Abstract
Background
Pediatric soft tissue sarcomas are rare and differ from those in adults regarding the spectrum of diagnoses and treatment. Sarcomas in extremities may have different prognoses from those located elsewhere.
Questions/purposes
We sought risk factors predicting local recurrence, metastasis, and overall survival and asked whether radiation and chemotherapy influenced local recurrence, metastasis, and overall survival.
Methods
We retrospectively reviewed all 98 patients aged 18 years or younger diagnosed with soft tissue sarcomas in extremities from 1990 to 2008. Age, tumor size, depth, location, bone or neurovascular involvement, histologic subtypes, unplanned excision, surgical margins, metastasis at diagnosis, and adjuvant treatments were reviewed for each patient. We determined the effect of each prognostic variable on local recurrence, metastasis, and overall survival.
Results
Ninety-four patients underwent surgical excision and seven patients had local recurrence at a median time of 18.6 months. Radiation therapy reduced the rate of local recurrence. Fourteen patients had metastasis at diagnosis and seven patients later developed metastasis. The median time to metastasis was 20.9 months. Six patients died and the median time to death was 28.0 months. Metastasis at diagnosis was a predictive factor for death.
Conclusions
When limited to extremities, radiation therapy reduced the rate of local recurrence in pediatric soft tissue sarcomas. Metastases at diagnosis predict death.
Level of Evidence
Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Soft tissue sarcomas are a heterogeneous group of malignant tumors that account for approximately 7% of all childhood tumors [9]. Soft tissue sarcomas in children differ from those of adults in the spectrum of diagnoses; the various histologic subtypes behave differently [4, 21, 22]. Rhabdomyosarcoma, for example, accounts for almost half of all soft tissue sarcomas in children [10, 13]; however, it is exceedingly rare in adults. Rhabdomyosarcoma in adults has a poorer survival than in children [4, 21] and adult patients with synovial sarcomas have substantially higher mortality rates than those in the pediatric population [22].
Treatment strategies for pediatric soft tissue sarcomas differ somewhat from those for adults. In a child, avoidance of radiotherapy is always preferred and surgical excision is the major therapeutic modality. While radiation reportedly enhances local control [11], it is associated with greater risks including growth retardation and secondary radiation-induced malignancy in children compared with adults. Adjuvant or neoadjuvant chemotherapy improves overall survival and is considered the standard of care of patients with rhabdomyosarcoma and Ewing’s sarcomas/primitive neuroectodermal tumor (PNET) [3, 5], whereas the value of chemotherapy in adult sarcoma populations and pediatric nonrhabdomyosarcomas is less clear [10].
Sarcomas in extremities also have a differing spectrum of diagnoses and prognoses than those located elsewhere. For example, nonextremity sarcomas have higher mortality than those located in extremities [6]. When sarcomas are not surgical by limb preservation resections, radiotherapy and amputation are alternative choices for obtaining local control.
Because soft tissue sarcomas in children are rare and histologically heterogeneous, few large studies document clinical behaviors or outcomes; thus, it is difficult to ascertain standardized therapy for pediatric soft tissue sarcomas. There are several case series for patients with soft tissue sarcomas [11, 18, 19] but none that specifically determine what factors influence recurrences, metastases, and survival in childhood extremity sarcomas. However, based on the literature, we would expect (1) positive margins after surgical resection would be associated with an increase in local recurrence [1, 17] and that radiation therapy would reduce local recurrence [2]; that (2) adjuvant chemotherapy would reduce the rate of metastasis [14] and that local recurrence would be associated with an increase in the rate of metastasis [8]; and that (3), local recurrence [20] and metastasis at diagnosis [15] are risk factors for death.
We therefore determined whether possible risk factors (age, tumor size, depth, location, bone or vascular involvement, histologic subtypes, unplanned excision, and surgical margins) influenced local recurrence, metastasis, and survival for pediatric soft tissue sarcomas in extremities.
Patients and Methods
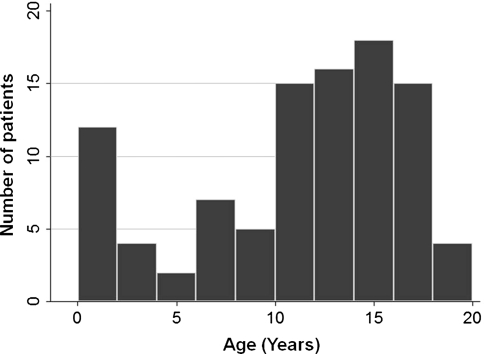
We retrospectively reviewed the charts of all 98 patients 18 years old or younger at diagnosis with soft tissue sarcomas in extremities from 1990 to 2008. Because treatment strategies, including adjuvant therapies, have been changed over the years, we excluded patients diagnosed before 1990. A tumor was considered to be in the upper extremity if it was at or beyond the shoulder and in the lower extremity if it was at or below the hip. Distal extremity tumors were those below the knee or elbow. We excluded patients with desmoid tumor or dermatofibrosarcoma protuberance in the study. Half of the patients (49 patients [50%]) received unplanned excision elsewhere before the initial visit. Fourteen patients had metastasis at diagnosis and seven patients subsequently developed metastasis. The median age of the study population was 12.5 years (Fig. 1). There were two peaks in the age distribution, one at age younger than 2 years and the other at the age of approximately 15 years. Therefore, we created two age categories: age younger than 2 years and age equal to or older than 2 years. There were 49 males (50%) and 49 females (50%). The most common anatomic site was the distal lower extremity (30%). Seventy-four percent of patients had tumors located beneath the fascia of the extremity. The most frequent histologic subtype in the extremity was synovial sarcoma (28 cases) followed by rhabdomyosarcoma (16 cases; Table 1). Other tumors consist of rare histologic subtypes, including malignant fibrous histiocytoma, spindle cell sarcoma, clear cell sarcoma, chondrosarcoma, liposarcoma, hemangiopericytoma, and undifferentiated sarcoma (Table 1). Six patients died during the followup period. The median time to death was 28.0 months (range, 7–52 months). The median time for followup was 61.5 months (range, 2–176 months). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from the medical records.
Fig. 1.
Age distributions are shown. There were two peaks in the age distribution: one is at age younger than 2 years, and the other is at the age of approximately 15 years.
Table 1.
Distribution of tumor and pathologic characteristics
| Tumor characteristic | Number | Percent |
|---|---|---|
| Age (years) | ||
| < 2 | 12 | 12 |
| ≥ 2 | 86 | 88 |
| Size (cm) | ||
| < 5 | 63 | 64 |
| ≥ 5 | 35 | 36 |
| Site | ||
| Upper extremity-proximal | 17 | 17 |
| Upper extremity-distal | 25 | 26 |
| Lower extremity-proximal | 27 | 28 |
| Lower extremity-distal | 29 | 30 |
| Depth | ||
| Superficial | 25 | 26 |
| Deep | 73 | 74 |
| Bone or neurovascular involvement | ||
| Absent | 62 | 63 |
| Present | 36 | 37 |
| Histology | ||
| Synovial sarcoma | 28 | 29 |
| Rhabdomyosarcoma | 16 | 16 |
| Fibrosarcoma | 9 | 9 |
| MPNST | 6 | 6 |
| Epithelioid sarcoma | 5 | 5 |
| Leiomyosarcoma | 5 | 5 |
| Ewing/PNET | 4 | 4 |
| Alveolar soft part sarcoma | 3 | 3 |
| Other tumors | 22 | 22 |
MPNST = malignant peripheral nerve sheath tumor; PNET = primitive neuroectodermal tumor.
Demographic data collected included gender, age at the time of diagnosis, and duration of followup. Tumor characteristics were tabulated with tumor size (less than 5 cm or 5 cm or greater), the site of primary tumor (upper proximal, upper distal, lower proximal, lower distal), depth (deep, superficial to fascia), invasiveness into bone or neurovascular structures (yes or no), histologic subtypes, and metastasis at diagnosis (yes or no). Tumor size was determined by the imaging studies at diagnosis using maximal diameter measured on CT or MRI. If patients had unplanned excision elsewhere, the tumor size was determined by outside images if available. Thirty-six percent of patients presented with tumors larger than or equal to 5 cm, whereas 64% had tumors less than 5 cm. We reviewed the pathology report when tumors were excised without preoperative imaging studies. We did not examine the histologic grade of the tumors, but the majority of the tumors were high-grade and 19 patients had low-grade tumors. We defined a tumor as invading bone or neurovascular structures if there was either gross or microscopic involvement by tumor at surgery or subsequent pathologic analysis. The most common histologic subtypes were synovial sarcoma (29%) followed by rhabdomyosarcoma (16%). Histologic diagnosis was confirmed by two pathologists (AR, AP-A) experienced in musculoskeletal pathology.
We recorded surgical procedures as well as adjuvant treatments administered. Data of unplanned tumor excision elsewhere before the initial visit (yes or no), surgical procedures (limb-sparing, amputation, none), chemotherapy (yes or no), and radiotherapy (yes or no) were collected. The indication for radiation varied over time and between institutions. Preoperative radiotherapy was used in instances when it was decided that a wide surgical margin could not be achieved without sacrificing neurovascular structures or important bony segments. Those who had surgical excision with positive margins were treated with either radiation or additional surgery. For patients who underwent definitive surgical resection of the primary tumor, we recorded microscopic margins (positive, negative). Surgery was the primary treatment in 94 patients. Limb-sparing surgery was performed in 90 patients (Table 2). Four patients were treated with amputations proximal to the wrist or ankle. Of 94 patients who were treated with surgical resection or amputation, 70 patients had microscopically negative margins. All patients treated by amputation had widely negative margins. Four patients did not receive surgical treatments, because one patient had unplanned excision with negative margins and three had metastasis elsewhere and surgery was not indicated.
Table 2.
Surgeries and adjuvant treatments
| Types of treatment | Number | Percent |
|---|---|---|
| Surgical procedure | ||
| Amputation | 4 | 4 |
| Limb-sparing procedure | 90 | 92 |
| None | 4 | 4 |
| Chemotherapy | ||
| Yes | 38 | 39 |
| No | 60 | 61 |
| Radiation | ||
| Yes | 44 | 45 |
| No | 54 | 55 |
Local recurrence of tumor, distant recurrence (metastasis), and overall survival were recorded with the time of failure. We defined local control as the time interval between surgical resection or amputation and the detection of local recurrence of disease. The duration of metastasis was the time between diagnosis and the detection of metastasis. The duration of survival was defined as the time interval between diagnosis and death from any cause or date of most recent followup. The effect of each variable (size, depth, location, bone or neurovascular involvement, unplanned tumor excision, margins) and treatments (chemotherapy and radiotherapy) on survival and event-free survival was examined in Cox proportional hazard models. Because all patients with rhabdomyosarcoma and Ewing’s sarcomas/PNET were treated with neoadjuvant and adjuvant chemotherapy, they were excluded from the Cox regression analysis to evaluate the effect of chemotherapy so as not to induce collinearity for histologic subtypes and indication for chemotherapy since they are strongly correlated and the results possibly biased by histologic subtypes. The effect of chemotherapy on overall survival was excluded from the analysis because all who died of tumor-related causes received chemotherapy. All variables significant at p ≤ 0.20 in the univariate analysis, age younger than 2 years or 2 years or older and gender were entered into a multivariate model. In multivariate Cox regression, p ≤ 0.05 was considered to be significant. We used Fisher’s exact test to compare two categorical groups when sample size was small and Cox regression was not applicable. Log-rank test was used to compare more than two categorical groups such as histologic subtypes and the effect of surgical margins on metastasis. All data were analyzed using statistical software Stata 10 (StataCorp, College Station, TX).
Results
Over the followup time, seven of the 94 patients who had surgical excision had recurrences. The median time to development of local recurrence was 18.6 months (range, 4–144 months). In the initial multivariate analysis, unplanned excision and radiation therapy reduced the rate for local recurrence (Table 3). Controlling for confounding factors, surgical margins did not predict local recurrence. None of the patients treated by amputation had a local recurrence.
Table 3.
Factors predictive of local recurrence
| Variables | Number of patients | Univariate p | RR | Multivariate p | RR |
|---|---|---|---|---|---|
| Age at diagnosis | |||||
| < 2 years | 12 | ||||
| ≥ 2 years | 82 | 0.67 | 0.62 | ||
| Tumor size | |||||
| < 5 cm | 61 | ||||
| ≥ 5 cm | 33 | 0.86 | 1.14 | ||
| Depth | |||||
| Superficial | 24 | ||||
| Deep | 70 | 0.74 | 1.44 | ||
| Primary tumor site | |||||
| Upper extremity | 55 | 0.22 | 2.55 | ||
| Lower extremity | 39 | ||||
| Proximity | |||||
| Proximal | 51 | 0.56 | 1.56 | ||
| Distal | 43 | ||||
| Bone or neurovascular involvement | |||||
| Absent | 59 | ||||
| Present | 35 | 0.76 | 1.27 | ||
| Histology | |||||
| Synovial sarcoma | 28 | ||||
| Rhabdomyosarcoma | 13 | ||||
| Fibrosarcoma | 9 | ||||
| MPNST | 6 | ||||
| Others | 38 | 0.14* | |||
| Unplanned excision | |||||
| Yes | 48 | 0.078 | 0.14 | 0.032 | 0.054 |
| No | 46 | ||||
| Surgical margins | |||||
| Negative | 70 | ||||
| Positive | 24 | 0.17 | 2.85 | 0.18 | 3.22 |
| Radiation therapy | |||||
| Yes | 42 | 0.09 | 0.14 | 0.037 | 0.045 |
| No | 52 | ||||
| Chemotherapy (without rhabdomyosarcoma and Ewing/PNET) | |||||
| Yes | 18 | 0.44 | 2.17 | ||
| No | 59 | ||||
* Log rank tests of no differences among categories versus any differences among categories; RR = rate ratio; MPNST = malignant peripheral nerve sheath tumor; PNET = primitive neuroectodermal tumor.
Of 84 patients who did not have metastasis at initial diagnosis, seven patients developed distant metastasis. The median time to development of metastasis was 20.9 months (range, 6–142 months). Two of these patients had distant metastasis that occurred synchronously with or subsequent to local recurrence. More patients (p = 0.02) developed metastasis with tumor in lower extremities compared with those in upper extremities. In the univariate Cox regression analysis, local recurrence was a major adverse prognostic factor for metastasis with a rate ratio (RR) of 3.13 (95% confidence interval [CI], 0.58–16.9; Table 4); however, when controlling other patient characteristics, including categorical age and gender in the regression model, local recurrence did not increase the rate for metastasis.
Table 4.
Factors predictive of metastasis
| Variables | Number of patients | Univariate p | RR | Multivariate p | RR |
|---|---|---|---|---|---|
| Age at diagnosis | |||||
| < 2 years | 9 | ||||
| ≥ 2 years | 75 | 0.44† | |||
| Tumor size | |||||
| < 5 cm | 56 | ||||
| ≥ 5 cm | 28 | 0.73 | 1.31 | ||
| Depth | |||||
| Superficial | 24 | ||||
| Deep | 60 | 0.28 | 0.41 | ||
| Primary tumor site | |||||
| Upper extremity | 35 | ||||
| Lower extremity | 49 | 0.02† | |||
| Proximity | |||||
| Proximal | 40 | 0.48 | 1.74 | ||
| Distal | 44 | ||||
| Bone or neurovascular involvement | |||||
| Absent | 56 | ||||
| Present | 28 | 0.82 | 0.83 | ||
| Histology | |||||
| Synovial sarcoma | 27 | ||||
| Rhabdomyosarcoma | 9 | ||||
| Fibrosarcoma | 9 | ||||
| MPNST | 6 | ||||
| Others | 33 | 0.74* | |||
| Unplanned excision | |||||
| Yes | 46 | 0.85 | 1.15 | ||
| No | 38 | ||||
| Surgical margins | |||||
| Negative | 62 | ||||
| Positive | 21 | ||||
| No surgery | 1 | 0.30* | |||
| Radiation therapy | |||||
| Yes | 33 | 0.96 | 0.96 | ||
| No | 51 | ||||
| Chemotherapy (without rhabdomyosarcoma and Ewing/PNET) | |||||
| Yes | 13 | 0.77 | 0.71 | ||
| No | 59 | ||||
| Local recurrence | |||||
| Yes | 7 | 0.18 | 3.13 | 0.11 | 4.14 |
| No | 77 | ||||
* Log rank tests of no differences among categories versus any differences among categories; †Fisher’s exact test of no differences between two categories versus any differences between two categories; RR = rate ratio; MPNST = malignant peripheral nerve sheath tumor; PNET = primitive neuroectodermal tumor.
Metastasis at diagnosis most strongly predicted death with a RR of 38.1 (95% CI, 3.42–424; Table 5). Tumor size and unplanned excision did not predict death after controlling confounding.
Table 5.
Factors predictive of survival
| Variables | Number of patients | Univariate p | RR | Multivariate p | RR |
|---|---|---|---|---|---|
| Age at diagnosis | |||||
| < 2 years | 12 | ||||
| ≥ 2 years | 86 | 0.78 | 0.73 | ||
| Tumor size | |||||
| < 5 cm | 63 | ||||
| ≥ 5 cm | 35 | 0.13 | 3.74 | 0.76 | 0.68 |
| Depth | |||||
| Superficial | 25 | ||||
| Deep | 73 | 0.74 | 0.75 | ||
| Primary tumor site | |||||
| Upper extremity | 56 | 0.68 | 0.70 | ||
| Lower extremity | 42 | ||||
| Proximity | |||||
| Proximal | 54 | 0.53 | 0.58 | ||
| Distal | 44 | ||||
| Bone or neurovascular involvement | |||||
| Absent | 62 | ||||
| Present | 36 | 0.36 | 2.10 | ||
| Histology | |||||
| Synovial sarcoma | 28 | ||||
| Rhabdomyosarcoma | 16 | ||||
| Fibrosarcoma | 9 | ||||
| MPNST | 6 | ||||
| Others | 39 | 0.13* | |||
| Unplanned excision | |||||
| Yes | 49 | 0.12 | 0.18 | 0.49 | 0.37 |
| No | 49 | ||||
| Surgical margins | |||||
| Negative | 70 | ||||
| Positive | 24 | 0.30† | |||
| Radiation therapy | |||||
| Yes | 44 | 0.73 | 1.33 | ||
| No | 54 | ||||
| Local recurrence | |||||
| Yes | 7 | 0.73† | |||
| No | 77 | ||||
| Metastasis at diagnosis | |||||
| Yes | 14 | 0.001 | 37.0 | 0.003 | 38.1 |
| No | 84 | ||||
* Log rank tests of no differences among categories versus any differences among categories; †Fisher’s exact test of no differences between two categories versus any differences between two categories; RR = rate ratio; MPNST = malignant peripheral nerve sheath tumor.
Discussion
Soft tissue sarcomas in children are rare and differ from those of adults in the spectrum of diagnoses and treatment strategies. We therefore determined whether possible risk factors (age, tumor size, depth, location, bone or vascular involvement, histologic subtypes, unplanned excision, and surgical margins) influenced recurrence, metastasis, and survival for pediatric soft tissue sarcomas in extremities.
We acknowledge limitations in this study. First is the limited number of patients with each histologic subtype. It would be ideal to evaluate different pathologic subtypes separately; however, pediatric soft tissue sarcomas are exceedingly rare and the numbers of each subtype are even rarer. We found the histologic subtypes did not predict local recurrence, metastasis, and survival but the chance of a Type II error is substantial. Nonetheless, ours is a relatively large series of soft tissue sarcomas in extremities in children and except for the caveat of histologic subtypes, 98 patients offered sufficient numbers to identify differences in other prognostic factors. Second, other reports have emphasized the histologic grade of the tumor; we did not use histologic grade as a potential prognostic factor. Histologic subtypes strongly correlate with grade. For example, all synovial sarcomas and rhabdomyosarcomas are considered high-grade. If we had used histologic grade as a prognostic factor as well, this would have induced collinearity in the analysis. Instead, we evaluated each histologic subtype as a variable because some histologic subtypes have known clinical outcomes that differ from others. For example, one adult study found leiomyosarcoma independently predicted metastasis and malignant peripheral nerve sheath tumor predicted overall survival [15]. Third, followup time for each patient varied in the study. This is because we evaluated risk factors for local recurrence, metastasis, and death using Cox regression and person-time data; we enrolled all patients seen between the study periods to avoid selection bias. The median time for followup was 61.5 months and it should be sufficiently long because the median time to develop local recurrence was 18.6 months. The median was 20.9 months for metastasis and 28.0 months for death.
We found local recurrence did not increase the rate for metastasis. The causal relationship between local recurrence and metastasis is controversial [23]. The relationship may be different for tumors in extremities than for tumors elsewhere because local recurrence can usually be treated with amputation for tumors in extremities, whereas radiation therapy may be the only modality for unresectable tumors in the trunk and other nonextremity sites. In our series, chemotherapy did not reduce the rate for metastasis.
We found only metastasis at diagnosis predicted death. Metastasis at presentation is reportedly a risk factor for death for synovial sarcoma [12] and adult soft tissue sarcoma of extremities [15]. One study suggested tumor size and positive surgical margin predicted death in truncal and retroperitoneal tumors [17]. In our series limited to the extremities, these and other factors may not have predicted death for two reasons. One is sarcomas in extremities can be excised with amputations if needed to achieve local control and that characteristics of the tumor itself do not affect the overall survival. Second is the limited power of this study to detect differences resulting from a small number of patients.
Radiation therapy reduced the rate of local recurrence. This result is consistent with previous literature [2]. Although we did not find any radiation-related complications in our patients, in a child, avoidance of radiotherapy is always preferred if adequate local control can be achieved by surgical resection alone. However, in the treatment of high-grade soft tissue sarcomas, especially those that are large and or inadequately resected, the addition of radiotherapy with surgery can maximize local control while avoiding amputation. Conformal techniques for treatment have been in use for many decades and these can limit toxicities. However, more technologic advances, including intensity-modulated radiation therapy and proton beam radiation therapy, can decrease further the late effects by minimizing the normal tissue exposure. Microscopically positive margins have been reported to increase the risk for local recurrence [1, 17]. In our study, positive margins did not increase the rate for local recurrence in multivariate analysis. We are relatively aggressive in using adjuvant therapies in patients with positive margins. Most such patients were treated with either another tumor bed excision or radiation therapy. In patients who received tumor bed excision or radiation therapy, a positive margin may lose its effect on local recurrence. Radiation therapy was effective in controlling local recurrence in our series. Surprisingly, those who received unplanned excision at outside facilities had a lower rate for local recurrence compared with those who received initial surgical treatment at our hospitals. In the literature, those who were referred to a cancer center and underwent a second operation had an improved disease-free survival rate [7]. This observation may be the result of the likelihood that large tumors in deep locations were more likely referred to us without any surgical treatments than smaller superficial tumors. Therefore, the data were reanalyzed adjusting for tumor size and depth in addition to age and gender and still patients who underwent unplanned excision experienced considerably fewer local recurrences (data not shown). Another possible explanation is most of patients who had unplanned excisions were treated with tumor bed excisions at our facilities, which achieved wider margins, but the precise explanation of this observation remains unclear. Of note, tumors located superficial to the fascia are not always benign as suggested by the observation that one-fourth of sarcomas located superficial to the fascia in our series were malignant. It is reported that duration of symptoms before diagnosis does not predict the sarcoma size and prognosis [16]. Although we did not find a higher local recurrence rate for those who had unplanned excisions, superficial tumors without longstanding symptoms should be treated with caution.
In conclusion, we observed that radiation therapy reduced the rate for local recurrence in pediatric soft tissue sarcoma in extremities and that metastasis at diagnosis predicted death. Histologic subtypes, tumor location, size, depth, bone or neurovascular involvement, and positive surgical margins were not risk factors for local recurrence, metastasis, or overall survival in this series of pediatric extremity sarcomas.
Acknowledgments
We thank Andrew E. Rosenberg, MD, for evaluation of pathologic specimens.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Massachusetts General Hospital, Children’s Hospital, Boston, MA, USA.
References
- 1.Bell RS, O’Sullivan B, Liu FF, Powell J, Langer F, Fornasier VL, Cummings B, Miceli PN, Hawkins N, Quirt I, et al. The surgical margin in soft-tissue sarcoma. J Bone Joint Surg Am. 1989;71:370–375. [PubMed] [Google Scholar]
- 2.Blakely ML, Spurbeck WW, Pappo AS, Pratt CB, Rodriguez-Galindo C, Santana VM, Merchant TE, Prichard M, Rao BN. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg. 1999;34:672–675. doi: 10.1016/S0022-3468(99)90353-6. [DOI] [PubMed] [Google Scholar]
- 3.Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, Breneman J, Qualman SJ, Wiener E, Wharam M, Lobe T, Webber B, Maurer HM, Donaldson SS. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari A, Dileo P, Casanova M, Bertulli R, Meazza C, Gandola L, Navarria P, Collini P, Gronchi A, Olmi P, Fossati-Bellani F, Casali PG. Rhabdomyosarcoma in adults. A retrospective analysis of 171 patients treated at a single institution. Cancer. 2003;98:571–580. doi: 10.1002/cncr.11550. [DOI] [PubMed] [Google Scholar]
- 5.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Miser JS. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 6.Guadagnolo BA, Zagars GK, Ballo MT, Strom SS, Pollock RE, Benjamin RS. Mortality after cure of soft-tissue sarcoma treated with conservation surgery and radiotherapy. Cancer. 2008;113:411–418. doi: 10.1002/cncr.23593. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effect of reresection in extremity soft tissue sarcoma. Ann Surg. 2000;231:655–663. doi: 10.1097/00000658-200005000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15:646–652. doi: 10.1200/JCO.1997.15.2.646. [DOI] [PubMed] [Google Scholar]
- 9.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113:2575–2596. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeb DM, Thornton K, Shokek O. Pediatric soft tissue sarcomas. Surg Clin North Am. 2008;88:615–627. doi: 10.1016/j.suc.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus KC, Grier HE, Shamberger RC, Gebhardt MC, Perez-Atayde A, Silver B, Tarbell NJ. Childhood soft tissue sarcoma: a 20-year experience. J Pediatr. 1997;131:603–607. doi: 10.1016/S0022-3476(97)70070-2. [DOI] [PubMed] [Google Scholar]
- 12.Palmerini E, Staals EL, Alberghini M, Zanella L, Ferrari C, Benassi MS, Picci P, Mercuri M, Bacci G, Ferrari S. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer. 2009;115:2988–2998. doi: 10.1002/cncr.24370. [DOI] [PubMed] [Google Scholar]
- 13.Pappo AS, Shapiro DN, Crist WM. Rhabdomyosarcoma. Biology and treatment. Pediatr Clin North Am. 1997;44:953–972. doi: 10.1016/S0031-3955(05)70539-3. [DOI] [PubMed] [Google Scholar]
- 14.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 15.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1, 041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 16.Rougraff BT, Davis K, Lawrence J. Does length of symptoms before diagnosis of sarcoma affect patient survival? Clin Orthop Relat Res. 2007;462:181–189. doi: 10.1097/BLO.0b013e3180f62608. [DOI] [PubMed] [Google Scholar]
- 17.Singer S, Corson JM, Demetri GD, Healey EA, Marcus K, Eberlein TJ. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg. 1995;221:185–195. doi: 10.1097/00000658-199502000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spunt SL, Hill DA, Motosue AM, Billups CA, Cain AM, Rao BN, Pratt CB, Merchant TE, Pappo AS. Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol. 2002;20:3225–3235. doi: 10.1200/JCO.2002.06.066. [DOI] [PubMed] [Google Scholar]
- 19.Spunt SL, Poquette CA, Hurt YS, Cain AM, Rao BN, Merchant TE, Jenkins JJ, Santana VM, Pratt CB, Pappo AS. Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children’s Research Hospital. J Clin Oncol. 1999;17:3697–3705. doi: 10.1200/JCO.1999.17.12.3697. [DOI] [PubMed] [Google Scholar]
- 20.Stotter AT, A’Hern RP, Fisher C, Mott AF, Fallowfield ME, Westbury G. The influence of local recurrence of extremity soft tissue sarcoma on metastasis and survival. Cancer. 1990;65:1119–1129. doi: 10.1002/1097-0142(19900301)65:5<1119::AID-CNCR2820650515>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2, 600 patients. J Clin Oncol. 2009;27:3391–3397. doi: 10.1200/JCO.2008.19.7483. [DOI] [PubMed] [Google Scholar]
- 22.Sultan I, Rodriguez-Galindo C, Saab R, Yasir S, Casanova M, Ferrari A. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer. 2009;115:3537–3547. doi: 10.1002/cncr.24424. [DOI] [PubMed] [Google Scholar]
- 23.Trovik CS, Bauer HC. Local recurrence after surgery for soft tissue sarcoma. The Scandinavian Sarcoma Group experience. Acta Orthop Scand Suppl. 1999;285:45–46. doi: 10.1080/17453674.1999.11744822. [DOI] [PubMed] [Google Scholar]