Abstract
Background
Unicameral bone cysts are benign lesions that usually spontaneously regress with skeletal maturity; however, the high risk of pathologic fractures often justifies treatment that could reinforce a weakened bone cortex. Various treatments have been proposed but there is no consensus regarding the best procedure.
Questions/purposes
We compared the healing rates and failures of two methods of cure based on multiple injections of corticosteroid or a single injection of demineralized bone matrix (DBM) in association with bone marrow concentrate (BMC).
Methods
We retrospectively reviewed 184 patients who had one of the two treatments for unicameral bone cysts with cortical erosion. Clinical records were reviewed for treatment failures and radiographs for healing in all patients. The minimum followup was 12 months for the Steroids Group (mean, 48 months; range, 12–120 months) and 12 months for the DBM + BMC Group (mean, 20 months; range, 12–28 months).
Results
After one treatment we observed a lower healing rate of cysts treated with multiple injections of steroids compared with the healing after the first injection of DBM + BMC (21% versus 58%, respectively). At last followup, 38% healed with steroids and 71% with DBM + BMC. The rate of failure after one steroid injection was higher than after a single injection of BDM + BMC (63% versus 24%, respectively). We observed no difference in fracture rates after treatment between the two groups.
Conclusions
A single injection of DBM added with autologous bone marrow concentrate appears to provide a higher healing rate with a lower number of failures compared with a single injection of steroids.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Unicameral bone cysts (UBCs) are benign self-limited lesions in long bones of skeletally immature individuals often located in the proximal humerus and proximal femur [39]. UBCs are frequently asymptomatic [7, 8, 12, 21] and may regress spontaneously [7, 8, 12, 21]. However, surgery is often advised because the bone cortex is usually eroded by the lesion and weakened, rendering the bone at risk for pathologic fracture [1, 22]. The main goals of surgery are therefore to decrease the risk of fracture and enhance cyst healing [40].
Curettage with removal of the cyst membrane and bone grafting has been considered for many years the gold standard procedure [8, 27] since it provides enough osteogenic stimulus to enhance healing. Although the bone grafts potentially promote new bone formation inside the cyst, the rate of recurrence is 5% to 50% by 1 to 8 years. Further, donor site morbidity has reduced the appeal of this procedure for some surgeons [11, 26, 35]. Thus, there was a need for less invasive treatments considering the benign and self-limited nature of the lesion.
A number of alternative, less invasive methods have been proposed in recent years [25]. Repeated percutaneous injection of corticosteroids was proposed by Scaglietti in 1979 [32]. Owing to the low morbidity, the simplicity, and the high healing rate reported by Scaglietti (90%), this treatment has been widely used [3, 4, 41]. However, subsequent studies reported a lower healing rate for this procedure with only 41% to 63% healing after the first injection [6, 13]. Sung and colleagues reported a failure rate of 84% after the initial treatment with steroids and 76% after the second procedure [35]. The injection of bone marrow (BM) alone [5, 6, 9, 21, 42, 43] or in combination with demineralized bone matrix (DBM) [17, 18, 29, 30, 35] has also been proposed as an alternative to steroids for treating UBC. BM should provide osteoprogenitor cells and DBM could stimulate new bone formation thanks to its osteoinductive and osteoconductive properties [20, 30].
We therefore (1) asked whether a single injection of autologous bone marrow concentrate (BMC) combined with DBM for treating UBC would produce comparable healing rates compared with multiple injections of corticosteroids at the first year of followup; (2) determined the healing rate at last followup independent from the number of treatments needed to obtain healing; and (3) evaluated the relationship of failure in relation to the site and the size of the cyst, the location of the cyst in relation to the epiphysis, and the age of the patients.
Patients and Methods
We retrospectively reviewed the records of all 230 patients present in our bone tumor register for UBCs in long bones from 1998 to 2009. Of these patients, 35 were not surgically treated, five were treated with open surgery, and six were lost to followup shortly after treatment. Of the remaining 184 patients, 143 were treated with repeated injections of methylprednisolone (Steroids Group) and 41 with a single injection of DBM associated with autologous BMC (DBM + BMC Group). There were 122 males (66%) and 62 females (34%). The mean age was 10 years (range, 2–21 years). The cyst was located in the humerus in 137 cases (74%), in the femur in 42 cases (23%), and in other sites such as the radius and tibia in five cases (3%). Clinical presentation was a pathologic fracture in 77% of the cases (143 of 184), pain in 5% (nine of 184), and incidental radiographic finding in the others (12% [32 of 184]). The lesion was considered active in 78% of the cases (144 of 184). There were no statistical differences between the two groups (steroids versus DBM + BMC) with respect to age, gender, site of the lesion, and clinical presentation (Table 2). The minimum followup was 12 months for the Steroids Group (mean, 48 months; range 12–120 months) and 12 months for the DBM + BMC Group (mean, 20 months; range, 12–28 months).
Table 2.
Descriptive baseline data*
| Data | Series (184) | Steroid Group (143) | DBM + CM Group (41) | p value |
|---|---|---|---|---|
| Mean age | 9.89 (± 3.9) | 9.9 (± 4) | 9.88 (± 3.3) | NS |
| Males (%) | 122 (66) | 95 | 27 | NS |
| Females (%) | 62 (34) | 48 | 14 | NS |
| Site of the lesion | ||||
| Humerus | 137 (74.5) | 108 (75.5) | 29 (70.7) | NS |
| Femur | 42 (22.8) | 31 (21.6) | 11 (26.8) | NS |
| Others | 5 (2.7) | 4 (2.7) | 1 (2.4) | NS |
| Clinical presentation (%) | ||||
| Pathologic fracture | 143 (77) | 112 (78.3) | 31 (75.6) | NS |
| Pain | 9 (4.8) | 7 (4.9) | 2 (4.8) | NS |
| Incidental radiograph | 32 (12) | 27 (18.8) | 5 (12.1) | NS |
| Active cysts | 144 (78.2) | 111 (77.6) | 33 (80.4) | NS |
* The two groups resulted similar for age, gender, site, and clinical presentation; DBM = demineralized bone matrix; BMC = bone marrow concentrate; NS = not significant.
In both groups, the injection was performed under general anesthesia with a fluoroscopy guide. In the Steroid Group, two needles were inserted proximally and distally inside the cyst; then after “washing” of the lesion with saline solution, we injected 80 mg methylprednisolone regardless of the cyst size. In this group, we considered as “treatment” a cycle of three repeated injections performed in less than a 6 months interval. In the DBM + BCM group, 18 to 20 mL BM was aspirated from the anterior iliac wing and centrifuged twice to obtain 9 to 10 mL BMC. BMC was prepared using the FIBRINET® Kit (Cascade Medical Enterprises, LLC, Wayne, PA) according to the manufacturer’s instructions. This procedure takes approximately 22 minutes. At the end of the procedure, the product included mononuclear progenitor cells, fibrin, and platelet-rich plasma [37]. When mixed with the demineralized bone powder (Musculoskeletal Tissue Bank of the Rizzoli Orthopaedic Institute), the marrow concentrate remains in a semisolid phase, thus maintaining this form also in the cystic cavity after injection. While the paste is being made, a single needle was percutaneously inserted inside the cyst allowing the outflow of the cystic liquid by aspiration. Thereafter, the needle was used to scrape out the inner wall of the cyst as well as the internal trabecular structure. Then the mixture of BMC and DBM was injected under pressure in the cystic cavity. The quantity of DBM varied from 5 to 15 mL in relation to the cyst size. In both cases, the procedure lasts from 30 to 45 minutes.
All patients were discharged the day of surgery. We recommended restricted activities in all patients for 15 days, after which they were allowed free movement and their usual physical activities.
Patients in both groups attended the outpatient clinic to assess cyst healing and clinical results at 2, 6, and 12 months after surgery and then annually until the end of skeletal growth. We obtained radiographs of the cyst at each control, and MRI assessment was performed at 12 and 24 months.
Three of us (CDB, TF, LC) independently evaluated the radiographic films using the modified Neer score system developed by other authors [6, 34]; the senior author (DD) confirmed all determinations. The Neer classification [6] has four categories: “Neer I,” complete cyst resolution; “Neer II,” partial cyst resolution; “Neer III,” persistence of the cyst with no immediate need for treatment; and “Neer IV,” nonresolution of the cyst with the need for treatment to prevent fracture (Table 1).
Table 1.
Modified Neer classification of radiologic results
| Score | Classification | Description | Treatment |
|---|---|---|---|
| I | Healed | Cyst filled with new bone, with or without small radiolucent area(s) < 1 cm in size | Not necessary |
| II | Healed with defects | Radiolucent area(s) < 50% of the diameter of the bone with enough cortical thickness to prevent fracture | Not necessary |
| III | Persistent cyst | Radiolucent area > 50% of the diameter of the bone and with a thin cortical rim; no increase of the size of the cyst | Continued restriction of activity, possible repeated treatment required |
| IV | Recurrent or nonresponsive cyst | Cyst reappeared in a previously obliterated area or a radiolucent area has increased in size | Need for repeated treatment |
The treatment was considered a failure when either (1) a fracture occurred; (2) there was no evidence of healing on radiographs after 6 months; or (3) a recurrent cyst required subsequent treatment (Neer IV).
We determined differences between the two groups of patients. Age was evaluated with one-way analysis of variance expressed in terms of mean ± SD of the mean, site of the lesion with Pearson’s chi square test, and gender and clinical presentation with Fisher’s exact test (Table 2). The same test was used to evaluate the results after the first treatment (Table 3) and at the last followup (Table 4). The differences in the two groups related to the number of treatment and number of injections to healing were evaluated with analysis of variance; when the Levene test for equality of variances was significant (p < 0.05), the Mann-Whitney test was used. We performed the survival to failure (defined above) using the Wilcoxon (Gehan) estimator of survival function [15]. The same analysis was performed to evaluate the influence of group, location, localization in relation to the growth plate, age, and dimensions to the survival. Then multivariate Cox regression with Wald backward method was performed to identify the more predictive model for failure. The odds ratio (OR) for each parameter with 95% confidence intervals (CIs) was used to express the Cox regression results. We used the Statistical Package for the Social Sciences (SPSS) software, Version 15.0 (SPSS Inc, Chicago, IL) for all analyses.
Table 3.
Comparison of the results after the first treatment (first year followup) in the two groups
| Results | Steroid group (143) | DBM + CM group (41) | p value |
|---|---|---|---|
| Cyst healing rate (Neer I, II) | 30 (21) | 24 (58.5) | < 0.0005 |
| Failures | 91 (63.6) | 10 (24.3) | < 0.0005 |
| Fracture | 24 (16.7) | 5 (12.2) | NS |
| No response to treatment (Neer IV) | 67 (46.8) | 5 (12.2) | < 0.0005 |
DBM = demineralized bone matrix; BMC = bone marrow concentrate; NS = not significant.
Table 4.
Comparison of the results at followup in the two groups
| Type of result | Steroids group (143) | DBM + BMC group (41) | p value |
|---|---|---|---|
| Healing rate (%) | 54 (38) | 29 (71) | < 0.001 |
| Number of treatments* needed to obtain healing (mean ± SD) | 1.66 (± 0.6) | 1.1 (± 0.3) | < 0.0005 |
| Number of injections needed to obtain healing (mean ± SD) | 4.3 (± 3.9) | 1.1 (± 0.3) | < 0.0005 |
| Final Neer score (%) | |||
| I | 11 (7.7) | 8 (19.5) | < 0.002 |
| II | 43 (30) | 21 (51.2) | < 0.002 |
| III | 56 (39.2) | 7 (17) | < 0.002 |
| IV | 33 (23) | 5 (12.2) | < 0.002 |
* In the DBM + BMC group, one treatment is equal to one injection; in the steroid group, one treatment can be more than one injection; DBM = demineralized bone matrix; BMC = bone marrow concentrate.
Results
After the first treatment, the healing rate was higher (p < 0.001) in the DBM + BMC Group than in the Steroid Group (24 of 41 [59%] versus 30 of 143 [21%], respectively) (Fig. 1). The number of failures was higher (p < 0.001) in the Steroid Group (91 of 143 [63%]) than in the DBM + BMC Group (10 of 41 [24%]), although the number of fractures occurring in the first year was similar in the two groups (Table 3).
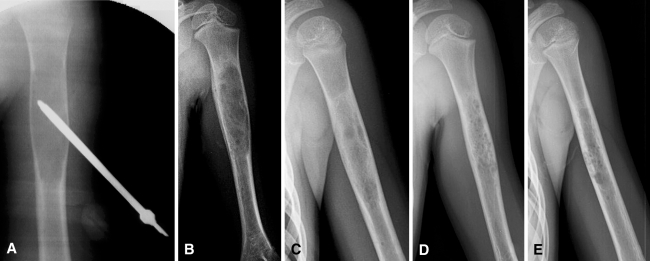
Fig. 1A–E.
Radiographs of a 6-year-old female patient treated after two fractures occurred earlier in the left proximal humerus. (A) Intraoperative check with the needle inserted. (B) Result at 2 months; intense periosteal reaction can be seen in the medial cortex of the cyst. (C) 6, (D) 12, and (E) 18 months followup. From 2 months to date, the patient was permitted to resume normal physical activity, including sports.
The healing rate independent from the number of treatments was higher (p < 0.001) in the DBM + BMC Group (29 of 41 [71%]) than in the Steroid Group (54 of 143 [38%]). Fifty-six of 143 patients (39.2%) in the Steroid Group and seven of 41 patients (17.1%) in the DBM + BMC Group obtained incomplete healing of the cyst (Neer III) with no immediate indication for subsequent surgery. These patients continued to be followed up but the cyst was not considered healed yet. The number of patients who needed more than one injection to heal as well as the number of treatments needed to obtain healing resulted statistically higher in the Steroid Group than in the DBM + BMC Group (Table 4).
The DBM + BMC Group had fewer (p = 0.001) failures than the Steroid Group during the first two year followup (Fig. 2). Cyst size larger than 21 cm2 had a higher (p = 0.04) failure risk although we observed no difference in failure rates among the different cyst locations (humerus, femur, others). Cysts located less than 1.5 cm from the growth plate had a higher (p = 0.005) failure rate than those located more than 1.5 cm (Fig. 3). Finally, patients younger than 8 years had a higher (p = 0.001) rate of failure (Fig. 4). Age influenced the failure rate (Wald statistic of 0.004; OR,.0.923; 95% CI, 0.87–0.97).
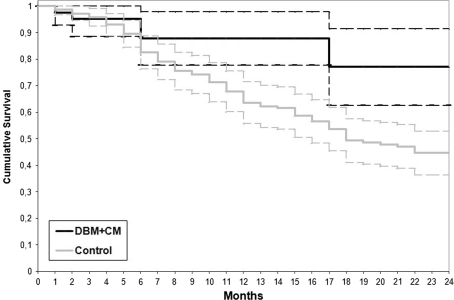
Fig. 2.
Lifetime table analysis of the treatment survival in the two groups. The end point is the failure of the procedure. The treatment was considered failed when a fracture occurred or when there was no response to treatment after 6 months or in the presence of recurrence of the cyst with the need for subsequent treatment (Neer IV). The DBM + BMC Group showed fewer failures compared to the Steroid Group (Wilcoxon-Gehan test; p = 0.001).
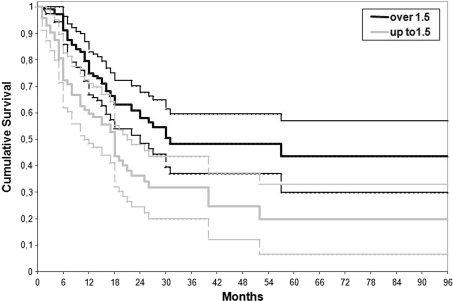
Fig. 3.
Lifetime table analysis of the treatment survival looking to the localization of the cyst in relation to the growth plate in the whole series. The end point is the failure of the procedure. Patients with cysts located less than 1.5 cm from the growth plate had a higher failure rate than those located more than 1.5 cm (Wilcoxon-Gehan test; p = 0.005). DBM + BMC = demineralized bone matrix + bone marrow concentrate.
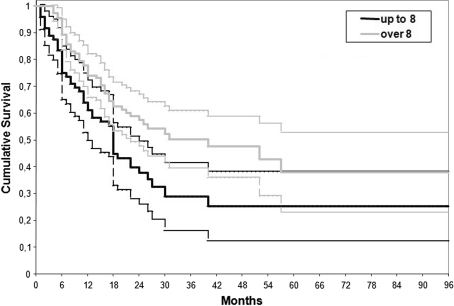
Fig. 4.
Lifetime table analysis of the treatment survival looking to the patient age in the whole series. The end point is the failure of the procedure. Patients younger than 8 years had a higher rate of failure than those older than 8 years (Wilcoxon-Gehan test; p = 0.001).
Discussion
UBCs are benign lesions that usually spontaneously regress with skeletal maturity [16]; however, when pathologic fractures occur with persistent radiographic signs of cyst, these young patients are strictly forbidden to take part in recreational physical activities. Treatment can reinforce the bone cortex. Numerous types of treatments have been proposed such as steroids, natural bone marrow, bone matrix or other injection materials [19, 24, 32, 36, 42, 43], curettage with bone grafting [34], subperiosteal resection [10, 23], and internal or external fixation [17, 28, 31]; however, until now, there was no consensus regarding the best procedure. Moreover, because the various literature reports all methods of treatment with a wide range of healing rates, it is difficult to derive clearcut criteria (Table 5). We therefore determined (1) whether during the first year of followup a single injection of autologous BMC combined with DBM for treating UBC would produce comparable healing rates than multiple steroid injections; (2) the healing rate at last followup; and (3) failure rate correlated with the site and size of the cyst with the location of the cyst in relation to the epiphysis and the age of the patients.
Table 5.
Healing rate (after first treatment and final) in major series reported in the literature according to different methods of treatment
| Author/year of publication | Number of patients | Type of treatment | Healing rate after first treatment (%) | Final healing rate (%) | Followup (years) | Observations |
|---|---|---|---|---|---|---|
| Neer et al./1966 [27] | 45 | Observation | – | 8 | 1–10 | 1–3 refractures occurred in 80% of cases |
| 129 | Curettage/grafting | NR | 52 | 2–10 | Any site and any age included | |
| Spence et al./1976 [34] | 144 | Curettage/grafting | 75 | NR | 1–4 | Underline the use of freeze-dried cancellous bone as packing material |
| Scaglietti et al./1979 [32] | 72 | Steroid injection | NR | 90 | 1–3 | More than one steroid injection of 40 to 200 mg according to the cyst volume |
| Scaglietti et al./1982 [33] | 163 | Steroid injection | NR | 24 | NR | Long-term results |
| Capanna et al./1982 [4] | 95 | Steroid injection | NR | 80 | 1–5 | From 2–7 injections every 2 months until evidence of cyst response. Neer modified |
| Campanacci et al./1986 [3] | 178 | Curettage/grafting | NR | 67 | 1–8 | Same as above. Longer followup |
| 141 | Steroid injection | NR | 68 | |||
| Farber and Stanton/1990 [11] | 19 | Curettage/grafting | 53 | 95 | 4.1 (av) | Younger age in curettage and bone grafting than steroid group |
| 17 | Steroid injection | 70 | 76 | 3.3 (av) | ||
| Lokiec et al./1996 [21] | 10 | Native BMI | 100 | 100 | 1–4 | Neer modified. Injection of average 25 ml (from 15 to 50) |
| Delloye et al./1998 [9] | 8 | Native BMI | 87.5 | 100 | 1–4.5 | Neer modified as Capanna et al. Quantity of bone marrow not reported |
| Yandow et al./1998 [42] | 12 | Native BMI | 42 | 67 | 1–6 | Personal classification. 18 ml av of bone marrow injection |
| Chang et al./2002 [5] | 14 | Native BMI | 43 | NR | 1–9 | Personal classification. 12 to 24 ml of bone marrow injection |
| 65 | Steroid injection | 51 | NR | |||
| Rougraff and Kling/2002 [30] | 23 | DBM + native BMI | 78 | 70 | 1–7 | Neer classification. 5 to 32 ml of bone marrow av 18 |
| Kanellopoulos et al./2005 [18] | 19 | DBM + native BMI | NR | 89.5 | 1–3.8 | Underline the role of reaming of the cyst. Neer classification |
| Cho et al./2007 [6] | 28 | Native BMI | 52 | 87 | 2–15 | Final 2.19 injections in steroid group versus 1.57 in the marrow injection group. 12 – 50 ml of bone marrow injection |
| 30 | Steroid injection | 23.3 | 92 | |||
| Sung et al./2008 [35] | 94 | Steroid injection | 16 | NR | 7 (av) | Neer classification. Quantity of bone marrow and steroid not specified |
| 39 | Curettage | 36 | NR | 9 (av) | ||
| 34 | Steroid injection + DBM + native BMI | 50 | NR | 4 (av) | ||
| Wright et al./2008 [41] | 45 | Steroid injection | 42 | 42 | 0–2 | Neer classification modified. 9 to 18 ml of bone marrow injection |
| 39 | Native BMI | 23 | 23 | |||
| Zamzam et al./2009 [43] | 28 | Native BMI | 57 | 82 | 2.5–3.5 | 20 ml av of bone marrow injection. Personal classification |
| Di Bella et al. [current study] | 143 | Steroid injection | 21 | 38 | 4 (av) | Neer classification. 10 cc of bone marrow concentrate |
| 41 | DBM + BMC | 59 | 71 | 1.8 (av) |
DBM = demineralized bone matrix; Native BMI = bone marrow injection without concentration; BMC = bone marrow concentrate injection; NR = not reported; av = average.
We recognize limitations to our study. First, the Steroid Group has a considerably longer followup compared with the DBM + BMC Group. Thus, we anticipate more failures in the DBM + BMC Group during followup. For this reason, a future study will be necessary to confirm these preliminary results. Second, the BMC used in our protocol is different from that in natural bone marrow, in which progenitor cells are diluted in a liquid media with possible dispersion in the soft tissue outside the cyst [14], thus explaining poor results reported in some studies [5, 41]. However, the study was not performed to prove different outcomes between natural BM and BMC. Rather, the rationale in the use of BMC in association with DBM is to provide enough factors such as stem cells and bone morphogenetic proteins, which are able to improve the osteogenic potential in the cystic area, contrasting the catabolic phenomena. Third, there are some differences in the surgical technique between the two methods of cure. In the Steroid Group we used high pressure cyst lavage through a double needle inserted in the cortex, while in the DBM + BMC Group we performed only one needle insertion with internal scraping.
During the first year, the number of cysts healed with a single injection of DBM + BMC (59%) was higher compared with multiple injections of corticosteroids (21%). These data are difficult to compare with data presented in the literature due to differences in the method of evaluation and type and number of bone marrow or steroid injection. However, most reports that specifically refer to the healing rate after the first treatment with bone marrow injection show lower percentages than achieved in our series [5, 6, 35, 41–43]. On the other hand, the percentage of healing rate following the first treatment with steroid injection in our series is lower than most of other reports [5, 6, 11, 34, 41]. Fracture of the cyst is one cause of failure after the first treatment; Neer et al. [27] reported up to 80% of fractures in patients followed without surgical treatment. In patients surgically treated, fracture has been reported in 2.6% of cases treated with curettage and bone grafting [35]; from 7.7% [4] to 28% [41] and 33% [42] in patients treated with marrow or steroid injection. We observed no difference in the number of fractures that occurred in our series in the two groups (12% in the DBM + BMC Group versus 17% in the Steroid Group), resulting in the average of fractures reported in series dealing with patients treated with either steroid or bone marrow injection. A good clinical and radiographic initial outcome is important to decrease the number of hospitalizations and surgeries. In some patients treated with steroids, more than eight injections were given, resulting in high costs for families and the healthcare system. On the other hand, in patients with partial or complete healing, daily activities, including sports, could be resumed.
Despite the longer followup in the Steroid Group, the final cyst healing rate was higher in the DBM + BMC Group than the Steroid Group (71% versus 38%). The life table analysis confirmed this difference between the two treatments. This is particularly important considering the smaller number of treatments (multiple injections in the Steroid Group) and injections needed to achieve this result (4.3 average in the Steroid Group versus 1.1 in the DBM + BMC Group). One series comparing steroid to bone marrow injection reported a higher number of steroid injections required to achieve the final healing rate [6]. However, despite the differences in the evaluation method, most of the failures occurred within the first two years of followup as reported by other series [11, 30], leaving a number of patients in the Neer III grade evaluation to be followed for a long time until evidence of definitive healing was achieved [3, 4, 27].
When considering factors with a negative effect on the treatment outcome, the humeral site did not score more fractures than other sites [5], although size, younger age and proximity to the growth plate confirmed their negative effect on the final healing rate requiring, despite fracture, a higher number of injections. These observations confirmed previous reports with age less than 10 years old be the major risk factor for failure in the UBC series [3–5, 11, 33–35]. As observed by Sung et al. [35] there is not likely an age at which risk increases, although younger patients are more likely to need subsequent treatments to achieve final healing. This is also likely true for the size and proximity of the growth plate as these are generally considered signs of active cyst [4, 16] although these differences would likely be significant only in larger study series. Finally, we believe it important to improve the operative technique by opening of the medullary canal from the diaphyseal side to reduce the pressure inside the lesion. This has been attempted using medullary nails or cannulated screws [2, 28]; however, in those series, the healing rate of the cyst was not sufficiently consistent because of the lack of osteogenic stimuli. When an adequate cyst opening procedure is associated with the bone formation enhancing agents such as BM and DBM, healing can be more consistent [18]. A number of cysts can be considered inactive and reasonably heal with no or minimal surgery. Therefore, we need a more accurate cyst index than previously reported [16] since this did not predict fracture [38].
The healing rate of unicameral bone cyst after the first treatment has been more frequently achieved with injection of DBM + BMC than steroid injection. This is also confirmed by the healing rate achieved in the last followup independent from the number of treatments required. We believe the data support the desirability of a more effective osteogenic material for bone regeneration in younger patients with larger cysts or cysts located close to the growth plate.
Acknowledgments
We thank Elettra Pignotti for contributions regarding statistical analysis and Enrico Lucarelli for manuscript review.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Rizzoli Orthopaedic Institute, Bologna, Italy.
References
- 1.Ahn JI, Park JS. Pathological fractures secondary to unicameral bone cysts. Int Orthop. 1994;18:20–22. doi: 10.1007/BF00180173. [DOI] [PubMed] [Google Scholar]
- 2.Brecelj J, Suhodolcan L. Continuous decompression of unicameral bone cyst with cannulated screws: a comparative study. J Pediatr Orthop B. 2007;16:367–372. doi: 10.1097/BPB.0b013e32826d1ad6. [DOI] [PubMed] [Google Scholar]
- 3.Campanacci M, Capanna R, Picci P. Unicameral and aneurysmal bone cysts. Clin Orthop Relat Res. 1986;204:25–36. [PubMed] [Google Scholar]
- 4.Capanna R, Dal Monte A, Gitelis S, Campanacci M. The natural history of unicameral bone cyst after steroid injection. Clin Orthop Relat Res. 1982;166:204–211. [PubMed] [Google Scholar]
- 5.Chang CH, Stanton RP, Glutting J. Unicameral bone cysts treated by injection of bone marrow or methylprednisolone. J Bone Joint Surg Br. 2002;84:407–412. doi: 10.1302/0301-620x.84b3.12115. [DOI] [PubMed] [Google Scholar]
- 6.Cho HS, Oh JH, Kim HS, Kang HG, Lee SH. Unicameral bone cysts. A comparison of injection of steroid and grafting with autologous bone marrow. J Bone Joint Surg Br. 2007;89:222–226. doi: 10.1302/0301-620X.89B2.18116. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Etiology of simple bone cysts. J Bone Joint Surg Am. 1970;52:1493–1497. [PubMed] [Google Scholar]
- 8.Cohen J. Unicameral bone cysts: current synthesis of reported cases. Orthop Clin North Am. 1977;8:715–736. [PubMed] [Google Scholar]
- 9.Delloye C, Docquier PL, Cornu O, Poilvache P, Peters M, Woitrin B, Rombouts JJ, Nayer P. Simple bone cysts treated with aspiration and single bone marrow injection. Int Orthop. 1998;22:134–138. doi: 10.1007/s002640050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahey JJ, O’Brien ET. Subtotal resection and grafting in selected cases of solitary unicameral bone cyst. J Bone Joint Surg Am. 1973;55:59–68. [PubMed] [Google Scholar]
- 11.Farber JM, Stanton RP. Treatment options in unicameral bone cysts. Orthopedics. 1990;13:25–32. doi: 10.3928/0147-7447-19900101-06. [DOI] [PubMed] [Google Scholar]
- 12.Garceau GJ, Gregory CF. Solitary unicameral bone cysts. J Bone Joint Surg Am. 1954;36:267–280. [PubMed] [Google Scholar]
- 13.Hashemi-Nejad A, Cole WG. Incomplete healing of simple bone cysts after steroid injections. J Bone Joint Surg Br. 1997;79:727–730. doi: 10.1302/0301-620x.79b5.7825. [DOI] [PubMed] [Google Scholar]
- 14.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 15.Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 2. New York, NY: Wiley; 2008. [Google Scholar]
- 16.Kaelin AJ, MacEwen GD. Unicameral bone cysts: natural history and risk of fracture. Int Orthop. 1989;13:275–282. doi: 10.1007/BF00268511. [DOI] [PubMed] [Google Scholar]
- 17.Kanellopoulos AD, Mavrogenis AF, Papagelopoulos PJ, Soucacos PN. Elastic intramedullary nailing and DBM-bone marrow injection for the treatment of simple bone cysts. World J Surg Oncol. 2007;5:111–118. doi: 10.1186/1477-7819-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanellopoulos AD, Yiannakopoulos CK, Soucacos PN. Percutaneous reaming of simple bone cysts in children followed by injection of demineralized bone matrix and autologous bone marrow. J Pediatr Orthop. 2005;25:671–675. doi: 10.1097/01.bpo.0000164874.36770.42. [DOI] [PubMed] [Google Scholar]
- 19.Killian JT, Wilkinson L, White S, Brassard M. Treatment of unicameral bone cyst with demineralized bone matrix. J Pediatr Orthop. 1998;18:621–624. doi: 10.1097/00004694-199809000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Komiya S, Minamitani K, Sasaguri Y, Hashimoto S, Morimatsu M, Inoue A. Simple bone cyst: treatment by trepanation and studies on bone resorptive factors in cyst fluid with a theory of its pathogenesis. Clin Orthop Relat Res. 1993;287:204–211. [PubMed] [Google Scholar]
- 21.Lokiec F, Ezra E, Khermosh O, Weintroub S. Simple bone cysts treated by percutaneous autologous bone marrow grafting. J Bone Joint Surg Br. 1996;78:934–937. doi: 10.1302/0301-620x78b6.6840. [DOI] [PubMed] [Google Scholar]
- 22.Makley JT, Joyce MJ. Unicameral bone cyst (simple bone cyst) Orthop Clin North Am. 1989;20:407–415. [PubMed] [Google Scholar]
- 23.McKay DW, Nason SS. Treatment of unicameral bone cysts by subtotal resection without grafts. J Bone Joint Surg Am. 1977;59:515–519. [PubMed] [Google Scholar]
- 24.Mik G, Arkader A, Manteghi A, Dormans JP. Results of a minimally invasive technique for treatment of unicameral bone cysts. Clin Orthop Relat Res. 2009;467:2949–2954. doi: 10.1007/s11999-009-1008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milbrandt T, Hopkins J. Unicameral bone cysts: etiology and treatment. Curr Opin Orthop. 2007;18:555–560. [Google Scholar]
- 26.Neer CS, Francis KC, Johnston AD, Kiernan HA. Current concepts on the treatment of solitary unicameral bone cyst. Clin Orthop Relat Res. 1973;97:40–51. doi: 10.1097/00003086-197311000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Neer C, Francis KC, Marcove RC, Terz J, Carbonara P. Treatment of unicameral bone cyst. A follow-up study of one hundred seventy-five cases. J Bone Joint Surg Am. 1966;48:731–745. [PubMed] [Google Scholar]
- 28.Roposch A, Saraph V, Linhart WE. Flexible intramedullary nailing for the treatment of unicameral bone cysts in long bones. J Bone Joint Surg Am. 2000;82:1447–1453. doi: 10.2106/00004623-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal RK, Folkman J, Glowacki J. Demineralized bone implants for nonunion fractures, bone cysts, and fibrous lesions. Clin Orthop Relat Res. 1999;364:61–69. doi: 10.1097/00003086-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Rougraff BT, Kling TJ. Treatment of active unicameral bone cysts with percutaneous injection of demineralized bone matrix and autogenous bone marrow. J Bone Joint Surg Am. 2002;84:921–929. doi: 10.2106/00004623-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Santori F, Ghera S, Castelli V. Treatment of solitary bone cysts with intramedullary nailing. Orthopedics. 1988;11:873–878. doi: 10.3928/0147-7447-19880601-06. [DOI] [PubMed] [Google Scholar]
- 32.Scaglietti O, Marchetti PG, Bartolozzi P. The effects of methylprednisolone acetate in the treatment of bone cysts. Results of three years follow-up. J Bone Joint Surg Br. 1979;61:200–204. doi: 10.1302/0301-620X.61B2.438272. [DOI] [PubMed] [Google Scholar]
- 33.Scaglietti O, Marchetti PG, Bartolozzi P. Final results obtained in the treatment of bone cysts with methylprednisolone acetate (Depo-Medro) and a discussion of results achieved in other bone lesions. Clin Orthop Relat Res. 1982;165:33–42. [PubMed] [Google Scholar]
- 34.Spence KF, Jr, Bright RW, Fitzgerald SP, Sell KW. Solitary unicameral bone cyst: treatment with freeze-dried crushed cortical-bone allograft: a review of one hundred and forty-four cases. J Bone Joint Surg Am. 1976;58:636–641. [PubMed] [Google Scholar]
- 35.Sung AD, Anderson ME, Zurakowski D, Hornicek FJ, Gebhardt MC. Unicameral bone cyst. A retrospective study of three surgeries. Clin Orthop Relat Res. 2008;466:2519–2526. doi: 10.1007/s11999-008-0407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thawrani D, Thai CC, Welch RD, Copley L, Johnston CE. Successful treatment of unicameral bone cyst by single percutaneous injection of α-BSM. J Pediatr Orthop. 2009;29:511–517. doi: 10.1097/BPO.0b013e3181aad704. [DOI] [PubMed] [Google Scholar]
- 37.Trombi L, Mattii L, Pacini S, D’Alessandro D, Battolla B, Orciuolo E, Buda G, Fazzi R, Galimberti S, Petrini M. Human autologous plasma-derived clot as a biological scaffold for mesenchymal stem cells in treatment of orthopedic healing. J Orthop Res. 2008;26:176–183. doi: 10.1002/jor.20490. [DOI] [PubMed] [Google Scholar]
- 38.Vasconcellos DA, Yandow SM, Grace AM, Moritz BM, Marley LD, Fillman RR. Cyst Index. A non predictor of simple bone cyst fracture. J Pediatr Orthop. 2007;27:307–310. doi: 10.1097/BPO.0b013e31803409e2. [DOI] [PubMed] [Google Scholar]
- 39.Virchow R. On the formation of bony cysts. In: Uber die Bilding von Knochencysten. Berlin, Germany: SB Akad Wiss; 1876:369–381.
- 40.Wilkins R. Unicameral bone cysts. J Am Acad Orthop Surg. 2000;8:217–224. doi: 10.5435/00124635-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Wright JG, Yandow S, Donaldson S, Marley L. A randomized clinical trial comparing intralesional bone marrow and steroid injection for simple bone cyst. J Bone Joint Surg Am. 2008;90:722–730. doi: 10.2106/JBJS.G.00620. [DOI] [PubMed] [Google Scholar]
- 42.Yandow SM, Lundeen G, Scott SM, Coffin C. Autogenic bone marrow injections as a treatment for simple bone cyst. J Pediatr Orthop. 1998;18:616–620. doi: 10.1097/00004694-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Zamzam MM, Abak AA, Bakarma KA, Aj-Jassir FF, Khoshhal KI, Zamzami MM. Efficacy of aspiration and autogenous bone marrow injection in the treatment of simple bone cysts. Int Orthop. 2009;33:1353–1358. doi: 10.1007/s00264-008-0619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]