann A, Lindner N, Rodl R, Hoffmann C, Burger H, Winkelmann W. Soft tissue reconstruction of megaprostheses using a Trevira tube. Clin Orthop Relat Res. 2001;393:264–271. doi: 10.1097/00003086-200112000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Jeon DG, Kim MS, Cho WH, Song WS, Lee SY. Clinical outcome of osteosarcoma with primary total femoral resection. Clin Orthop Relat Res. 2007;457:176–182. doi: 10.1097/BLO.0b013e31802ba4af. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 17.Katznelson A, Nerubay J. Total femur replacement in sarcoma of the distal end of the femur. Acta Orthop Scand. 1980;51:845–851. doi: 10.3109/17453678008990883. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie G, Healey JH, Lane JM, Marcove RC. Prosthetic total femur replacement following massive resection for sarcoma. In: Brown KLB, ed. Complications of Limb Salvage. Montreal, Quebec: ISOLS; 1991:129–132.
- 19.Mankin HJ. The changes in limb reconstruction as a result of the development of allografts. Chir Org Mov. 2003;88:101–113. [PubMed] [Google Scholar]
- 20.Mankin HJ, Hornicek FJ, Harris M. Total femur replacement procedures in tumor treatment. Clin Orthop Relat Res. 2005;438:60–64. doi: 10.1097/00003086-200509000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Marcove RC, Lewis MM, Huvos AG. Total femur and total knee replacement. A preliminary report. Clin Orthop Relat Res. 1977;126:147–152. [PubMed] [Google Scholar]
- 22.Morris HG, Capanna R, Campanacci D, Del Ben M, Gasbarrini A. Modular endoprosthetic replacement after total resection of the femur for malignant tumour. Int Orthop. 1994;18:90–95. doi: 10.1007/BF02484417. [DOI] [PubMed] [Google Scholar]
- 23.Nerubay J, Katznelson A, Tichler T, Rubinstein Z, Morag B, Bubis JJ. Total femoral replacement. Clin Orthop Relat Res. 1988;229:143–148. [PubMed] [Google Scholar]
- 24.Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, Marcove RC, Lane JM, Mehta B, Urban C. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::AID-CNCR2820490625>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 25.Sajadi KR, Heck RK, Neel MD, Rao BN, Daw N, Rodriguez-Galindo C, Hoffer FA, Stacy GS, Peabody TD, Simon MA. The incidence and prognosis of osteosarcomas skip metastases. Clin Orthop Relat Res. 2004;426:92–96. doi: 10.1097/01.blo.0000141493.52166.69. [DOI] [PubMed] [Google Scholar]
- 26.Schindler OS, Cannon SR, Briggs TW, Blunn GW, Grimer RJ, Walker PS. Use of extendable total femoral replacements in children with malignant bone tumors. Clin Orthop Relat Res. 1998;357:157–170. doi: 10.1097/00003086-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Unni KK. Dahlin’s Bone Tumors. General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996. [Google Scholar]
- 28.Ward WG, Dorey F, Eckardt JJ. Total femoral endoprosthetic reconstruction. Clin Orthop Relat Res. 1995;316:195–206. [PubMed] [Google Scholar]
- 29.Wuisman P. Prognosis for patients who have osteosarcoma with skip metastasis. J Bone Joint Surg Am. 1990;72:60–68. [PubMed] [Google Scholar]