Abstract
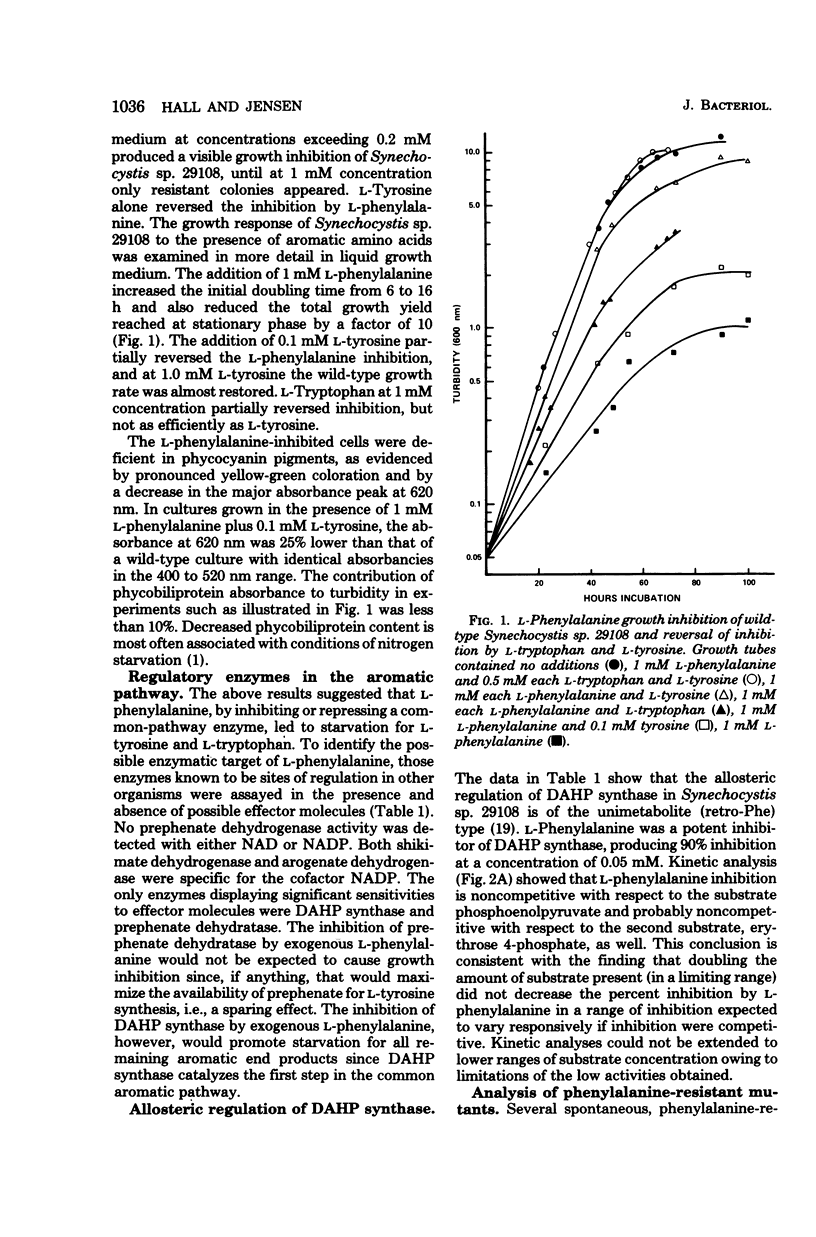
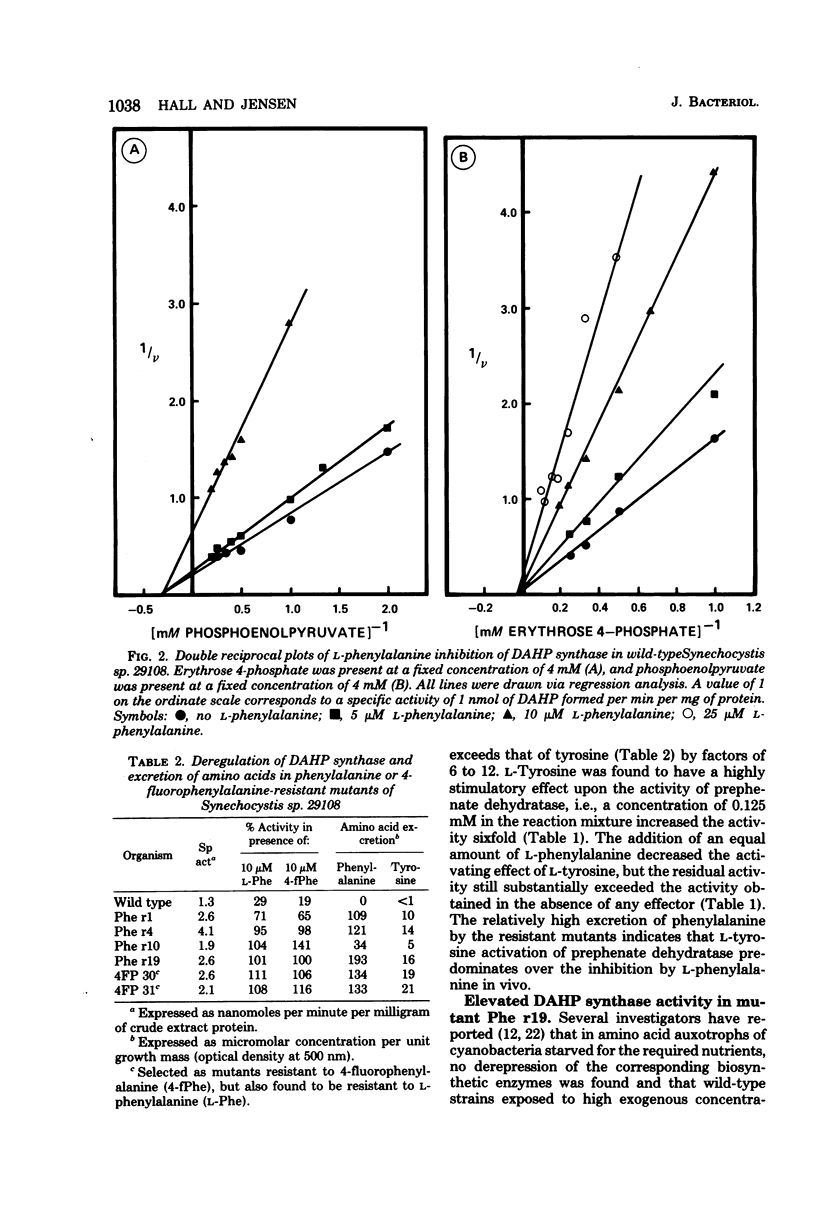
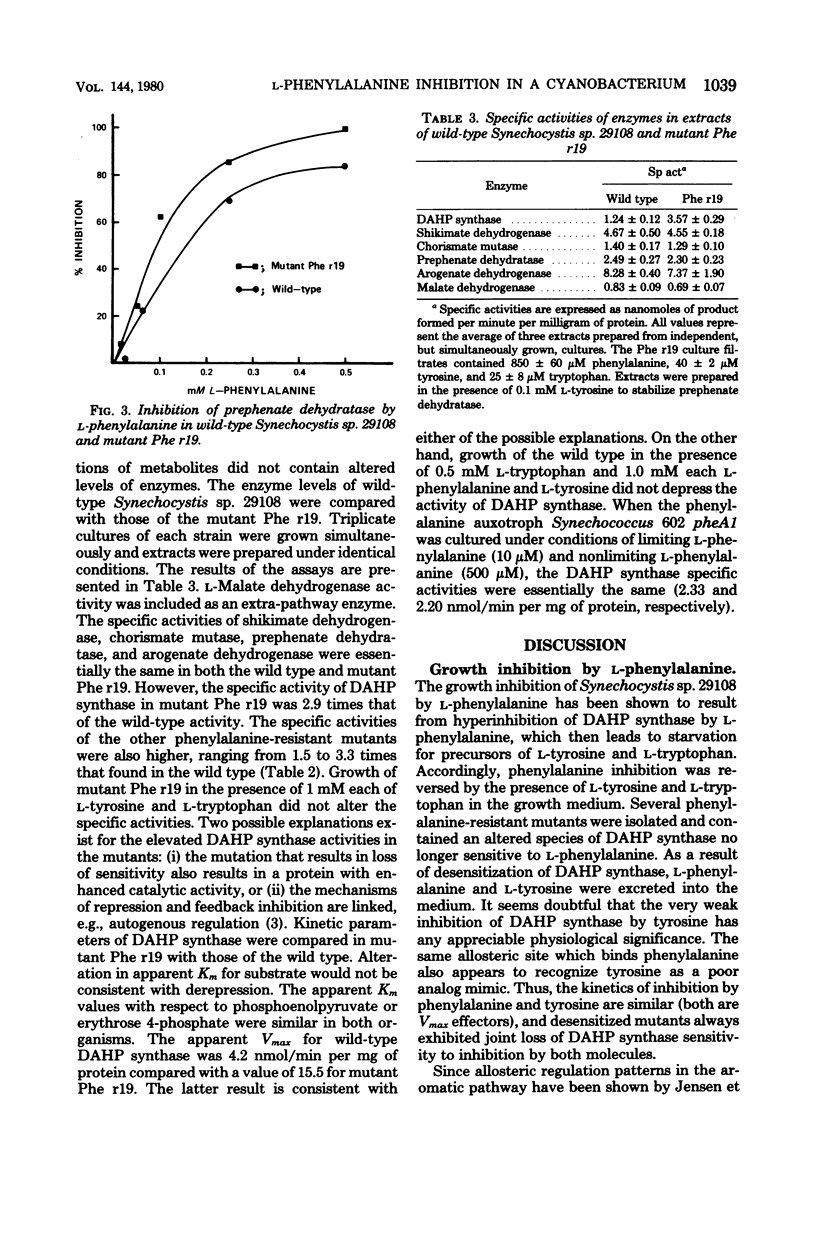
The pattern of allosteric control in the biosynthetic pathway for aromatic amino acids provides a basis to explain vulnerability to growth inhibition by l-phenylalanine (0.2 mM or greater) in the unicellular cyanobacterium Synechocystis sp. 29108. We attribute growth inhibition to the hypersensitivity of 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase to feedback inhibition by l-phenylalanine. Hyperregulation of this initial enzyme of aromatic biosynthesis depletes the supply of precursors needed for biosynthesis of l-tyrosine and l-tryptophan. Consistent with this mechanism is the total reversal of phenylalanine inhibition by a combination of tyrosine and tryptophan. Inhibited cultures also contained decreased levels of phycocyanin pigments, a characteristic previously correlated with amino acid starvation in cyanobacteria. l-Phenylalanine is a potent noncompetitive inhibitor (with both substrates) of 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase, whereas l-tyrosine is a very weak inhibitor. Prephenate dehydratase also displays allosteric sensitivity to phenylalanine (inhibition) and to tyrosine (activation). Both 2-fluoro and 4-fluoro derivatives of phenylalanine were potent analog antimetabolites, and these were used in addition to l-phenylalanine as selective agents for resistant mutants. Mutants were isolated which excreted both phenylalanine and tyrosine, the consequence of an altered 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase no longer sensitive to feedback inhibition. Simultaneous insensitivity to l-tyrosine suggests that l-tyrosine acts as a weak analog mimic of l-phenylalanine at a common binding site. Prephenate dehydratase in the regulatory mutants was unaltered. Surprisingly, in view of the lack of regulation in the tyrosine branchlet of the pathway, such mutants excrete more phenylalanine than tyrosine, indicating that l-tyrosine activation dominates l-phenylalanine inhibition of prephenate dehydratase in vivo. In mutant Phe r19 the loss in allosteric sensitivity of 3-deoxy-d-arabinoheptulosonate 7-phosphate synthase was accompanied by a threefold increase in specific activity. This could suggest that existence of a modest degree of repression control (autogenous) over 3-deoxy-d-arabinoheptulosonate synthase, although other explanations are possible. Specific activities of chorismate mutase, prephenate dehydratase, shikimate/nicotinamide adenine dinucleotide phosphate dehydrogenase, and arogenate/nicotinamide adenine dinucleotide phosphate dehydrogenase in mutant Phe r19 were identical with those of the wild type.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H. Autoregulation of gene expression. Annu Rev Microbiol. 1975;29:275–299. doi: 10.1146/annurev.mi.29.100175.001423. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. Channel-shuttle mechanism for the regulation of phenylalanine and tyrosine synthesis at a metabolic branch point in Pseudomonas aeruginosa. J Bacteriol. 1973 Jan;113(1):241–251. doi: 10.1128/jb.113.1.241-251.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R. S., Sokatch J. R., Jensen R. A. Relationship of metabolite inhibition of growth to flow-of-carbon patterns in nature. Life Sci. 1976 Aug 1;19(3):299–320. doi: 10.1016/0024-3205(76)90034-5. [DOI] [PubMed] [Google Scholar]
- De Felice M., Levinthal M., Iaccarino M., Guardiola J. Growth inhibition as a consequence of antagonism between related amino acids: effect of valine in Escherichia coli K-12. Microbiol Rev. 1979 Mar;43(1):42–58. doi: 10.1128/mr.43.1.42-58.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel A. M., Jensen R. A. Obligatory biosynthesis of L-tyrosine via the pretyrosine branchlet in coryneform bacteria. J Bacteriol. 1979 Jun;138(3):805–815. doi: 10.1128/jb.138.3.805-815.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel A. M., Jensen R. A. Regulation of prephenate dehydratase in Coryneform species of bacteria by L-phenylalanine and by remote effectors. Arch Biochem Biophys. 1980 Mar;200(1):165–176. doi: 10.1016/0003-9861(80)90343-4. [DOI] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G., Flick M. B., Jensen R. A. Approach to recognition of regulatory mutants of cyanobacteria. J Bacteriol. 1980 Aug;143(2):981–988. doi: 10.1128/jb.143.2.981-988.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood W., Carr N. G. Apparent lack of control by repression of arginine metabolism in blue-green algae. J Bacteriol. 1971 Jul;107(1):365–367. doi: 10.1128/jb.107.1.365-367.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Jensen R. A. Growth inhibition by L-phenylalanine in Agmenellum quadruplicatum. A clue to some amino acid interrelationships. Arch Mikrobiol. 1973 Jun 6;91(3):221–233. doi: 10.1007/BF00408909. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Pierson D., Kane J. F., Van Baalen C., Jensen R. A. Documentation of auxotrophic mutation in blue-green bacteria: characterization of a tryptophan auxotroph in Agmenellum quadruplicatum. J Bacteriol. 1972 Jul;111(1):112–118. doi: 10.1128/jb.111.1.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S. Comparative regulation of isoenzymic 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetases in microorganisms. J Bacteriol. 1968 Jan;95(1):188–196. doi: 10.1128/jb.95.1.188-196.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. I. Purification and properties of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3365–3372. [PubMed] [Google Scholar]
- Jensen R. A., Pierson D. L. Evolutionary implications of different types of microbial enzymology for L-tyrosine biosynthesis. Nature. 1975 Apr 24;254(5502):667–671. doi: 10.1038/254667a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Stenmark-Cox S., Ingram L. O. Mis-regulation of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase does not account for growth inhibition by phenylalanine in Agmenellum quadruplicatum. J Bacteriol. 1974 Dec;120(3):1124–1132. doi: 10.1128/jb.120.3.1124-1132.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaney A. R., Jhabvala P. Absence of repression in a phenylalanine auxotroph of Synechococcus cedrorum. J Gen Microbiol. 1975 Apr;87(2):370–372. doi: 10.1099/00221287-87-2-370. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Regulation of chemoautotrophic metabolism. 3. DAHP synthetase in Thiobacillus neapolitanus. Arch Mikrobiol. 1969;69(4):360–369. doi: 10.1007/BF00408576. [DOI] [PubMed] [Google Scholar]
- Kelly D. P. Regulation of chemoautotrophic metabolism. I. Toxicity of phenylalanine to thiobacilli. Arch Mikrobiol. 1969;69(4):330–342. doi: 10.1007/BF00408574. [DOI] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Pierson D. L., Jensen R. A. Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem. 1977 Aug 25;252(16):5839–5846. [PubMed] [Google Scholar]
- Phares W., Chapman L. F. Anacystis nidulans mutants resistant to aromatic amino acid analogues. J Bacteriol. 1975 Jun;122(3):943–948. doi: 10.1128/jb.122.3.943-948.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. L., Jensen R. A. Enzymology of l-Tyrosine Biosynthesis in Mung Bean (Vigna radiata [L.] Wilczek). Plant Physiol. 1979 Nov;64(5):727–734. doi: 10.1104/pp.64.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- Sagan L. On the origin of mitosing cells. J Theor Biol. 1967 Mar;14(3):255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Specialist phototrophs, lithotrophs, and methylotrophs: a unity among a diversity of procaryotes? Bacteriol Rev. 1977 Jun;41(2):419–448. doi: 10.1128/br.41.2.419-448.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



