Abstract
Background
Multimodality treatment of primary soft tissue sarcoma by expert teams reportedly affords a low incidence of local recurrence. Despite advances, treatment of local recurrence remains difficult and is not standardized.
Questions/purposes
We (1) determined the incidence of local recurrence from soft tissue sarcoma; (2) compared characteristics of the recurrent tumors with those of the primary ones; (3) evaluated local recurrences, metastases and death according to treatments; and (4) explored the relationship between the diagnosis of local recurrence and the occurrence of metastases.
Methods
From our prospective database, we identified 618 soft tissue sarcomas. Thirty-seven of the 618 patients (6%) had local recurrence. Leiomyosarcoma was the most frequent diagnosis (eight of 37). The mean delay from original surgery was 22 months (range, 2–75 months). Mean size was 4.8 cm (range, 0.4–28.0 cm). Median followup after local recurrence was 16 months (range, 0–98 months).
Results
Recurrent tumors had a tendency toward becoming deeper seated and higher graded. Nineteen of the 37 patients with recurrence underwent limb salvage (nine free flaps) and six had an amputation. Twenty-two (59%) had metastases, including 10 occurring after the local recurrence event at an average delay of 21 months (range, 1–34 months). Six patients developed additional local recurrences, with no apparent difference in risk between amputation (two of six) and limb salvage (four of 19).
Conclusions
Patients with a local recurrence of a soft tissue sarcoma have a poor prognosis. Limb salvage and additional radiotherapy remain possible but with substantial complications. Amputation did not prevent additional local recurrence or death.
Level of Evidence
Level IV, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Specialized multidisciplinary teams, using sophisticated imaging modalities, appropriate limb salvage techniques, and neoadjuvant or adjuvant therapies, have reported local recurrence for soft tissue sarcoma of the trunk and extremities ranging from 7% to 15% [6, 9, 17, 27]. Overall survival rates, disappointingly, have not improved along with local control [1, 15, 29]. The treatment of local recurrence remains even today quite challenging [1, 11, 19, 24–26]. Recurrences are associated with a poor prognosis, with a 2-year survival rate ranging from 50% to 70% [7, 10, 13, 16, 25, 27, 28, 30]. Local recurrences resulting from inadequate treatment have reported better prognosis than those occurring after a more comprehensive approach, with 5-year survival figures of 50% and 32%, respectively [1, 11, 22]. Moreover, certain patient characteristics such as male gender, high histologic grade, larger size of the primary tumor, number of recurrences, and short time delay to recurrence are reportedly signs of worse prognosis [7, 9, 11, 14, 16]. A nomogram and other prognostic index were recently proposed to help predict survival after local recurrence [14, 21]. Little is known about the interplay of local recurrence and distant metastases, and it remains unclear what real impact local recurrences have on the overall survival of soft tissue sarcoma patients. It could be argued a second attempt to eradicate disease locally should be at least, if not more, aggressive than the first and thus historically amputation was favored [3, 23], but amputation does not prevent the occurrence of metastases [8] and death. More recently, repeated attempt at limb salvage has become a reasonable option for many of these patients. This approach has also been our practice.
The management of local recurrence is complicated by prior adjuvant treatments. The increased potential for late treatment morbidity often limits the repeat use of therapeutic modalities. Thus, the treatment plan used for patients with recurrences has often differed from the primary treatment, and the extent of these variations remains poorly documented. The local control after a second attempt at limb salvage is less than what is expected for up-front therapy [22, 25–27]. Thus, the best way to manage these recurrences is unclear and literature on the subject is scarce. We suspected that optimally treated soft tissue sarcoma patients with a local recurrence have an increased incidence of local recurrences, metastases and death.
We therefore (1) determined the incidence of local recurrence from soft tissue sarcoma initially treated by a devoted multidisciplinary team; (2) compared characteristics of the recurrent tumors with those of the primary ones; (3) evaluated local recurrences, metastases and death according to treatments provided; and (4) explored the relationship between the diagnosis of local recurrence and the occurrence of metastases.
Patients and Methods
Patients for this collaborative and retrospective study were identified using a prospective database of patients with soft tissue sarcomas followed and treated within musculoskeletal oncology units at our two institutions. Our data came from the database and medical records; no patient was recalled specifically for this study. We obtained local ethics board approval. We excluded patients diagnosed with intraosseous tumors (317), extraskeletal osteosarcoma (10) or primitive neuroectodermal tumor\Ewing’s sarcoma (15), dermatofibrosarcoma protuberans (45), well-differentiated liposarcoma (103), and cutaneous angiosarcoma (7) because they are dissimilar in their characteristics, management, and prognosis. We also excluded 87 patients referred to us for local recurrence after initial management performed outside our institution as we presumed they were more likely to have recurred after suboptimal primary treatment and thus likely to have a better prognosis. From the database, we identified 618 patients with soft tissue sarcomas fulfilling our criteria treated from July 1989 to January 2008.
Cross-sectional imaging studies were performed in all patients at time of diagnosis and repeated prior surgery if given neoadjuvant treatments. We routinely used MRI for local imaging or, if contraindicated, CT scan. Postoperatively, we did not perform routine imaging of the tumor site. Chest CT was performed at staging and every 3 months after surgery for the following 2 years. Thereafter, we relied on plain chest radiographs. Followup assessments were every 3 months for the first 2 years, every 6 months up to year 5, and then yearly up to 10 years, after which the patients were usually discharged if no other event occurred since initial treatment. No additional imaging studies were routinely performed unless the questionnaire replies and physical examination were suspicious. Tumors were classified histologically into low or high grade according initially to the classification of Broders et al. [2] (Grades 1 and 2 as low and grades 3–4 as high grade) and in recent years to the French Federation classification (FNCLCC) [25] (grade 1 as low grade and grades 2 and 3 as high grade). Tumors were characterized for depth as superficial or deep according to their location respective to the deep fascia demarcating the subcutaneous tissue from the muscles. The American Joint Committee on Cancer TNM Version 6 was used for staging [10]. This staging is based on tumor size, histological grade, depth and the presence of metastases.
For limb salvage procedures we aimed for negative margins recognizing that wide margins are often not possible and marginal resection considered satisfactory with subsequent radiotherapy. Sacrifice of major structures such as large nerve, blood vessels or bone were avoided if they were not encased or grossly involved on preoperatives studies and at time of surgery. We accepted planned micropositive margins, with radiotherapy, through adventitial, epineurial or periosteal dissection. In cases where radiotherapy could not be used and positive margins were expected we resorted to amputation. Amputations were performed with the goal of obtaining wide margins. Limb salvage procedures required free tissue transfer for closure in nine patients (47%). Surgical margins were reported as negative if the minimal distance from tumor to the ink surface was 1 mm or more [12, 20]. In 24 patients (65%), the margins of the initial resection were considered positive.
From the medical histories, we collected information about the characteristics of the primary tumor, local recurrence and, if applicable, metastasis that developed. Moreover, the management received at each step of disease and complications was also identified. The status of the patient at last followup was also recorded. We compared the initial tumor depth with that at recurrence based on cross-sectional imaging appearance.
We determined differences in patient characteristics, modalities of treatment, and morbidity and mortality of the patients between the primary tumor and the local recurrence using chi-square analysis (Excel 2007, Microsoft Corporation, Seattle, USA).
Results
As of their last followup, 37 of the 618 patients (6%) had a local recurrence and these were retained for this report (Table 1). Local recurrences were only found in patients who underwent limb salvage for their primary tumor. There were 21 males and 16 females. The median age of these patients at diagnosis was 54 years (range, 15–92 years). The diagnosis of local recurrence was made at an average of 22 months after surgery to the primary tumor (range, 2–75 months). The recurrent tumor presented in the lower limbs in 24 patients (65%), in the upper limbs in 11 patients (30%), and in the trunk in two patients (5%). The median followup of patients after the appearance of the local recurrence was 16 months (range, 0–98 months). Two patients were lost to followup after the diagnosis of local recurrence was made as they refused any further treatment and chose palliative treatment within their community.
Table 1.
Main characteristics in primary tumor, local recurrence, and metastatic disease
| Variable | Primary tumor | Local recurrence | Metastasis | P-value |
|---|---|---|---|---|
| Total | 37 | 37 | 22 (59%) | reference |
| Gender | ||||
| Male | 21 (57%) | 14 (64%) | ||
| Female | 16 (43%) | 8 (36%) | ||
| Age at diagnosis (years) | ||||
| Median | 54 | 56 | 58 | |
| Range | 15–92 | 16–96 | 20–86 | |
| Mean delay time (months) | ||||
| Between primary tumor and local recurrence | 23 [SD = 20] | |||
| Between primary tumor and metastasis | 29 [SD = 27] | |||
| Between local recurrence and metastasis | 12 [SD = 13] | |||
| Tumor location | ||||
| Upper limb | 11 (30%) | |||
| Lower limb | 24 (65%) | |||
| Trunk | 2 (5%) | |||
| Tumor depth | ||||
| Superficial | 4 (11%) | 8 (22%) | 0.110 | |
| Deep | 33 (89%) | 29 (78%) | 0.110 | |
| Tumor grade | ||||
| Low | 7 (19%) | 5 (14%) | 0.085 | |
| High | 30 (81%) | 28 (76%) | 0.085 | |
| Histologic type | ||||
| Malignant fibrous histiocytoma | 7 (19%) | 8 (22%) | 0.519 | |
| Liposarcoma | 5 (13.5%) | 4 (11%) | 0.519 | |
| Synovial sarcoma | 4 (11%) | 5 (13.5%) | 0.519 | |
| Fibrosarcoma | 3 (8%) | 2 (5%) | 0.519 | |
| Leiomyosarcoma | 8 (22%) | 6 (16%) | 0.519 | |
| Malignant peripheral nerve sheath tumor | 5 (13.5%) | 4 (11%) | 0.519 | |
| Other | 5 (13.5%) | 4 (11%) | 0.519 | |
| Unknown | 0 (0.0%) | 4 (11%) | 0.519 | |
| Size (cm) | ||||
| Mean | 7.9 | 4.8 | 0.025 | |
| Range | 1.0–25.8 | 0.4–28.0 | ||
| Stage (AJCC staging system) | ||||
| I | 3 (8%) | |||
| II | 4 (11%) | |||
| III | 21 (57%) | |||
| IV | 2 (5%) | |||
| Surgical procedure | ||||
| Limb salvage | 37 (100%) | 19 (52%) | ||
| Amputation | 0 (0%) | 6 (17%) | ||
| No surgery | 0 (0%) | 12 (31%) | ||
| Surgical margins | ||||
| Positive | 24 (65%) | 11 (44%) | < 0.0001 | |
AJCC = American Joint Committee on Cancer.
Local recurrences were smaller (p = 0.025) than the original tumors. The mean size of the sarcomas at initial diagnosis was 7.9 cm (range, 1.0–25.8 cm) while the mean size of the local relapses was 4.8 cm (range, 0.4–28.0 cm) (Table 1). The most common initial histologic subtypes were leiomyosarcoma (22%), undifferentiated sarcoma (19%), liposarcoma (14%), and malignant peripheral nerve sheet tumor (14%). The histologic subtypes at recurrence were similarly distributed (p = 0.519). Primary tumors were superficial in four patients (11%) and in eight patients (22%) at the time of local recurrence. Seven (21%) of 33 initially deep-seated tumors recurred superficially, whereas three of four (75%) initially superficial tumors developed deep-seated local relapses. The majority (81%) of local recurrences occurred in patients initially diagnosed with high-grade tumors. At recurrence, five patients (14%) had a low-grade tumor, 28 (75%) had a high-grade recurrence, and four patients (11%) had undetermined histologic grade because a biopsy was not performed or was refused and the recurrence was established clinically and with proper imaging (unoperated patients). The grade infrequently changed from the initial diagnosis: three patients (43%) with initially low-grade primary tumor had high-grade local recurrences and one initially high-grade tumor was scored as low grade at recurrence (3%). Increase in grade was thus more likely (p = 0.016) than decrease.
Limb salvage was considered for all patients who presented local recurrence; however, 12 patients (32%) did not undergo any surgery because the patient (1) had metastatic disease and was managed with chemotherapy or supportive care (seven patients); (2) died before any treatment was initiated (one patient); (3) refused surgery (two patients); or (4) was lost to followup (two patients). For patients who received surgical treatment, 19 (76%) had a second limb salvage procedure whereas six (24%) underwent amputation. Amputation was selected when gross residual disease was unavoidable after surgery; positive margins were to occur in the context that no radiotherapy would be given or when expected limb function would have been worse than with amputation. All patients without prior radiotherapy who underwent a second limb salvage were given radiotherapy. Five patients had standard fractionated external beam radiotherapy at primary diagnosis and for recurrence. Of these, two patients had no complications, one developed secondary neuritis resulting in severe chronic pain, and two patients had serious wound-healing complications that required free flap coverage. We could not determine if local recurrences occurred within or outside the previous radiation fields. All of the patients who underwent amputation were free of metastases at the time of the procedure and all had received prior radiotherapy (Table 2). The median age of patients undergoing amputation was 36 years, with three of six patients younger than 24 years. Five of the six amputations were for large deep high-grade recurrent tumors (Table 3). Six patients presented further local recurrences (24%). The treatment of the second local recurrence was amputation in two patients, whereas it was additional limb salvage in the other four. All patients who underwent amputation and 10 of the 19 patients with limb salvage died of their disease within the followup time. After local recurrence, the 1-year actuarial overall survival rate was 57% and the 5-year rate 11%. The disease-free survival was similar (Fig. 1).
Table 2.
Surgical treatment for local recurrence in relation to prior use of radiotherapy
| Primary treatment | Local recurrence surgical treatment | Total | ||
|---|---|---|---|---|
| No surgery | Second limb salvage | Amputation | ||
| No radiotherapy | 2 (18%) | 7 (100%) | 0 (0%) | 9 (24%) |
| Radiotherapy | 9 (82%) | 12 (67%) | 6 (33%) | 28 (76%) |
| Total | 11 (30%) | 19 (54%) | 6 (16%) | 37 (100%) |
Table 3.
Characteristics versus surgical management for local recurrence
| Characteristic | Amputation | Limb salvage | P value |
|---|---|---|---|
| Number of patients | 6 | 19 | Reference |
| Age (years)* | 42 (15–72) | 53 (15–80) | 0.338 |
| Tumor size (cm)* | 10.4 | 3.2 | 0.007 |
| Tumor depth at local recurrence | |||
| Superficial | 0 | 7 | 0.080 |
| Deep | 6 | 12 | 0.080 |
| Radiation at primary tumor | 6 | 12 | 0.080 |
| Tumor location | |||
| Proximal | 3 | 8 | 0.703 |
| Distal | 3 | 9 | 0.703 |
| Trunk | 0 | 2 | 0.703 |
| Tumor location | |||
| Upper limb | 2 | 9 | 0.546 |
| Lower limb | 4 | 10 | 0.546 |
* Values are expressed as means or means, with ranges in parentheses.
Fig. 1A–B.
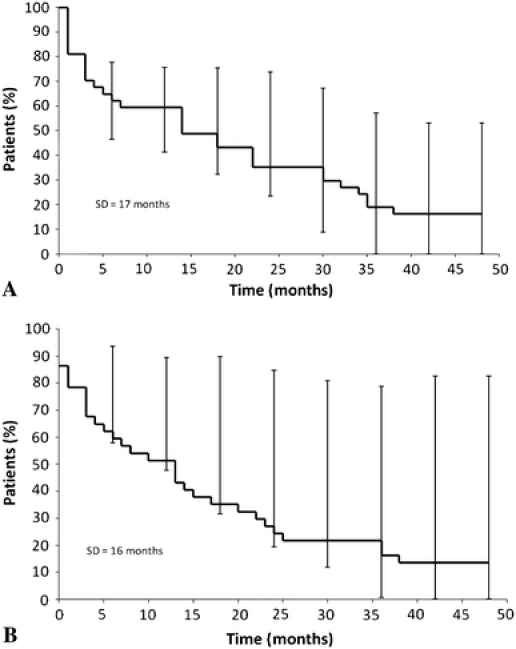
(A) Kaplan-Meier analysis of survival and (B) disease-free survival following local recurrence from soft-tissue sarcoma are shown.
Ultimately, 22 of 37 patients (59%) had distant metastases. Seven patients had metastases diagnosed before the local recurrence. For these the mean delay from the diagnosis of metastases and local recurrence was 14 months (range, 4–35 months). Five had metastases synchronous (within 30 days) to the local recurrence and underwent limb salvage with curative intent. For the other 10 patients, metastases occurred after the local recurrence: six (of 13) after limb salvage and four (of six) after amputation. The mean time to the occurrence of metastases for this group was 21 months (range, 1–34 months).
Discussion
Contemporary multimodal treatment of primary soft tissue sarcoma by expert teams reportedly affords a low incidence of local recurrence. Despite advances, however, the treatment of local recurrence remains difficult and is not standardized. We presumed the prognosis would be poor in patients who underwent comprehensive management of their initial tumor but nevertheless failed local control of their disease. Recurrences after aggressive initial local treatment are likely to be more difficult to manage because of the prior treatments given and possibly also because recurrent tumor may possess some inherent characteristics that can explain its recurrence and make it more resistant to cure [12]. Our intent was therefore to: (1) determine the incidence of local recurrence from soft tissue sarcoma initially addressed by a devoted multidisciplinary team; (2) compare characteristics of the recurrent tumors with those of the primary ones; (3) document the treatment modalities and their related complications; and (4) correlate the advent of local recurrence and metastases. Although some reports have addressed these issues, they usually included cases where the initial tumor management was inadequate or insufficient or excluded cases where local recurrence occurred after metastatic dissemination.
Our study is subject to a number of limitations. First, despite a substantial soft tissue sarcoma practice, our group found only 37 cases that met our inclusion criteria. Second, although we followed established guidelines in the management of primary tumors there are no such principles for local recurrence. Subjectivity, although from an expert team, may have occurred as these were addressed on case by case basis. Third, four patients (11%) were lost early in followup and 12 (32%) did not undergo surgical treatment thus restricting further our conclusions about treatment. Fourth, longer followup could have led to the detection of additional cases recognizing that most of recurrences occur within the first 3 years after surgery. Fifth, as local imaging was not routinely performed small local recurrence might have gone unnoticed. All these factors impacted our ability to provide for statistical analyses and strong conclusions.
Local recurrence occurred in 6% of our patients, which stands at the lower end of previously reported numbers [6, 9, 17, 27]. Our success in achieving local control reflects the comprehensive approach of a multidisciplinary team and illustrates the special character recurrent tumors exhibit. However, it may also relate to our frequent use of radiotherapy. From a recent internal review, we found 78% of our soft tissue sarcoma population receives radiotherapy. Similar low recurrence was reported by specialized teams, but radiotherapy was given to all their patients [18].
Unlike others [6], we did not see any major difference in depth, grade, and histologic subtype between primary and recurrent tumors. Although changes in tumor grade were rare, the tendency was for higher grade at recurrence. Local recurrences from initial superficial tumors commonly occurred in the muscle layer exposed at the time of initial tumor resection. Deep tumors that recurred superficially may represent subcutaneous layer seeding at time of surgery with positive margins but could also represent satellite lesions from the primary tumors. Although most recurrences are deep, risk of a superficial relapse should not be ignored. Superficial tumors are usually easier to manage than deep-seated ones. Tumor size at the time of local failure has been reported typically smaller than the size at primary diagnosis [6, 20], a finding confirmed by our observation. The smaller size of recurrent tumors can be explained by increased awareness of patients and serial followups in specialized clinics. One patient had a huge recurrent tumor. It developed rapidly between two clinical evaluations. Despite smaller size of recurrent tumor, the prognosis is reportedly worse than for primary tumor of similar dimensions [5, 15].
Wide surgical margins remain the most important factor in preventing further local relapse from sarcoma. Unfortunately, positive margins are reported with a high incidence ranging from 15–36% after surgery for local recurrence [4, 6, 15, 16, 26]. Positive margins were recorded in 44% of our cases and demonstrated our inability to correctly predict margins preoperatively. This, in our opinion, reflects our reluctance to amputate and the general difficulty of managing local relapses after aggressive initial local tumor treatment. We found patients undergoing limb salvage for their local recurrence had a high risk of locally recurring again (four of 19 or 21%). Despite our difficulty in achieving negative margins, the incidence of iterative recurrence was similar to what has been reported previously (18%–49%) [13, 16, 22, 23, 25, 26]. As many of these patients died shortly after their local relapse it is likely that the real local relapse figures would have been higher with longer survival period. We were committed to providing limb salvage whenever possible and in respect of the patient’s preference. Radiation therapy is known as a useful treatment modality to reduce the incidence of local recurrence but, to be effective, requires the delivery of 40 to 70 Gy. Radiation should certainly be used in most patients who failed limb salvage without prior use of this modality [27]. Administering a full course of radiotherapy to an area previously irradiated remains controversial. One could argue recurrence after irradiation is a suggestion of tumor radioresistance while others may believe it should be repeated in view of the limited prognosis and general ability of radiotherapy to improve local control [4, 25]. When performed twice in the same area, the risk of late complications is high. Some authors reported improved local control [4], but others found similar results whether radiotherapy was used or not [23]. Again, the quality of the surgical margins is critical. We evaluated whether amputation provided for better results than limb salvage. The rate of amputation for recurrence is reportedly higher than for primary tumor management [7, 12, 25, 26]. This has been our experience. Our recurrent tumors managed with amputation were larger than those managed with a second limb salvage procedure. With our limited patient numbers, we did not find a correlation with age, tumor location, depth, and former use of radiotherapy, although there were trends to amputation in young patients with previously irradiated deep tumors. Both these findings may represent a selection bias within the multidisciplinary decision process and are limited by the retrospective nature of this study. Unfortunately, we found amputation did not protect against additional local recurrence and death. Our definition of local recurrence was tumor in the vicinity of the former tumor bed or at the end of the amputation stump. Thus, it remains unclear to us whether limb salvage or amputation is most beneficial to the patient.
We found 34% of local recurrences occurred at or after the diagnosis of systemic metastases. This has been infrequently reported and appears higher than the 16% to 19% from previous literature [21, 26]. This finding supports the hypothesis that local recurrence is rarely the source of metastasis but rather an indicator of aggressive tumor biology, especially when it occurs after multimodality treatment within a specialized team [1, 11, 14, 23, 25]. There is a spectrum of locally recurrent tumors: some will have metastasized early and the local recurrence will be an epiphenomenon, whereas others may recur locally (sometimes more than once) without systemic dissemination [26]. As metastases are frequently associated with local recurrences there is no good way to determine if some represent secondary reseeding of the former tumor bed by systemic disease.
Modern multimodality treatment of soft tissue sarcoma yields a low incidence of local recurrence. Local relapses were difficult to manage and associated with a poor prognosis. Metastases were frequently found before or at the time the diagnosis of local failure. New approaches to locally recurrent soft tissue sarcoma need to be developed as current treatment modalities offered no satisfactory way out in the prevention of additional local relapses or metastases.
Acknowledgments
We thank Ms Cindy Wong, RN, for her invaluable help with the management of the sarcoma database and the data collection.
Footnotes
One of the authors (SA) was supported by a McGill medical student summer research bursary from the Mach Gaensslen Foundation of Canada. The institution of one or more of the authors (RET, MIH) has received funding from Stryker Canada. Stryker Canada has been unaware of both the data and the results and has no rights to oversee the conclusions.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This study was conducted within the musculoskeletal oncology units at McGill University Health Centre and Maisonneuve-Rosemont Hospital.
References
- 1.Brennan MF. Local recurrence in soft tissue sarcoma: more about the tumor, less about the surgeon. Ann Surg Oncol. 2007;14:1528–1529. doi: 10.1245/s10434-006-9340-1. [DOI] [PubMed] [Google Scholar]
- 2.Broders AC, Hargrave R, Meyerding HW. Pathological features of soft tissue fibrosarcoma: with special reference to the grading of its malignancy. Surg Gynecol Obstet. 1939;69:267. [Google Scholar]
- 3.Cantin J, McNeer GP, Chu F, Booker RJ. The problem of local recurrence after treatment of soft tissue sarcoma. Ann Surg. 1968;168:47–53. doi: 10.1097/00000658-196807000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catton C, Davis A, Bell R, O’Sullivan B, Fornasier V, Wunder J, McLean M. Soft tissue sarcoma of the extremity: limb salvage after failure of combined conservative therapy. Radiother Oncol. 1996;41:209–214. doi: 10.1016/S0167-8140(96)01856-7. [DOI] [PubMed] [Google Scholar]
- 5.Choong PF, Gustafson P, Rydholm A. Size and timing of local recurrence predicts metastasis in soft tissue sarcoma: growth rate index retrospectively analyzed in 134 patients. Acta Orthop Scand. 1995;66:147–152. doi: 10.3109/17453679508995509. [DOI] [PubMed] [Google Scholar]
- 6.Eilber FC, Brennan MF, Riedel E, Alektiar KM, Antonescu CR, Singer S. Prognostic factors for survival in patients with locally recurrent extremity soft tissue sarcomas. Ann Surg Oncol. 2005;12:228–236. doi: 10.1245/ASO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Eilber FC, Rosen G, Nelson SD, Selch M, Dorey F, Eckardt J, Eilber FR. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–226. doi: 10.1097/00000658-200302000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghert MA, Abudu A, Driver N, Davis AM, Griffin AM, Pearce D, White L, O’Sullivan B, Catton CN, Bell RS, Wunder JS. The indications for and the prognostic significance of amputation as the primary surgical procedure for localized soft tissue sarcoma of the extremity. Ann Surg Oncol. 2005;12:1–2. doi: 10.1245/ASO.2005.10.907. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs JF, Lee RJ, Driscoll DL, McGrath BE, Mindell ER, Kraybill WG. Clinical importance of late recurrence in soft-tissue sarcomas. J Surg Oncol. 2000;73:81–86. doi: 10.1002/(SICI)1096-9098(200002)73:2<81::AID-JSO5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano A, Eilber F, Morton D. The management of locally recurrent soft tissue sarcoma. Ann Surg. 1982;196:87–91. doi: 10.1097/00000658-198207000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobmyer SR, Brennan MF. Predictive variables detailing the recurrence rate of soft tissue sarcomas. Curr Opin Oncol. 2003;15:319–326. doi: 10.1097/00001622-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Gronchi A, Miceli R, Fiore M, Collini P, Lozza L, Grosso F, Mariani L, Casali PG. Extremity soft tissue sarcoma: adding to the prognostic meaning of local failure. Ann Surg Oncol. 2007;14:1583–1590. doi: 10.1245/s10434-006-9325-0. [DOI] [PubMed] [Google Scholar]
- 13.Karakousis CP, Proimakis C, Rao U, Velez AF, Driscoll DL. Local recurrence and survival in soft-tissue sarcomas. Ann Surg Oncol. 1996;3:255–260. doi: 10.1007/BF02306280. [DOI] [PubMed] [Google Scholar]
- 14.Kattan MW, Heller G, Brennan MF. A competing-risks nomogram for sarcoma-specific death following local recurrence. Stat Med. 2003;22:3515–3525. doi: 10.1002/sim.1574. [DOI] [PubMed] [Google Scholar]
- 15.Kaytan E, Yaman F, Cosar R, Eralp Y, Saip P, Darendeliler E. Prognostic factors in localized soft-tissue sarcomas. Am J Clin Oncol. 2003;26:411–415. doi: 10.1097/00000421-200308000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15:646–652. doi: 10.1200/JCO.1997.15.2.646. [DOI] [PubMed] [Google Scholar]
- 17.Nori D, Schupak K, Shiu MH, Brennan MF. Role of brachytherapy in recurrent extremity sarcoma in patients treated with prior surgery and irradiation. Int J Radiat Oncol Biol Phys. 1991;20:1229–1233. doi: 10.1016/0360-3016(91)90232-S. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, Pater J, Zee B. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 19.Pearlstone DB, Janjan NA, Feig BW, Yasko AW, Hunt KK, Pollock RE, Lawyer A, Horton J, Pisters PW. Re-resection with brachytherapy for locally recurrent soft tissue sarcoma arising in a previously radiated field. Cancer J Sci Am. 1999;5:26–33. [PubMed] [Google Scholar]
- 20.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan RC, A’Hern R, Fisher C, Thomas JM. Prognostic index for extremity soft tissue sarcomas with isolated local recurrence. Ann Surg Oncol. 2001;8:278–289. doi: 10.1007/s10434-001-0278-z. [DOI] [PubMed] [Google Scholar]
- 22.Robinson M, Barr L, Fisher C, Fryatt I, Stotter A, Harmer C, Wiltshaw E, Westbury G. Treatment of extremity soft tissue sarcomas with surgery and radiotherapy. Radiother Oncol. 1990;18:221–233. doi: 10.1016/0167-8140(90)90058-5. [DOI] [PubMed] [Google Scholar]
- 23.Shiu MH, Castro EB, Hajdu SI, Fortner JG. Surgical treatment of 297 soft tissue sarcomas of the lower extremity. Ann Surg. 1975;182:597–602. doi: 10.1097/00000658-197511000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobin LH, Gospodarowicz MK, Wittekind CH, eds, for the International Union Against. Cancer (UICC). Soft tissues. In: TNM Classification of Malignant Tumors. 7th ed. Oxford, UK: Wiley-Blackwell; 2009:157–156.
- 25.Torres MA, Ballo MT, Butler CE, Feig BW, Cormier JN, Lewis VO, Pollock RE, Pisters PW, Zagars GK. Management of locally recurrent soft tissue sarcoma after prior surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:1124–1129. doi: 10.1016/j.ijrobp.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Trovik CS, Gustafson P, Bauer HC, Saeter G, Klepp R, Berlin O, Erlanson M, Wahlström O, Raabe N. Consequences of local recurrence of soft tissue sarcoma: 205 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand. 2000;71:488–495. doi: 10.1080/000164700317381199. [DOI] [PubMed] [Google Scholar]
- 27.Trovik CS, Scandinavian Sarcoma Group Project Local recurrence of soft tissue sarcoma: a Scandinavian Sarcoma Group Project. Acta Orthop Scand Suppl. 2001;72:1–31. doi: 10.1080/000164701753606608. [DOI] [PubMed] [Google Scholar]
- 28.Ueda T, Yoshikawa H, Mori S, Araki N, Myoui A, Kuratsu S, Uchida A. Influence of local recurrence on the prognosis of soft-tissue sarcomas. J Bone Joint Surg Br. 1997;79:553–557. doi: 10.1302/0301-620X.79B4.7487. [DOI] [PubMed] [Google Scholar]
- 29.Vraa S, Keller J, Nielsen OS, Jurik AG, Jensen OM. Soft-tissue sarcoma of the thigh: surgical margin influences local recurrence but not survival in 152 patients. Acta Orthop Scand. 2001;72:72–77. doi: 10.1080/000164701753606734. [DOI] [PubMed] [Google Scholar]
- 30.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:739–747. doi: 10.1016/S0360-3016(03)00714-4. [DOI] [PubMed] [Google Scholar]


