Abstract
Background
The shoulder is commonly affected by primary and metastatic tumors. Current surgical techniques for complex shoulder reconstruction frequently result in functional deficits and instability. A synthetic mesh used in vascular surgery has the biological properties to provide mechanical constraint and improve stability after tumor related shoulder reconstruction.
Questions/purposes
We describe (1) surgical technique using a synthetic mesh during humerus reconstructions; (2) functional level defined as shoulder ROM of patients undergoing the procedure; (3) incidence of postoperative dislocation and shoulder instability; and (4) complications associated with the use of the device.
Methods
We retrospectively reviewed 16 patients with proximal humerus replacements reconstructed with a synthetic mesh from February 2006 to July 2008. Patients were followed clinically and radiographically for a minimum of 13 months (mean, 26 months; range, 13–43 months).
Results
There were no shoulder dislocations at the latest followup. The mean shoulder flexion was 43° (range, 15°–170°) and mean shoulder abduction of 38 (range, 15°–110°). The mean operative time was 121 minutes (range, 80–170 minutes) and the mean blood loss was 220 mL (range, 50–750 mL). One patient had a superficial wound infection and none a deep infection requiring removal of the graft or prosthesis.
Conclusions
The data suggest the use of a synthetic vascular mesh for proximal humerus reconstruction may reduce dislocations and facilitate soft tissue attachment and reconstruction after tumor resection.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Modern advances in orthopaedic oncology have allowed limb salvage in the treatment of tumors of the appendicular skeleton [3]. However, the preservation of the affected limb with metallic endoprostheses or allografts after tumor resection is often associated with substantial soft tissue loss and functional deficits [42, 43, 46]. Functional limitations such as instability and increased risk for joint dislocation [4, 9, 18], gait abnormalities [4, 29, 41], postoperative stiffness [49, 51], and pain [4, 9] after reconstruction of tumors involving the lower extremity are well documented. The shoulder is commonly affected by primary and metastatic tumors [37]. Functional deficits and instability are particularly frequent after any type of tumor reconstruction about the shoulder [4, 5, 11, 22, 24, 25, 35, 37, 44, 47, 52].
The concept of limb salvage for tumors of the upper extremity is not new [35]. Multiple surgical techniques such as the Tikhoff-Linberg procedure [15, 31, 32], shortening arthrodeses [2, 6], fibular transplants [19], en bloc shoulder allografts [8, 41], and metallic implants [9, 28, 34] have been described to reconstruct the shoulder after tumor resection and avoid the need of forequarter amputations. All of these procedures have been associated with joint instability and recurrent dislocations [25], “flail extremity” [13], traction neuropraxia [4, 5, 11], donor site morbidity [2, 19, 35], or multiple revision surgeries [35]. The development of novel biomaterials has allowed reliable limb reconstructions with endoprostheses and the advances in diagnostic imaging techniques have allowed precise tumor resections preserving neurovascular bundles [40].
A synthetic mesh commonly used in vascular surgery could be used to reduce the rate of dislocation and facilitate soft tissue attachment after proximal or total humerus reconstruction in the treatment of tumors of the upper extremity. By creating a sleeve that anchors in the native glenoid and wraps around the reconstructed proximal humerus, a synthetic mesh may improve postoperative stability and function and may provide a biocompatible scaffold for soft tissue ingrowth.
We describe (1) the surgical technique using a synthetic mesh during humerus reconstructions; (2) the functional level and shoulder ROM of patients undergoing the procedure; (3) the incidence of postoperative dislocation and shoulder instability; and (4) the complications associated with the use of the device.
Patients and Methods
We retrospectively reviewed the hospital records of 16 patients with proximal humerus replacements supplemented with the use of an aortograft mesh between February 2006 and July 2008. The study included patients who had a bone malignancy of the proximal aspect of the humerus. All patients included in this study had type IA or IB resections using the Malawer classification of shoulder girdle resections [30]. Patients with soft tissue sarcomas or bone tumors of the clavicle, scapula, or only the humeral diaphysis were excluded. Patients underwent limb-sparing resection and reconstruction with metallic endoprostheses when the preoperative imaging studies suggested that a satisfactory surgical margin could be achieved. Preoperative evaluation included a complete physical examination with special attention to neurovascular and functional status of the affected limb (in an attempt to document involvement of the major neurovascular bundles and overall status of the shoulder girdle musculature). Staging studies included plain radiography, computerized tomography, magnetic resonance imaging, and angiography of the shoulder girdle and upper extremity as described by Bickels and colleagues [4]. Core needle biopsy was performed after the completion of all the staging studies and attention was given to the location of the biopsy site as to not interfere with the resection and reconstruction surgical incision. Staging distribution was as follows: five patients Stage IIB, four patients Stage IIIA, four patients Stage IIIB, and three patients Stage IV (Table 1). The aortograft was used in 11 primary proximal humerus replacements and five revision cases after failed shoulder reconstruction for metastatic renal cell carcinoma in three cases, Hodgkin’s lymphoma in one case and osteosarcoma in one patient. There were 11 women and five men who had a mean age of 51 years (range, 14–71 years) at the time of surgery. The mean body mass index was 24.67 (range, 16.3–42.9). We excluded one patient who died of metastatic disease 1 month following reconstruction. The minimum followup of the remaining 15 patients was 13 months (mean, 26 months; range, 13–43 months; median 24 months). The study was approved by our Institutional review board (IRB) and Scientific Review Committee (SRC).
Table 1.
Demographic variables
| Patient | Age (years) | Deceased | Age at death (years) | Gender | Diagnosis | Weight (kg) | Height (cm) | Body mass index | Side | Prosthesis | Length of resection (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | No | NA | Male | Multiple myeloma | 152 | 177 | 42.9 | Right | Stryker | 10 |
| 2 | 56 | No | NA | Female | Metastatic carcinoma | 78.5 | 168.9 | 23.2 | Right | Stryker | 12 |
| 3 | 64 | No | NA | Female | Multiple myeloma | 62 | 163 | 19.0 | Right | Stryker | 10 |
| 4 | 14 | No | NA | Female | Osteosarcoma | 66 | 162.6 | 20.3 | Right | Stryker | 24 |
| 5 | 15 | No | NA | Female | Osteosarcoma | 63 | 187.6 | 16.8 | Right | Stryker | 13 |
| 6 | 62 | No | NA | Female | Lymphoma | 95 | 170 | 27.9 | Right | Stryker | 11 |
| 7 | 56 | No | NA | Male | Osteosarcoma | 95 | 177.8 | 26.7 | Left | Stryker | 10 |
| 8 | 67 | Yes | 68 | Male | Metastatic lung cancer | 76.7 | 170 | 22.6 | Right | Stryker | 11 |
| 9 | 70 | No | NA | Male | Metastatic renal cell carcinoma | 108 | 177 | 30.5 | Right | Stryker | 13 |
| 10 | 24 | No | NA | Female | Osteosarcoma | 56.6 | 172.7 | 16.4 | Right | Biomet | 12 |
| 11 | 63 | No | NA | Female | Large B cell lymphoma | 74 | 170 | 21.8 | Right | Stryker | 13 |
| 12 | 50 | Yes | 52 | Female | Chondrosarcoma | 86.6 | 177.8 | 24.4 | Left | Stryker | 10 |
| 13 | 64 | Yes | 65 | Male | Metastatic renal cell carcinoma | 107 | 179 | 29.9 | Left | Stryker | 12 |
| 14 | 33 | No | NA | Female | Metastatic breast cancer | 55.7 | 154.9 | 18.0 | Left | Stryker | 12 |
| 15 | 64 | No | NA | Female | Multiple myeloma | 62 | 163 | 19.0 | Right | Stryker | 12 |
| 16 | 71 | No | NA | Female | Metastatic renal cell carcinoma | 71 | 177 | 20.1 | Right | Stryker | 12 |
| Mean | 51 | 82 | 172 | 24 | 12.5 |
NA = not applicable.
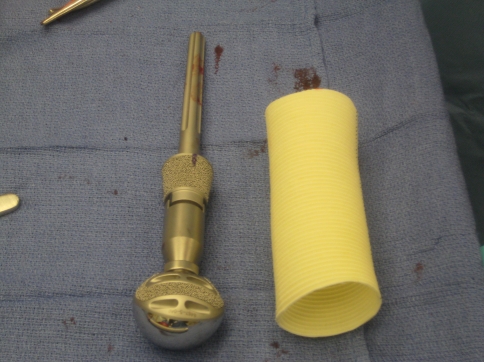
Several studies have reported in detail the surgical technique for proximal humerus replacement using metallic endoprostheses after tumor excision [29, 37, 47, 50, 51]. As the humeral insertions for the rotator cuff complex and deltoid muscle are often involved in the resection, all of these techniques have stressed the importance of conserving a sufficient soft tissue envelope for reconstruction and the need to recreate sites for attachment of remaining structures. We supplemented these approaches with a synthetic mesh (Gore Graft; Gore Medical, Flagstaff, AZ) commonly used in cardiovascular procedures [10, 12, 38, 39, 49]. The “aortograft” is a flexible, self-expanding tube of polytetrafluoroethylene (ePTFE) used for the treatment of aortic aneurysms. The graft is available in diameters of 26 to 45 mm and in lengths of 10 to 20 cm (Fig. 1). All of the synthetic tubes used in this study had 38-mm diameters and 20-cm lengths (the initial length was further adjusted intraoperatively depending on the amount of humerus resection and overall status of residual soft tissue envelope). The ePTFE is biologically inert [18] and there are no reports of allergic or host versus graft responses with the use of the graft.
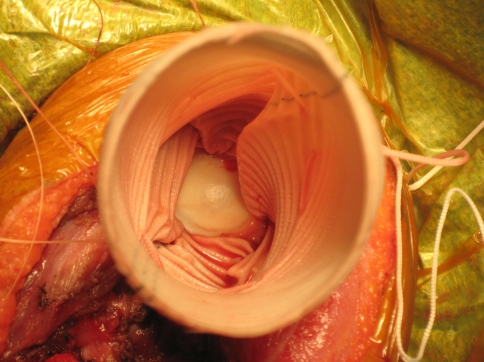
Fig. 1.
Aortograft mesh with proximal humerus replacement is shown.
All but one of the procedures was performed with the use of Stryker (Global Modular Replacement System (GMRS) Stryker Orthopaedics, Mahwah, NJ) prostheses and instruments. The remaining patient received a Biomet (Biomet Mosaic Humeral Replacement System, Biomet Inc, Warsaw, IN) proximal humerus replacement. We determined the length of the humerus resection based on pre- and intraoperative planning, with the use of magnetic resonance imaging and/or computerized tomography scans and intraoperative biopsy of the medullary canal sampled after the initial osteotomy.
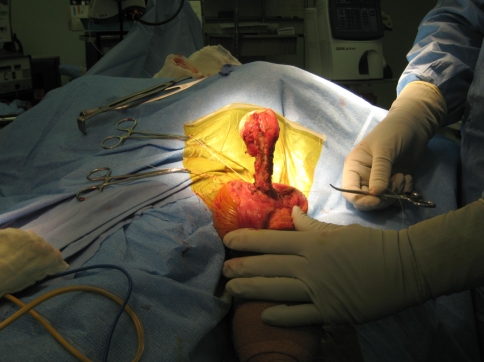
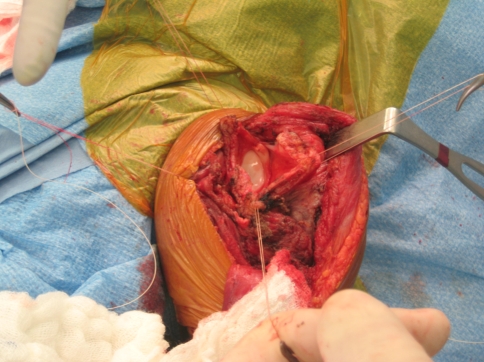
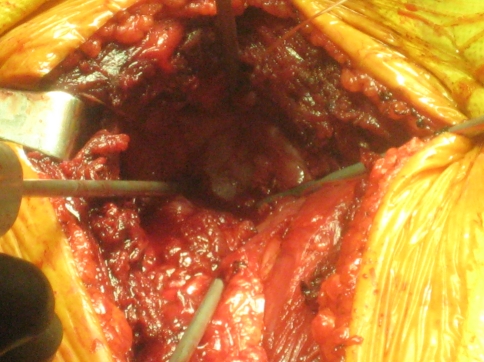
All of the procedures were performed through an extended anterior approach [36]. The skin incision starts superior to the coracoid process, follows the line of the deltopectoral groove and extends distally depending on the expected level of humerus resection. The soft tissue dissection is dependent on the presence or absence of tumor compromise (extraskeletal involvement) and it is carried down to the humeral periosteum. Once the proposed level of humeral resection is identified, an oscillating saw is used to make the osteotomy (Fig. 2). The main soft tissue attachments to the proximal humerus (such as the rotator cuff tendons and joint capsule) are identified and tagged (Fig. 3). Curette samples from the medullary canal are sent for pathology analysis and confirmation of disease free remaining bone. Using four evenly spaced bioabsorbable screws (Fig. 4), the end of the aortograft is anchored and secured to the glenoid (Fig. 5). The endoprosthesis is cemented in place and the prosthetic humeral head is introduced through the free end of the aortograft. A purse-string suture is passed at the level of the neck-head junction to further secure the graft to the prosthesis (Fig. 6). The remaining soft tissues are attached to the aortograft, covering it entirely, and the surgical incision is closed following anatomic planes. (Fig. 7) Hemostasis is maintained throughout the procedure using a bipolar sealer device (Aquamantys™; Salient Surgical, Dover, NH) and standard electrocautery (Electrosurgical Pencil; Valleylab, Boulder, CO).
Fig. 2.
Identification of the level of resection of proximal humerus is shown.
Fig. 3.
Soft tissue structures, capsule and rotator cuff muscles are tagged and identified for repair.
Fig. 4.
The glenoid is visualized for anchoring of the aortograft mesh.
Fig. 5.
The glenoid view with the aortograft secured in place is shown.
Fig. 6.
A pursestring suture on the aortograft securing the neck of the prosthesis is shown.
Fig. 7.
Attachment of soft tissue structures on the aortograft mesh and layered closure of the surgical incision are shown.
Postoperatively, patients received intravenous vancomycin for the duration of the hospital stay (mean, 3 days; range, 1–9 days). Patients were allowed to ambulate freely with a sling for the affected upper extremity and were instructed to perform pendulum exercises on postoperative day 1.
The physical therapy team assisted with all patient activities after the procedure. During the hospital stay and for the first 4 weeks (home or in-patient physical therapy) only passive range of motion exercises to less than 100º elevation, 20º external rotation, and 35º abduction were initiated. During this stage no active range of motion, resistance or strengthening exercises were performed with the involved upper extremity. Active range of motion exercises were initiated after week 7 to include light strengthening maneuvers. Over the head activities and heavy lifting were not recommended in all cases. Medications upon discharge for all patients included oral ciprofloxacin and doxycycline for 3 weeks and a short course of narcotic pain medication.
Patients were followed clinically and radiographically at 3 weeks after discharge, followed by visits at 7 weeks and 6 months after discharge. ROM was assessed during the postoperative clinic visits.
Results
At latest followup, mean shoulder flexion was 43° (range, 15°–170°). The patient with shoulder flexion of 15° was a 50-year-old woman with a diagnosis of chondrosarcoma. She succumbed to her disease 20 months after the proximal humerus replacement. The mean shoulder abduction was 38° (range, 15°–110°). In this case, the patient with 15° of abduction was a 67-year-old man with a diagnosis of metastatic lung cancer who died of the disease 6 months after the surgical intervention.
None of the patients had a shoulder dislocation or instability requiring repeat surgery. One of the patients in the subgroup of revision surgery presented with clinical and radiographic evidence of anterior subluxation of the proximal humerus replacement. This patient is able to perform most of his daily activities and has not had any further surgery.
There were no revision surgeries. A patient with a diagnosis of metastatic renal cell cancer had a superficial wound infection with dehiscence that required irrigation and débridement in the operating room. There was no intraoperative evidence of deep extension of the infection and it was controlled with a postoperative course of intravenous and oral antibiotics. The patient died 1 year after the surgical procedure. The use of the aortograft represents an additional surgical step during the proximal humerus reconstruction. The mean operative time was 121 minutes (range, 80–170 minutes). Blood loss is an important variable in orthopaedic tumor reconstructions. There was a mean blood loss of 220 mL (range, 50–750 mL). The patient with 750 mL of blood loss was a 64-year-old patient with a diagnosis of metastatic renal cell cancer and hypertension. Of note, the patient had a past medical history of multiple symptomatic deep venous thromboses that required long-term anticoagulation.
Discussion
The shoulder has the largest ROM in the human skeleton. In contrast with the hip, the glenohumeral joint has six degrees of freedom and a major component of the overall stability is provided by soft tissue attachments [1, 21]. Modern metallic endoprostheses permit the orthopaedic surgeon to reconstruct large segments of diseased bone. However, these constructs lack the soft tissue-bone interface required for function. Several studies have focused on the proximal femur to try to understand the complex relationship of tendon-bone interfaces [16, 27, 33]. Osteoconductive scaffolds [53], metallic anchors for soft tissues [20], predetermined suture sites embedded in the metallic prostheses [50], and coating of the prostheses with novel materials [14, 16, 45], have been described to promote soft tissue ingrowth and improve function after limb salvage with endoprostheses. All of the surgical options for proximal humerus reconstruction have been associated with instability [25], recurrent dislocation [25, 38], and overall poor function [36, 38]. In the present study, the authors describe the surgical technique using an aortograft during humerus reconstructions, the functional level and shoulder ROM of patients undergoing the procedure, and the incidence of postoperative dislocation and shoulder instability.
We acknowledge several limitations to our study. First, it is a small sample of patients. Proximal humerus reconstruction for tumors is a relatively rare surgical procedure and it is difficult to accumulate large series in a single institution. We report on 16 patients (accrual over 17 months) who underwent a proximal humerus reconstruction with the use of an aortograft mesh at a large National Cancer Institute designated cancer center. Second, we had a relatively short followup of a mean of 26 months (range 13 to 43 months). Patients with osteosarcoma have a short life expectancy (68% at 5 years) 40 and in the case of other primary tumors (such as renal cell) with metastasis to bone, the survival decreases even more to a median life expectancy of 12 months [48]. Although only one of the patients described in this series died (13 months after the procedure), the authors believe that some of the most important functional deficits (dislocations and instability) are evident within the present study followup and adversely affect quality of life during the remaining survival [23]. A mean followup of 26 months allows adequate time to assess soft tissue attachment, restoration of function, and occurrence of dislocations [22, 37]. In other series [7] three of 36 patients (8%) presented with dislocations after proximal humeral replacements at a mean followup of 26 months (minimum followup of 6 months). Third, we lacked a control group. The authors continued using the aortograft mesh in all patients after the first procedure was performed in February of 2006. An attempt to compare the present series to previous procedures performed by the same surgeon without the use of the aortograft mesh was abandoned due to difficulty with matching of diagnoses, preoperative limb function, and number of cases available.
Kumar et al. [26] reported on 10 patients who underwent limb salvage procedures of the upper extremity. Patients were followed for a mean of 18 months and they observed acceptable function (defined as patients free of disease and pain and able to return to occupational activities but not sports) in 30% of their patients [26]. None of the patients described in that series was able to perform over the head activities. O’Connor et al. [37] published the functional results of 57 patients who had a limb salvage procedure for a tumor of the shoulder girdle region. The average followup was 5.3 years (median, 4.6 years). They concluded that proximal humeral replacements produced symptomatic instability that leads to a secondary arthrodesis in some patients [37]. Camnasio et al. [7] reported on 37 proximal humerus reconstructions. At a mean followup of 26 months, 30.6% of patients had satisfactory functional results (compared to 74% of patients with proximal femur replacements) [7]. Finally, Mourikis et al. [35] concluded all reconstruction approaches to the proximal humerus, resulted in functional problems for the patient (Table 2).
Table 2.
Comparison of results
| Author | Year | Patients | Followup | Dislocations | Comments |
|---|---|---|---|---|---|
| Bickels et al. [4] | 2002 | 92 | 75 | NA | Poor functional results in 7.5% of patients |
| Gosheger et al. [17] | 2001 | 16 | 31 | 0 | Use of trevira tube for soft tissue reconstruction |
| Camnasio et al. [7] | 2008 | 36 | 26 | 3 | Good results (Enneking score) in 30% of patients |
| O’Connor et al. [37] | 1996 | 11 | 63 | 6 | Two patients had arthrodesis due to symptomatic instability at 22 and 48 months respectively |
| Mourikis et al. [35] | 2007 | 26 | 108 | NA | No patient had postoperative normal range of motion and all were limited in flexion, abduction, and external rotation |
| Wittig et al. [50] | 2002 | 23 | 120 | 0 | MSTS scores 80% to 90% |
| Kiss et al. [24] | 2007 | 14 | 51 | 11* | One patient required revision surgery |
| Kitagawa et al. [25] | 2007 | 10 | 21 | 2 | Mention in text of use of a “mesh” attached to the glenoid for soft tissue reconstruction (no further description) |
| Marulanda et al. [current study] | 2010 | 16 | 26 | 0 |
NA = not available; *1 Dislocation and 10 symptomatic subluxations.
Several studies report the use of modified surgical techniques and new biomaterials with improved function after proximal humerus reconstruction. Wittig et al. [50] analyzed the long-term survival and function and complications associated with limb-sparing surgery for 23 patients with osteosarcoma of the proximal humerus. In all patients, the endoprosthetic replacement was stabilized by static suspension (Dacron® tapes) and dynamic suspension (muscle transfers). At latest followup (median, 10 years), 15 patients (65%) were alive without evidence of disease. The Musculoskeletal Tumor Society upper extremity functional score ranged from 24 to 27 (80%–90%). All shoulders were stable and pain-free. The most common complication was a transient neuropraxia (n = 8) [50]. Gosheger et al. [17] studied 69 megaprostheses implanted with the use of a polyethylene terephthalate (Trevira®) tube to support reconstruction of the capsule and soft tissues. In cases of proximal humerus replacement (16 patients), the tube allowed for reconstruction of the capsule and refixation of the muscles. No dislocation was observed in patients with a proximal humerus endoprosthesis. They analyzed the implanted trevira tube in six patients who had revision surgery for aseptic loosening of a distal femur (one patient), revised periprosthetic fracture (one patient), amputations for local recurrence (three patients), and low-grade late infection (one patient). The histopathologic findings in those patients showed tissue ingrowth into the tube. They concluded, in soft tissue reconstruction of megaprostheses, the reattachment of soft tissues and joint capsules is essential and can be adequate with the use of biomaterials such as polyethylene terephthalate [17].
Our data suggest the use of an aortograft mesh during proximal humerus reconstruction may reduce dislocations and facilitate soft tissue attachment and reconstruction after tumor resection. Our findings should prompt larger investigations to confirm the safety and clinical benefits of this straightforward surgical technique. In addition to tumor control, surgical procedures should provide an adequate quality of life with reduced complications and repeat surgery. The sleeve created by the aortograft allows for both mechanical restraint and ingrowth potential for soft tissue attachments.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Moffitt Cancer Center.
References
- 1.Amadi HO, Hansen UN, Bull AM. A numerical tool for the reconstruction of the physiological kinematics of the glenohumeral joint. Proc Inst Mech Eng H. 2009;223:833–837. doi: 10.1243/09544119JEIM551. [DOI] [PubMed] [Google Scholar]
- 2.Amin SN, Ebeid WA. Shoulder reconstruction after tumor resection by pedicled scapular crest graft. Clin Orthop Relat Res. 2002;397:133–142. doi: 10.1097/00003086-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Ferrari S, Lari S, Mercuri M, Donati D, Longhi A, Forni C, Bertoni F, Versari M, Pignotti E. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84:88–92. doi: 10.1302/0301-620X.84B1.12211. [DOI] [PubMed] [Google Scholar]
- 4.Bickels J, Wittig JC, Kollender Y, Kellar-Graney K, Meller I, Malawer MM. Limb-sparing resections of the shoulder girdle. J Am Coll Surg. 2002;194:422–435. doi: 10.1016/S1072-7515(02)01124-9. [DOI] [PubMed] [Google Scholar]
- 5.Bos G, Sim F, Pritchard D, Shives T, Rock M, Askew L, Chao E. Prosthetic replacement of the proximal humerus. Clin Orthop Relat Res. 1987;224:178–191. [PubMed] [Google Scholar]
- 6.Bruck JC, Weber U. Tumor-related arthrodesis. Reconstruction of the shoulder contour using a free TRAM (Transverse Rectus Abdominis Musculocutaneous) flap [in German] Orthopade. 1998;27:441–444. doi: 10.1007/s001320050253. [DOI] [PubMed] [Google Scholar]
- 7.Camnasio F, Scotti C, Peretti GM, Fontana F, Fraschini G. Prosthetic joint replacement for long bone metastases: analysis of 154 cases. Arch Orthop Trauma Surg. 2008;128:787–793. doi: 10.1007/s00402-007-0464-y. [DOI] [PubMed] [Google Scholar]
- 8.Cheng EY, Gebhardt MC. Allograft reconstructions of the shoulder after bone tumor resections. Orthop Clin North Am. 1991;22:37–48. [PubMed] [Google Scholar]
- 9.Dal Monte A, Manes E. Osteosarcoma of the proximal femur and humerus in children treated by resection, endoprosthesis and complementary chemotherapy. Ital J Orthop Traumatol. 1983;9:151–156. [PubMed] [Google Scholar]
- 10.Deutsch M, Meinhart J, Zilla P, Howanietz N, Gorlitzer M, Froeschl A, Stuempflen A, Bezuidenhout D, Grabenwoeger M. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg. 2009;49:352–362. doi: 10.1016/j.jvs.2008.08.101. [DOI] [PubMed] [Google Scholar]
- 11.Wilde LF, Plasschaert FS, Audenaert EA, Verdonk RC. Functional recovery after a reverse prosthesis for reconstruction of the proximal humerus in tumor surgery. Clin Orthop Relat Res. 2005;430:156–162. doi: 10.1097/01.blo.0000146741.83183.18. [DOI] [PubMed] [Google Scholar]
- 12.Doble M, Makadia N, Pavithran S, Kumar RS. Analysis of explanted ePTFE cardiovascular grafts (modified BT shunt) Biomed Mater. 2008;3:034118. doi: 10.1088/1748-6041/3/3/034118. [DOI] [PubMed] [Google Scholar]
- 13.Eggers IM, Mennen U. The EFFUL (Evaluation of Function in the Flail Upper Limb) system. A ranking score system to measure improvement achieved by surgical reconstruction and rehabilitation. J Hand Surg Br. 1997;22:388–394. doi: 10.1016/S0266-7681(97)80410-X. [DOI] [PubMed] [Google Scholar]
- 14.Fernie GR, Kostuik JP, Lobb RJ. A percutaneous implant using a porous metal surface coating for adhesion to bone and a velour covering for soft tissue attachment: results of trials in pigs. J Biomed Mater Res. 1977;11:883–891. doi: 10.1002/jbm.820110608. [DOI] [PubMed] [Google Scholar]
- 15.Francis KC, Marchetti PG, Caruso M. Inter-scapulo-thoracic resection (Tikhoff-Linberg Operation) [in Italian] Arch Putti Chir Organi Mov. 1964;19:331–340. [PubMed] [Google Scholar]
- 16.Freels DB, Kilpatrick S, Gordon ES, Ward WG. Animal model for evaluation of soft tissue ingrowth into various types of porous coating. Clin Orthop Relat Res. 2002;397:315–322. doi: 10.1097/00003086-200204000-00036. [DOI] [PubMed] [Google Scholar]
- 17.Gosheger G, Hillmann A, Lindner N, Rodl R, Hoffmann C, Burger H, Winkelmann W. Soft tissue reconstruction of megaprostheses using a trevira tube. Clin Orthop Relat Res. 2001;393:264–271. doi: 10.1097/00003086-200112000-00030. [DOI] [PubMed] [Google Scholar]
- 18.Greisler HP, Tattersall CW, Henderson SC, Cabusao EA, Garfield JD, Kim DU. Polypropylene small-diameter vascular grafts. J Biomed Mater Res. 1992;26:1383–1394. doi: 10.1002/jbm.820261009. [DOI] [PubMed] [Google Scholar]
- 19.Heitmann C, Erdmann D, Levin LS. Treatment of segmental defects of the humerus with an osteoseptocutaneous fibular transplant. J Bone Joint Surg Am. 2002;84:2216–2223. doi: 10.2106/00004623-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Higuera CA, Inoue N, Lim JS, Zhang R, Dimaano N, Frassica FJ, Chao EY. Tendon reattachment to a metallic implant using an allogenic bone plate augmented with rhOP-1 vs. autogenous cancellous bone and marrow in a canine model. J Orthop Res. 2005;23:1091–1099. doi: 10.1016/j.orthres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Huffman GR, Tibone JE, McGarry MH, Phipps BM, Lee YS, Lee TQ. Path of glenohumeral articulation throughout the rotational range of motion in a thrower’s shoulder model. Am J Sports Med. 2006;34:1662–1669. doi: 10.1177/0363546506287740. [DOI] [PubMed] [Google Scholar]
- 22.Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. [Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up] Rev Chir Orthop Reparatrice Appar Mot. 2005;91:15–23. doi: 10.1016/s0035-1040(05)84271-0. [DOI] [PubMed] [Google Scholar]
- 23.Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI) Am J Sports Med. 1998;26:764–772. doi: 10.1177/03635465980260060501. [DOI] [PubMed] [Google Scholar]
- 24.Kiss J, Sztrinkai G, Antal I, Szendroi M. Functional results and quality of life after shoulder girdle resections in musculoskeletal tumors. J Shoulder Elbow Surg. 2007;16:273–279. doi: 10.1016/j.jse.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Kitagawa Y, Thai DM, Choong PF. Reconstructions of the shoulder following tumour resection. J Orthop Surg (Hong Kong) 2007;15:201–206. doi: 10.1177/230949900701500216. [DOI] [PubMed] [Google Scholar]
- 26.Kumar VP, Satku SK, Mitra AK, Pho RW. Function following limb salvage for primary tumors of the shoulder girdle. 10 patients followed 4 (1–11) years. Acta Orthop Scand. 1994;65:55–61. doi: 10.3109/17453679408993719. [DOI] [PubMed] [Google Scholar]
- 27.LaBerge M, Bobyn JD, Rivard CH, Drouin G, Duval P. Study of soft tissue ingrowth into canine porous coated femoral implants designed for osteosarcomas management. J Biomed Mater Res. 1990;24:959–971. doi: 10.1002/jbm.820240712. [DOI] [PubMed] [Google Scholar]
- 28.Lukin AV, Kiss EE, Grishkin VA, Chernova VI. Individual endoprosthesis in a malignant tumor of the humerus [in Russian] Vestn Khir Im I I Grek. 1987;139:65–66. [PubMed] [Google Scholar]
- 29.Malawer M. Surgical technique and results of limb sparing surgery for high grade bone sarcomas of the knee and shoulder. Orthopedics. 1985;8:597–607. doi: 10.3928/0147-7447-19850501-14. [DOI] [PubMed] [Google Scholar]
- 30.Malawer MM. Tumors of the shoulder girdle. Technique of resection and description of a surgical classification. Orthop Clin North Am. 1991;22:7–35. [PubMed] [Google Scholar]
- 31.Malawer MM, Sugarbaker PH, Lampert M, Baker AR, Gerber NL. The Tikhoff-Linberg procedure: report of ten patients and presentation of a modified technique for tumors of the proximal humerus. Surgery. 1985;97:518–528. [PubMed] [Google Scholar]
- 32.Marcove RC, Lewis MM, Huvos AG. En bloc upper humeral interscapulo-thoracic resection. The Tikhoff-Linberg procedure. Clin Orthop Relat Res. 1977;124:219–228. [PubMed] [Google Scholar]
- 33.Mazurkiewicz T, Warda E, Kopacz J, Mazurkiewicz M. Results of the megaprosthesis replacement reconstruction proximal femoral resection bone tumors. Ortop Traumatol Rehabil. 2005;7:595–599. [PubMed] [Google Scholar]
- 34.Moran M, Stalley PD. Reconstruction of the proximal humerus with a composite of extracorporeally irradiated bone and endoprosthesis following excision of high grade primary bone sarcomas. Arch Orthop Trauma Surg. 2009;129:1339–1345. doi: 10.1007/s00402-008-0752-1. [DOI] [PubMed] [Google Scholar]
- 35.Mourikis A, Mankin HJ, Hornicek FJ, Raskin KA. Treatment of proximal humeral chondrosarcoma with resection and allograft. J Shoulder Elbow Surg. 2007;16:519–524. doi: 10.1016/j.jse.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Neer CS., 2nd Prosthetic Replacement of the Humeral Head: Indications and Operative Technique. Surg Clin North Am. 1963;43:1581–1597. doi: 10.1016/s0039-6109(16)37147-x. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor MI, Sim FH, Chao EY. Limb salvage for neoplasms of the shoulder girdle. Intermediate reconstructive and functional results. Bone Joint Surg Am. 1996;78:1872–1888. doi: 10.2106/00004623-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Ochiai Y, Imoto Y, Sakamoto M, Kajiwara T, Sese A, Watanabe M, Ohno T, Joo K. Mid-term follow-up of the status of Gore-Tex graft after extracardiac conduit Fontan procedure. Eur J Cardiothorac Surg. 2009;36:63–67. doi: 10.1016/j.ejcts.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Petrovic P, Lotina S, Djordjevic M, Avramov S, Pfau J, Velimirovic D, Fabri M, Stojanov P, Savic D. Results of 132 PTFE (Gore-Tex) bifurcated graft implantations. J Cardiovasc Surg (Torino) 1989;30:897–901. [PubMed] [Google Scholar]
- 40.Picci P, Mercuri M, Ferrari S, Alberghini M, Briccoli A, Ferrari C, Pignotti E, Bacci G. Survival in high-grade osteosarcoma: improvement over 21 years at a single institution. Ann Oncol. 2009 Nov 4. [Epub ahead of print]. [DOI] [PubMed]
- 41.Probyn LJ, Wunder JS, Bell RS, Griffin AM, Davis AM. A comparison of outcome of osteoarticular allograft reconstruction and shoulder arthrodesis following resection of primary tumours of the proximal humerus. Sarcoma. 1998;2:163–170. doi: 10.1080/13577149877920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quill G, Gitelis S, Morton T, Piasecki P. Complications associated with limb salvage for extremity sarcomas and their management. Clin Orthop Relat Res. 1990;260:242–250. [PubMed] [Google Scholar]
- 43.Renard AJ, Veth RP, Schreuder HW, Loon CJ, Koops HS, Horn JR. Function and complications after ablative and limb-salvage therapy in lower extremity sarcoma of bone. J Surg Oncol. 2000;73:198–205. doi: 10.1002/(SICI)1096-9098(200004)73:4<198::AID-JSO3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 44.Ross AC, Wilson JN, Scales JT. Endoprosthetic replacement of the proximal humerus. J Bone Joint Surg Br. 1987;69:656–661. doi: 10.1302/0301-620X.69B4.3611177. [DOI] [PubMed] [Google Scholar]
- 45.Runne WC, Sambeek KJ, Stierum JL, Tongerloo RB. Femoral endoprosthesis fixation with a soft, flexible low modulus stem coating. Four to six year clinical results. Orthopedics. 1989;12:529–535. doi: 10.3928/0147-7447-19890401-07. [DOI] [PubMed] [Google Scholar]
- 46.Sim IW, Tse LF, Ek ET, Powell GJ, Choong PF. Salvaging the limb salvage: management of complications following endoprosthetic reconstruction for tumours around the knee. Eur J Surg Oncol. 2007;33:796–802. doi: 10.1016/j.ejso.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Thai DM, Kitagawa Y, Choong PF. Outcome of surgical management of bony metastases to the humerus and shoulder girdle: a retrospective analysis of 93 patients. Int Semin Surg Oncol. 2006;3:5. doi: 10.1186/1477-7800-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toyoda Y, Shinohara N, Harabayashi T, Abe T, Akino T, Sazawa A, Nonomura K. Survival and prognostic classification of patients with metastatic renal cell carcinoma of bone. Eur Urol. 2007;52:163–168. doi: 10.1016/j.eururo.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 49.Verhagen HJ, Blankensteijn JD, Groot PG, Heijnen-Snyder GJ, Pronk A, Vroom TM, Muller HJ, Nicolay K, Vroonhoven TJ, Sixma JJ, Eikelboom BC. In vivo experiments with mesothelial cell seeded ePTFE vascular grafts. Eur J Vasc Endovasc Surg. 1998;15:489–496. doi: 10.1016/S1078-5884(98)80108-1. [DOI] [PubMed] [Google Scholar]
- 50.Wittig JC, Bickels J, Kellar-Graney KL, Kim FH, Malawer MM. Osteosarcoma of the proximal humerus: long-term results with limb-sparing surgery. Clin Orthop Relat Res. 2002;397:156–176. doi: 10.1097/00003086-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 51.Wodajo FM, Bickels J, Wittig J, Malawer M. Complex reconstruction in the management of extremity sarcomas. Curr Opin Oncol. 2003;15:304–312. doi: 10.1097/00001622-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Yang Q, Li J, Yang Z, Li X, Li Z. Limb sparing surgery for bone tumours of the shoulder girdle: the oncological and functional results. Int Orthop. 2009 Aug 23. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 53.Yang X, Tare RS, Partridge KA, Roach HI, Clarke NM, Howdle SM, Shakesheff KM, Oreffo RO. Induction of human osteoprogenitor chemotaxis, proliferation, differentiation, and bone formation by osteoblast stimulating factor-1/pleiotrophin: osteoconductive biomimetic scaffolds for tissue engineering. J Bone Miner Res. 2003;18:47–57. doi: 10.1359/jbmr.2003.18.1.47. [DOI] [PubMed] [Google Scholar]