Abstract
Background
Established prognostic factors influencing survival in soft tissue sarcomas include tumor stage, histopathologic grade, size, depth, and anatomic site. The presence of tumor near or at the margin of resection increases the risk of local recurrence but whether a positive surgical margin or local recurrence affect overall survival is controversial.
Questions/purposes
We explored the impact of microscopic margin on local recurrence, metastasis, and overall survival in patients with intermediate- to high-grade soft tissue sarcomas of the extremities. We then determined whether local recurrence decreases overall survival.
Methods
We retrospectively reviewed the medical records of 248 patients who had soft tissue sarcomas of the extremities treated surgically from 1995 to 2008. We estimated survival, local recurrence, and distant metastasis and examined factors potentially influencing these outcomes. The minimum followup was 0.4 years (median, 4.4 years; range, 0.4–13 years).
Results
The 5-year cumulative incidence of local recurrence was 4.1%. Patients who presented with positive margins or a margin of 2 mm or less had a worse survival than patients who had margins of greater than 2 mm and wide margins (5-year survival, 47% versus 70% and 72%). In addition to surgical margin, developing metastasis, tumor response of less than 90% necrosis, high histopathologic grade, high AJCC stage (Stage III), increasing age, and male gender were associated with decreased overall survival. Local recurrence independently predicted decreased overall survival.
Conclusions
Microscopic surgical margin and local recurrence after surgical treatment should be included as risk factors predicting decreased overall survival for intermediate- to high-grade soft tissue sarcomas of the extremities.
Level of Evidence
Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of relatively rare malignant neoplasms that arise in mesenchymal tissue. In the United States, it is estimated around 10,600 new cases will be diagnosed and 3820 patients will die of soft tissue sarcomas in the year 2009 [18]. Conventional treatment of soft tissue sarcomas of the extremities includes wide margin surgery and radiotherapy. This multimodality approach has replaced amputation as the primary surgical treatment of choice [5, 11, 22, 25, 26]. Several analyses of prognostic factors influencing both local recurrence and overall survival in patients with extremity soft tissue sarcomas show tumor stage, grade, size, depth, and anatomic site are most important for patient survival [6, 13, 24, 26]. Negative surgical margin, low histologic grade, and use of radiotherapy are the most important factors in achieving local disease control [6, 8, 10, 12, 23, 27, 30, 33]. However, the effect of positive margin and local recurrence as independent prognostic factors for metastasis-free survival and overall survival is still a matter of debate [9, 10, 16, 21, 23, 25, 28, 29].
Several studies in the past have addressed these issues with conflicting conclusions. Some authors believe a negative microscopic surgical margin enhances local disease control but does not measurably improve overall survival [3, 4, 28]. In contrast, recent reports argued surgical excision with negative margins not only improves local control but also enhances overall survival [9, 23, 27]. In one of the largest published series of 2084 patients, Stojadinovic et al. [27] reported a positive margin nearly doubled the risk of local recurrence and increased the risk of distant metastasis and disease-related death [27].
Local recurrence is a devastating event associated with substantial morbidity and thus should be avoided. However, the influence of local recurrence on the overall survival in soft tissue sarcomas of the extremities is not clear [10, 16, 25, 31]. Two previous prospective studies have concluded local recurrences had no influence on survival. Rosenberg et al. [25] prospectively randomized patients with extremity soft tissue sarcomas and compared amputation with limb-sparing surgery associated with radiation. There was a 20% rate of local recurrence in the limb salvage group and none in the amputation group. However, there was no difference in overall survival. Brennan et al. [3] showed, despite lower rates of local recurrence in patients who underwent surgical resection with adjuvant brachytherapy, the overall survival was not different from patients who received surgical resection alone. In contrast, several other studies have recognized local recurrence as a major factor associated with decreased overall survival [10, 21, 23]. The question therefore remains as to whether increasing morbidity and cost with a multimodality aggressive approach to obtain appropriate local tumor control would have an impact on local and distant disease-free survival and overall survival in patients with soft tissue sarcomas. Ideally we would want to precisely identify patients with high risk for distant recurrence and for poor survival to justify the use of these aggressive therapies.
The purposes of this investigation were to (1) define the effect of microscopic surgical margins on local recurrence, distant metastasis, and overall survival in patients with extremity soft tissue sarcoma; (2) determine whether various characteristics of the patient and tumor and local recurrence influenced overall survival in patients with intermediate- to high-grade soft tissue sarcomas of the extremities.
Patients and Methods
We identified 726 patients who underwent surgery for soft tissue sarcoma between 1995 and 2008 in the tumor registry of our institution. We included in the study only patients with a primary, deep, and intermediate- or high-grade soft tissue sarcoma located at the lower or upper extremity. Of the 726 patients, we excluded those with metastatic disease, secondary sarcoma, superficial located in relation to the compartment fascia, low histologic grade, and axial, pelvic, or retroperitoneal sarcoma. We also excluded patients younger than 18 years and those who received initial surgical treatment outside our institution. These exclusions left 248 patients with intermediate- or high-grade soft tissue sarcoma of the extremities (Table 1). We then reviewed the medical records including operative and pathologic reports and recorded the following information: patient demographics, treatment received, tumor anatomic location, size, histopathologic subtype, American Joint Committee on Cancer (AJCC) stage [15], and tumor response to adjuvant therapy. No patients were recalled specifically for this study; all data were obtained from the medical records. Approval from the Institutional Review Board at our institution was obtained before this investigation was begun.
Table 1.
Demographics and tumor characteristics
| Characteristic | Number of patients |
|---|---|
| Total | 248 |
| Gender | |
| Male | 134 (54%) |
| Female | 114 (46%) |
| Age group | |
| 18–29 years | 18 (7.3%) |
| 30–39 years | 24 (9.7%) |
| 40–49 years | 38 (15.3%) |
| 50–59 years | 38 (15.3%) |
| 60–69 years | 50 (20.2%) |
| 70–79 years | 49 (19.8%) |
| 80–89 years | 27 (10.9%) |
| 90 + years | 4 (1.6%) |
| Tumor location | |
| Lower extremity | 195 (78.6%) |
| Upper extremity | 53 (21.4%) |
| Histologic grade | |
| Grade 2 | 41 (16.5%) |
| Grade 3 | 68 (27.4%) |
| Grade 4 | 139 (56%) |
| Tumor size | |
| 0–4.99 cm | 18 (7.3%) |
| 5–9.99 cm | 89 (39.5%) |
| 10.0 + cm | 132 (53.2%) |
| AJCC clinical stage | |
| 1A | 2 (0.8%) |
| 2A | 38 (15.3%) |
| 2B | 20 (8.1%) |
| 3 | 188 (75.8%) |
| Margin | |
| Positive margin (inside the tumor) | 5 (2%) |
| ≤ 2 mm but clear margin | 44 (17.7%) |
| > 2 mm but ≤ 2 cm | 164 (66.1%) |
| Wide margin (> 2 cm) | 35 (14.1%) |
| Viability | |
| No viable tumor | 25 (10.1%) |
| ≥ 90% necrosis | 113 (45.6%) |
| 50%–89% necrosis | 65 (26.2%) |
| 0%–49% necrosis | 45 (18.1%) |
AJCC = American Joint Committee on Cancer.
Tumor size was determined as the greatest diameter of the resected tumor. Tumors were divided into three sizes: small (< 5 cm), intermediate (5–9.9 cm), or large (≥ 10 cm).
The closest surgical margin was determined by the pathologist after gross examination of the specimen. The entire external surface of the specimen was then inked and the specimen was cut perpendicularly to the inked margin. Under microscopic examination, the distance between the tumor and the inked margin was measured. The surgical margins, as described in the pathologic reports, were recorded and categorized as follows: positive for tumor present at the inked border of the specimen; close but clear for margins of 2 mm or less; negative for margins between 2 mm and 1.9 cm; and wide for margins of greater than 2 cm. For the purpose of this study, we elected to consider positive and close but clear margins as a group (inadequate margin) to compare with negative margin and wide margin (adequate margin). Tumor viability was determined by the percentage of viable tumor cells in relation to necrotic tumor in histologic examination. We divided the tumors in two categories by percentage of viable tumor cells: (1) those with greater than or equal to 90 percent of necrosis and (2) those with less than 90 percent of necrosis.
Of the 248 patients, 195 (79%) presented with lower extremity soft tissue sarcoma and 53 (21%) with tumor located on the upper extremity. One hundred fifty-one patients (61%) had the tumor in the thigh, which was the most common anatomic site followed by the calf. The most frequent histopathologic type was malignant fibrous histiocytoma, diagnosed in 68 (27.4%) of the patients. The mean tumor size (greatest diameter measured in the excised specimen) was 11.9 cm. The tumor measured less than 5 cm in 18 (7%) patients, between 5 and 9.9 cm in 98 (40%), and 10 cm or more in 132 (53%). We used a four-grade scale of histopathologic malignancy grading: 41 tumors (17%) were intermediate-grade (Grade II) and 207 (83%) were high-grade (Grades III or IV) lesions. The AJCC stage of disease at presentation to our institution was I in two (1%) patients, II in 58 (23%), and III in 188 (76%). Ninety-five percent of the surgical procedures involved limb-sparing techniques. Eleven patients were initially treated with an amputation. The adjuvant therapy applied during the study period was not uniform. The decision to use either chemotherapy or radiation varied during the long time period and was made by a multidisciplinary team. Surgery alone was performed on 55 (22%) patients. Surgical treatment was supplemented with radiotherapy in 85 (34%) and chemotherapy in 19 (8%). Eighty-nine (36%) patients received preoperative chemotherapy and radiation therapy, and surgical treatment. In 110 patients (44%) less than 90% of tumor necrosis was observed while 138 (56%) patients had more than 90% of tumor necrosis. Among the 248 patients in the cohort, the minimum followup was 0.4 years (median, 4.4 years; range, 0.4–13 years).
The data were summarized and reported using conventional methods: variables comprised of continuous data are reported as mean ± SD, and variables based on discrete data are reported as count (percentage). The analysis focused on the end points of local recurrence, metastasis, and survival (all-cause death). The rate of occurrence of these end points was estimated using the method of Kaplan and Meier [19]. The association of potential prognostic factors with local recurrence, metastasis, and survival was analyzed using Cox proportional-hazards regression models [7]. All risk factors (gender, age, histology, viability, grade, AJCC stage, tumor size, radiation, and margin) were initially analyzed univariately. Local recurrence and metastasis were also evaluated as risk factors for decreased survival; this was accomplished by incorporating these outcomes into the Cox models as time-dependent covariates. The effect of local recurrence on survival was displayed graphically using a landmark survival curve [1] in which patients who were alive and under observation at 1 year postsurgery were divided into two groups: those who had experienced a local recurrence within the first year postsurgery and those who had not; the Kaplan-Meier curve was then drawn for these groups starting at the 1-year landmark. In addition, multivariable analysis was conducted for survival and for metastasis (the small number of local recurrences precluded meaningful multivariable analysis for this end point). Starting with the risk factors with p < 0.05 univariately, variable selection for the final models was performed using stepwise selection. All statistical tests were two-sided.
Results
Seven of the 248 patients developed a local recurrence (Table 2). Twenty-nine patients died without evidence of recurrence or metastasis and 48 patients died with evidence of local recurrence or metastasis. The cumulative probability of local recurrence at 5 years was 4.1%. Inadequate microscopic margin (positive margin or a margin of 2 mm or less) and female gender were the only prognostic factors associated with higher local recurrence rate among all factors univariately analyzed. No multivariate model was applied because we had a low number of local recurrences. Patients with an inadequate margin (positive margin or a margin of 2 mm or less) had a greater risk (p = 0.002) of local recurrence (Table 3). The Kaplan-Meier 5-year estimated rate of local recurrence was 11.6% (95% confidence interval [CI], 0%–22.4%) for patients with an inadequate margin compared to 2.4% (95% CI, 0%–6%) in patients with a margin of between 2 mm and 1.9 cm. No patient with a wide margin developed local recurrence.
Table 2.
Description of patients who developed local recurrence
| Histology | Grade | Size (cm) | Location | Radiation therapy | Chemotherapy | Margin | Time to local recurrence (months) | Treatment | Metastasis | Vital status |
|---|---|---|---|---|---|---|---|---|---|---|
| Leiomyosarcoma | High 4/4 | 10.2 | Forearm | None | Yes | > 2 mm margin but ≤ 2 cm | 48 | Amputation | No | Alive |
| Leiomyosarcoma | High 4/4 | 21.0 | Thigh | Preoperative Intraoperative | Yes | ≤ 2 mm but clear margin | 15 | None | Yes | Dead |
| Synovial sarcoma | High 3/4 | 3.0 | Thigh | Intraoperative | No | ≤ 2 mm but clear margin | 13 | Reexcision | No | Dead |
| Myxoid liposarcoma | Intermediate 2/4 | 15.0 | Thigh | Intraoperative | No | ≤ 2 mm but clear margin | None | No | Alive | |
| Myxoid fibrosarcoma | Intermediate 2/4 | 6.3 | Leg | None | No | ≤ 2 mm but clear margin | Reexcision | No | Alive | |
| Pleomorphic spindle cell sarcoma | High 4/4 | 12.4 | Thigh | Preoperative Intraoperative | Yes | > 2 mm margin but ≤ 2 cm | 27 | Reexcision | Yes | Dead |
| Malignant fibrous histiocytoma | High 3/4 | 14.3 | Thigh | Preoperative Intraoperative | Yes | ≤ 2 mm but clear margin | 7 | Amputation | Yes | Dead |
Table 3.
Surgical margin and local recurrence, metastasis, and survival
| Outcome | Margins | Number | Events | 1-year rate (95% CI) | 5-year rate (95% CI) | 10-year rate (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| Local recurrence | Positive or ≤ 2 mm | 49 | 5 | 4.2% (0, 9.8) | 11.6% (0, 22.4) | 11.6% (0, 22.4) | 0.002 |
| > 2 mm and ≤ 2 cm | 164 | 2 | 0.6% (0, 1.8) | 2.4% (0, 6) | 2.4% (0, 6) | ||
| > 2 cm | 35 | 0 | 0% (0, 0) | 0% (0, 0) | 0% (0, 0) | ||
| Metastasis | Positive or ≤ 2 mm | 49 | 19 | 21.3% (8.6, 32.3) | 52.2% (28.3, 68.1) | 52.2% (28.3, 68.1) | 0.07 |
| > 2 mm and ≤ 2 cm | 164 | 34 | 13.9% (8, 19.4) | 26.5% (17.9, 34.2) | 28.1% (19, 36.2) | ||
| > 2 cm | 35 | 7 | 11.2% (0, 22.4) | 29.7% (8, 46.3) | 29.7% (8, 46.3) | ||
| Overall survival | Positive or ≤ 2 mm | 49 | 25 | 89.1% (80.5, 98.6) | 46.6% (32.1, 67.5) | 18.3% (7.1, 47.1) | 0.005 |
| > 2 mm and ≤ 2 cm | 164 | 42 | 93.5% (89.5, 97.7) | 69.7% (61.5, 78.9) | 54.6% (43.7, 68.2) | ||
| > 2 cm | 35 | 10 | 100% (100, 100) | 72.3% (55.3, 94.4) | 39.4% (19.1, 81.2) |
CI = confidence interval.
Sixty patients developed distant metastasis. The metastasis rate at 5 years was 32.1%. Histopathologic Grade III and IV (hazard ratio [HR], 4.2; 95% CI, 1.3–13.3) and tumor viability with less than 90% necrosis (HR, 1.9; 95% CI, 1.1–3.2) were risk factors for distant metastasis. Inadequate surgical margin (positive margin or a margin of 2 mm or less) tended to be associated with higher (p = 0.07) rate of metastasis. The Kaplan-Meier 5-year estimated rate of metastasis was 52.2% (95% CI, 28%–68%) for patients with an inadequate margin compared to 27% (95% CI, 8%–46%) in patients with a margin of between 2 mm and 1.9 cm and 30% (95% CI, 8%–46%) in patients with a wide margin. Local recurrence was not a risk factor for distant metastasis.
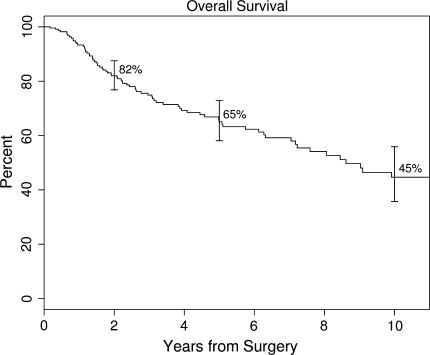
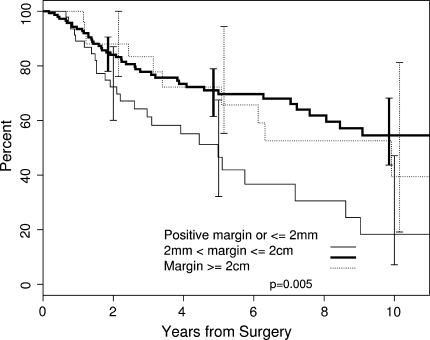
The median overall survival was 8.6 years. The Kaplan-Meier 5-year estimated rate of survival for the entire cohort was 65.1% (95% CI, 58.1%–72.9%) (Fig. 1). Inadequate surgical margin, local recurrence, developing metastasis, tumor response of less than 90% necrosis, high histopathologic grade, high AJCC stage (Stage III), increasing age, and male gender were associated with decreased overall survival. Neither tumor location (upper extremity versus lower extremity) nor tumor size was a risk factor for overall survival. The 5-year estimated rate of overall survival was lower (p = 0.005) for patients with an inadequate margin (46.6%, 95% CI, 32.1%–67.5%) compared to those with a margin of between 2 mm and 1.9 cm (69.7%, 95% CI, 61.5%–78.9%) and with a wide margin (72.3%, 95% CI, 55.3%–94.4%) (Fig. 2).
Fig. 1.
A Kaplan-Meier survivorship curve shows overall survival rates with 95% CIs at 2, 5, and 10 years postsurgery. The Kaplan-Meier 5-year estimated rate of survival for the entire cohort was 65.1% (95% CI, 58.1%–72.9%).
Fig. 2.
A Kaplan-Meier survivorship curve illustrates the effect of microscopic surgical margin on overall survival. The 5-year estimated rate of overall survival was lower (p = 0.005) for patients with an inadequate margin (46.6%, 95% CI, 32.1%–67.5%) compared to those with a margin between 2 mm and 1.9 cm (69.7%, 95% CI, 61.5%–78.9%) and with a wide margin (72.3%, 95% CI, 55.3%–94.4%).
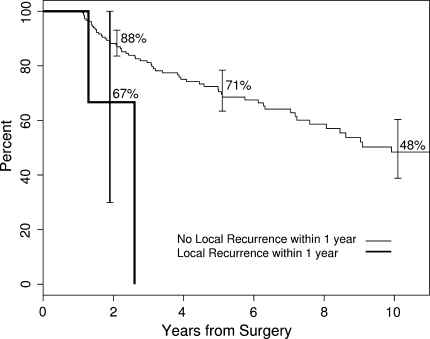
Local recurrence, metastasis, male gender, increasing age at diagnosis, and AJCC stage independently predicted decreased survival (Table 4). Patients who had local recurrence within the first 12 months after surgery had worse survival than those who did not (Fig. 3).
Table 4.
Multivariate analysis: factors associated with decreased overall survival
| Factor | Hazard ratio (95% CI) | P value |
|---|---|---|
| Metastases | 20.2 (11.8, 34.5) | < 0.001 |
| Local recurrence | 32.4 (9.9, 105.3) | < 0.001 |
| Male versus female | 0.4 (0.2, 0.6) | < 0.001 |
| Age at diagnosis | 1.1 (1.0, 1.1) | < 0.001 |
| AJCC Stage III versus Stages I and II | 2.6 (1.4, 4.8) | 0.004 |
CI = confidence interval; AJCC = American Joint Committee on Cancer.
Fig. 3.
A Kaplan–Meier survivorship curve shows overall survival using a 12-month landmark. Patients were grouped into those who had local recurrence within the first year and those who were free of local recurrence at 12 months. Patients who had local recurrence within the first 12 months had worse survival than those who did not.
Discussion
Since the development of limb salvage treatment, tumor size, depth, histologic grade, and anatomic site have been well-accepted prognostic factors affecting local recurrence and survival in patients with STS. The role of microscopic surgical margin and local recurrence is still controversial. We defined the effect of microscopic surgical margin on local recurrence, distant metastasis, and overall survival in patients with intermediate- to high-grade STS of the extremities. We also analyzed local recurrence and tumor and patient related characteristics as predictors of decreased overall survival.
We note some limitations to our study. First is the lack of standardization of adjuvant therapy. Nevertheless, radiotherapy was used in 70% and chemotherapy in 36% of our patients. This is higher than previous investigations [8, 23, 28]. Second is the small number of local recurrences. We included only patients with primary tumors treated at our institution. This limited the number of local recurrences; however, it avoided confounding factors associated with presentation after inadequate surgical resection. Our median followup of 4.4 years is consistent with previous studies that showed 2/3 of patients will present with a local recurrence within 2 years after resection of a STS and 90% will recur within 4 years [10].
An inadequate microscopic surgical margin (positive margin or a margin of 2 mm or less) was associated with local recurrence when compared to negative (between 2 mm and 2 cm) and wide margins (> 2 cm). The ideal margin width that enhances local control is controversial [14, 16, 17, 23, 28]. Most studies classify margin as positive or negative [10, 20, 27]. Others emphasized the extent and quality of the margin [9, 16] (Table 5). Dickinson et al. [9] reported increasing the width of resection improves local control and overall survival. We elected to consider positive and close margins (< 2 mm) as one group, which included 20% of our patients. The simple presence of residual disease does not imply the tumor will locally recur. Despite the 20% rate of inadequate margin, the cumulative rate of local recurrence was only 4.1% at 5 years. This is inferior to the 12% to 15% recurrence rate previously reported [11, 20, 28]. One explanation may be 70% of our patients received either radiation therapy compared to 40% in the series reported by Pisters et al. [23].
Table 5.
Results of microscopic surgical margin on local recurrence, metastasis, and survival for patients with STS
| Study | Number of patients | Sarcoma site | Margin categories | Local recurrence at 5 years (%) | Metastasis | Overall survival at 5 years (%) | Comments |
|---|---|---|---|---|---|---|---|
| Dickinson et al. [9] | 279 | All sites | Contaminated > 20 mm 10–19 mm 5–9 mm 1–4 mm < 1 mm |
NA | NA | NA | Contaminated margins led to higher rate of local recurrence; failure to achieve a noncontaminated margin led to decrease overall survival |
| Eilber et al. [10] | 753 | Extremity | Positive Negative |
18 | NA | NA | Positive versus negative surgical margin (HR = 2.1; p = 0.2; 95% CI = 0.67–6.61) for patients with primary STS; positive surgical margin was not a factor for developing local recurrence. |
| Herbert et al. [17] | 74 | Extremity | Grossly positive | 45 | 45 | 58 | In a multivariate Cox regression analysis, the importance of surgical resection margins (negative versus close or positive) was confirmed (p = 0.02) |
| Microscopically positive | |||||||
| Close (5 mm) | 20 | 28 | 72 | ||||
| Negative | 0 | 10 | 89 | ||||
| p = 0.003 | p = 0.05 | p = 0.09 | |||||
| Lewis et al. [21] | 911 | Extremity | p = 0.0019 RR = 1.6 CI = 1.1–2.5 |
p = 0.007 | p = 0.009 RR = 1.6 CI = 1.1–2.2 |
||
| Lewis et al. [20] | 495 | Extremity | Positive Negative |
Overall 13 p = 0.01 |
25 p = 0.009 |
79 p = 0.01 |
A positive microscopic margin was adversely associated with local recurrence and 5-year disease-specific survival and a predictor for development of metastasis |
| Pisters et al. [23] | 1041 | Extremity | Positive (1 mm) | 80 | 74 | 77.6 | Included patients who presented with primary sarcomas and patients who presented with a local recurrence |
| Negative | 59.9 | 70.3 | 68.6 | ||||
| p = 0.001 | p = 0.13 | p = 0.001 | |||||
| Stojadinovic et al. [27] | 2084 | All sites | Positive | 35 | 32 | 70 | By multivariate analysis, microscopic margin remained associated with the rate of local recurrence and metastasis |
| Negative | 18 | 24 | 80 | ||||
| p < 0.01 | p < 0.01 | p < 0.01 | |||||
| Tanabe et al. [28] | 95 | Extremity | Positive | 38 | NA | 30 | A negative surgical margin enhanced local disease control but did not measurably improve survival |
| Negative | 9 | 35 | |||||
| p = 0.005 | p = 0.9 | ||||||
| Trovik et al. [30] | 559 | Trunk Extremity | Inadequate (intralesional or marginal) | 25 | NA | An inadequate surgical margin was a risk factor for local recurrence but not for metastasis | |
| Wide | 75 | ||||||
| p < 0.001 | p = 0.6 | ||||||
| Novais et al. [current study] | 248 | Extremity | Inadequate: positive and ≤ 2 mm) | 11.6 | 52.2 | 46.6 | A surgical margin ≤ 2 mm was a risk factor for local recurrence and decreased overall survival; the association of inadequate margin and metastasis was not significant |
| > 2 mm but ≤ 2 cm | 2.4 | 27 | 69.7 | ||||
| Wide (> 2 cm) | 0 | 30 | 72.3 | ||||
| p = 0.002 | p = 0.07 | p = 0.0045 |
STS = soft tissue sarcoma; NA = not available, HR = hazards ratio; CI = confidence interval.
We observed only a trend toward the association of inadequate margin and development of metastasis. The observed HR for development of metastasis between patients with an inadequate margin and patients with a wide margin was 1.86. A power analysis revealed to have 80% power to detect this HR as being statistically significant would have required 76 patients with inadequate margins and 28 with wide margins. Nevertheless, this large HR and the trend toward statistical significance (p = 0.07) suggest inadequate margin may be an important factor in the development of metastasis. Pisters et al. [24] reported the prognostic factors associated with an increased risk for local recurrence were different from those associated with an increased risk of metastasis. In their series of 1041 patients with STS of the extremities, positive margin was associated with local recurrence and tumor-related mortality but not directly to metastasis. In contrast, in a long-term study of patients with extremity STS, positive margin was adversely associated with disease-specific survival and was a predictor of metastasis in patients who were alive at 5 years [20].
We found an inadequate surgical margin was associated with decreased overall survival. This association has been questioned in the past in a series of 95 patients that found no difference in the 5-year overall survival for patients with negative and positive margins after surgical excision of a STS of the extremities [28]. However our findings are supported by others [9, 17, 20, 23, 27]. In the largest single-institution investigation, microscopic resection margin was reported as an independent predictor of distant recurrence-free and disease-specific survival [27]. In another series of extremity STS, patients with positive surgical margins were at increased risk for subsequent tumor-related mortality [23]. The goal to obtain an adequate surgical margin may be limited by anatomic constraints, such as tumor abutting critical neurovascular structures. In fact, we were not able to achieve an adequate margin in 20% of our patients. Treatment options to improve local control may increase the rate of treatment complications. Some authors have questioned the role of increasing morbidity to obtain an adequate margin favoring the biologic aggressiveness of the tumor as a more important predictor of survival [2, 32]. Nonetheless, we showed a microscopic surgical margin of greater than 2 mm is a factor reducing the risk of local recurrence and improving overall survival in intermediate- to high-grade STS of the extremities.
Local recurrence remained an independent predictor of survival after adjusting for all major risk factors by multivariate analysis. Whether this is due to local control directly affecting survival or higher biologic tumor aggressiveness is speculative. We did not find an association between local recurrence and metastasis. This may be related to the low number of local recurrences. We cannot draw any conclusions about the causal relationship between local recurrence and decreased overall survival, as already reported by several authors [10, 16, 21, 23]. A strong correlation between local recurrence and metastasis was reported in a series of patients with STS treated exclusively by surgical resection [30]. However, the association of local recurrence with overall survival was questioned in the past (Table 6). Rosenberg et al. [25] prospectively randomized 43 patients with extremity STS and compared amputation with limb-sparing surgery and radiation. They reported a 20% rate of local recurrence in the limb salvage group and none in the amputation group, but the higher rate of local recurrence in the limb salvage group was not significant since the number of patients available for comparison was small and the study likely underpowered for that question. A similar consideration of lack of statistical power apply to the study by Brennan et al. [3] showing, despite lower rates of local recurrence in patients who underwent surgical resection with adjuvant brachytherapy, the overall survival was not different from that in patients who received surgical resection alone.
Table 6.
Results of local recurrence on metastasis and survival for patients with STS
| Study | Number of patients | Sarcoma site | Recurrence (%) | Metastasis (%) | Overall survival at 5 years (%) | Comments |
|---|---|---|---|---|---|---|
| Eilber et al. [10] | 607 (primary) | Extremity | Yes 10 | 29 | ||
| No 90 | 77 | |||||
| p < 0.0001 | ||||||
| Lewis et al. [21] | 911 | Extremity | Yes 33 | 38 (at 24 months) | 72 (at 24 months) | Local recurrence is strongly associated with metastasis and disease-specific death |
| Lewis et al. [20] | 495 | Extremity | NA | 25 | 79 | Development of local recurrence did not influence metastasis-free or overall survival |
| p = 0.009 | p = 0.01 | |||||
| Pisters et al. [23] | 1041 | Extremity | Yes 20 | 73.6 | 75.1 | Included patients who presented with primary sarcomas and patients who presented with a local recurrence |
| No 80 | 71.9 | 74.7 | ||||
| p = 0.015 | p = 0.003 | |||||
| Rosenberg et al. [25] | 43 | Extremity | Difference in local recurrence rate between the groups did not result in improved survival | |||
| Amputation | Yes (3/16) | 78 | 88 | |||
| (n = 16) | No (13/16) | |||||
| Limb sparing | Yes (6/27) | 71 | 83 | |||
| No (21/27) | ||||||
| (n = 27) | ||||||
| Stojadinovic et al. [27] | 2084 | All sites | Yes 18 | |||
| No 82 | ||||||
| Tanabe et al. [28] | 95 | Extremity | Yes 15 | 57 | Local control did not have a measurable impact on overall survival | |
| No 85 | 68 | |||||
| p = 0.16 | ||||||
| Trovik et al. [30] | 559 | Trunk | Yes 18 | p < 0.001 | NA | Local recurrence was associated with an increased risk of metastasis (HR = 4.4; 95% CI = 2.9–6.8) |
| Extremity | No 82 | |||||
| Novais et al. [current study] | 248 | Extremity | Yes 3 | |||
| No 97 |
STS = soft tissue sarcoma; NA = not available, HR = hazards ratio; CI = confidence interval.
The diagnosis of positive and close surgical margins (≤ 2 mm) and of a local recurrence should be included as prognostic factors in patient management decisions and in clinical trials. The results of this study drive us in the direction of further investigating the best strategy to manage patients with positive and close margins after surgery and patients who develop a local recurrence in moderate- and high-grade STS of the extremities.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 2.Brennan MF. Local recurrence in soft tissue sarcoma: more about the tumor, less about the surgeon. Ann Surg Oncol. 2007;14:1528–1529. doi: 10.1245/s10434-006-9340-1. [DOI] [PubMed] [Google Scholar]
- 3.Brennan MF, Hilaris B, Shiu MH, Lane J, Magill G, Friedrich C, Hajdu SI. Local recurrence in adult soft-tissue sarcoma: a randomized trial of brachytherapy. Arch Surg. 1987;122:1289–1293. doi: 10.1001/archsurg.1987.01400230075014. [DOI] [PubMed] [Google Scholar]
- 4.Chang AE, Kinsella T, Glatstein E, Baker AR, Sindelar WF, Lotze MT, Danforth DN, Jr, Sugarbaker PH, Lack EE, Steinberg SM, et al. Adjuvant chemotherapy for patients with high-grade soft-tissue sarcomas of the extremity. J Clin Oncol. 1988;6:1491–1500. doi: 10.1200/JCO.1988.6.9.1491. [DOI] [PubMed] [Google Scholar]
- 5.Choong PF, Petersen IA, Nascimento AG, Sim FH. Is radiotherapy important for low-grade soft tissue sarcoma of the extremity? Clin Orthop Relat Res. 2001;387:191–199. doi: 10.1097/00003086-200106000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Collin C, Hajdu SI, Godbold J, Friedrich C, Brennan MF. Localized operable soft tissue sarcoma of the upper extremity: presentation, management, and factors affecting local recurrence in 108 patients. Ann Surg. 1987;205:331–339. doi: 10.1097/00000658-198704000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox DR. Regression models and life-tables (with discussion) J R Stat Soc B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 8.Davis AM, Kandel RA, Wunder JS, Unger R, Meer J, O’Sullivan B, Catton CN, Bell RS. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1997;66:81–87. doi: 10.1002/(SICI)1096-9098(199710)66:2<81::AID-JSO2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson IC, Whitwell DJ, Battistuta D, Thompson B, Strobel N, Duggal A, Steadman P. Surgical margin and its influence on survival in soft tissue sarcoma. ANZ J Surg. 2006;76:104–109. doi: 10.1111/j.1445-2197.2006.03615.x. [DOI] [PubMed] [Google Scholar]
- 10.Eilber FC, Rosen G, Nelson SD, Selch M, Dorey F, Eckardt J, Eilber FR. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237:218–226. doi: 10.1097/00000658-200302000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eilber FR, Eckardt J. Surgical management of soft tissue sarcomas. Semin Oncol. 1997;24:526–533. [PubMed] [Google Scholar]
- 12.Espat NJ, Lewis JJ. The biological significance of failure at the primary site on ultimate survival in soft tissue sarcoma. Semin Radiat Oncol. 1999;9:369–377. doi: 10.1016/S1053-4296(99)80031-9. [DOI] [PubMed] [Google Scholar]
- 13.Gaynor JJ, Tan CC, Casper ES, Collin CF, Friedrich C, Shiu M, Hajdu SI, Brennan MF. Refinement of clinicopathologic staging for localized soft tissue sarcoma of the extremity: a study of 423 adults. J Clin Oncol. 1992;10:1317–1329. doi: 10.1200/JCO.1992.10.8.1317. [DOI] [PubMed] [Google Scholar]
- 14.Gerrand CH, Wunder JS, Kandel RA, O’Sullivan B, Catton CN, Bell RS, Griffin AM, Davis AM. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br. 2001;83:1149–1455. doi: 10.1302/0301-620X.83B8.12028. [DOI] [PubMed] [Google Scholar]
- 15.Green FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, editors. AJCC Cancer Staging Manual. 6. New York, NY: Springer; 2002. [Google Scholar]
- 16.Gronchi A, Miceli R, Fiore M, Collini P, Lozza L, Grosso F, Mariani L, Casali PG. Extremity soft tissue sarcoma: adding to the prognostic meaning of local failure. Ann Surg Oncol. 2007;14:1583–1590. doi: 10.1245/s10434-006-9325-0. [DOI] [PubMed] [Google Scholar]
- 17.Herbert SH, Corn BW, Solin LJ, Lanciano RM, Schultz DJ, McKenna WG, Coia LR. Limb-preserving treatment for soft tissue sarcomas of the extremities: the significance of surgical margins. Cancer. 1993;72:1230–1238. doi: 10.1002/1097-0142(19930815)72:4<1230::AID-CNCR2820720416>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 20.Lewis JJ, Leung D, Casper ES, Woodruff J, Hajdu SI, Brennan MF. Multifactorial analysis of long-term follow-up (more than 5 years) of primary extremity sarcoma. Arch Surg. 1999;134:190–194. doi: 10.1001/archsurg.134.2.190. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15:646–652. doi: 10.1200/JCO.1997.15.2.646. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor MI, Pritchard DJ, Gunderson LL. Integration of limb-sparing surgery, brachytherapy, and external-beam irradiation in the treatment of soft-tissue sarcomas. Clin Orthop Relat Res. 1993;289:73–80. [PubMed] [Google Scholar]
- 23.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 24.Pisters PW, Pollock RE. Staging and prognostic factors in soft tissue sarcoma. Semin Radiat Oncol. 1999;9:307–314. doi: 10.1016/S1053-4296(99)80025-3. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, DeMoss EV, Seipp C, Sindelar WF, Sugarbaker P, Wesley R. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sim FH, Pritchard DJ, Reiman HM, Edmonson JH, Schray MF. Soft-tissue sarcoma: Mayo Clinic experience. Semin Surg Oncol. 1988;4:38–44. doi: 10.1002/ssu.2980040109. [DOI] [PubMed] [Google Scholar]
- 27.Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2, 084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–434. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanabe KK, Pollock RE, Ellis LM, Murphy A, Sherman N, Romsdahl MM. Influence of surgical margins on outcome in patients with preoperatively irradiated extremity soft tissue sarcomas. Cancer. 1994;73:1652–1659. doi: 10.1002/1097-0142(19940315)73:6<1652::AID-CNCR2820730617>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 29.Trovik CS. Local recurrence of soft tissue sarcoma. A Scandinavian Sarcoma Group Project. Acta Orthop Scand Suppl. 2001;72:1–31. doi: 10.1080/000164701753606608. [DOI] [PubMed] [Google Scholar]
- 30.Trovik CS, Bauer HC, Alvegard TA, Anderson H, Blomqvist C, Berlin O, Gustafson P, Saeter G, Wallöe A. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36:710–716. doi: 10.1016/S0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 31.Trovik CS, Gustafson P, Bauer HC, Saeter G, Klepp R, Berlin O, Gustafson P, Saeter G, Wallöe A. Consequences of local recurrence of soft tissue sarcoma: 205 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand. 2000;71:488–495. doi: 10.1080/000164700317381199. [DOI] [PubMed] [Google Scholar]
- 32.Ueda T, Yoshikawa H, Mori S, Araki N, Myoui A, Kuratsu S, Uchida A. Influence of local recurrence on the prognosis of soft-tissue sarcomas. J Bone Joint Surg Br. 1997;79:553–557. doi: 10.1302/0301-620X.79B4.7487. [DOI] [PubMed] [Google Scholar]
- 33.Wilson RB, Crowe PJ, Fisher R, Hook C, Donnellan MJ. Extremity soft tissue sarcoma: factors predictive of local recurrence and survival. Aust N Z J Surg. 1999;69:344–349. doi: 10.1046/j.1440-1622.1999.01569.x. [DOI] [PubMed] [Google Scholar]