History and Physical Examination
A 30-year-old woman with a history of chronic congenital lymphedema and overgrowth of the right upper extremity presented with a visibly inflamed, reddened, nodular, palpable mass arising from the skin on the dorsal aspect of her right forearm of 9 months duration. The patient described the initial appearance as three small pimples on the skin of the forearm that coalesced and became larger. There were no fevers, weight loss, or night sweats. The patient was otherwise healthy. Eventually the mass became tender and painful, which caused her to seek medical attention. On presentation, there was an irregular, nodular, nontender, cutaneous mass that was approximately 3 × 3 cm surrounded by a 1-cm halo of erythema. Sensation and motor function were grossly intact. She had no history of malignancy or radiation treatments. Surgical history was significant for several surgical debulking procedures and liposuction of the right forearm and arm. The entire upper extremity from shoulder through the fingers was diffusely enlarged approximately four times the circumference of her contralateral normal upper extremity. It seemed to involve the skin and subcutaneous tissue. There were no palpable enlarged lymph nodes in the axilla or epitrochlear region although these areas were difficult to palpate because of the chronic lymphedema. Laboratory studies showed complete blood count, chemistries, coagulation analysis, erythrocyte sedimentation rate, and C-reactive protein were all normal.
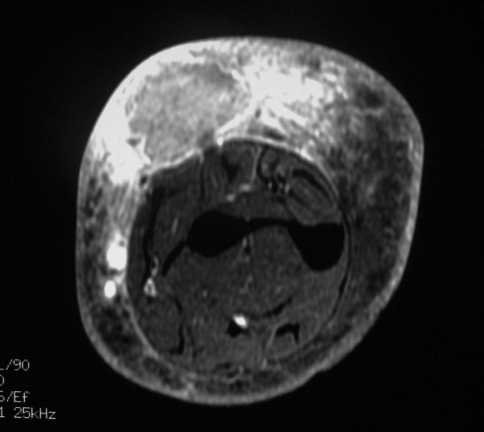
MRI was performed (Figs. 1–3).
Fig. 1.
An axial T1-weighted MR image shows the somewhat poorly defined mass in the dorsal subcutaneous soft tissues of the forearm, which is isointense to low in signal compared with the underlying muscle. The high signal centrally likely attributable to hemorrhage and the prominent surrounding low signal edema can be seen.
Fig. 3.
An axial T1-weighted MR image with fat saturation after the intravenous administration of gadolinium shows heterogeneous enhancement of the mass and exuberant enhancement in the surrounding soft tissues.
Based on the history, physical examination, and imaging studies, what is the differential diagnosis?
Imaging Interpretation
MRI of the right forearm showed a mass involving the skin and subcutaneous tissue along the extensor surface of the midforearm that measured approximately 2.7 × 2.4 × 1.8 cm. The mass abutted but did not penetrate the superficial fascia along the posterior compartment of the forearm. The mass was heterogeneous but predominantly intermediate signal on T1 (Fig. 1) and high signal on T2 with a thin low signal intensity rim on the T2-weighted images (Fig. 2). High signal in the center of the mass on both sequences suggested blood products from hemorrhage. There was extensive edema in the surrounding subcutaneous soft tissues and thickening of the overlying skin with no involvement of the intramuscular compartments. The mass enhanced heterogeneously after gadolinium administration, with substantial enhancement in the surrounding subcutaneous soft tissues (Fig. 3).
Fig. 2.
An axial T2-weighted MR image with fat saturation shows the heterogeneous, but predominantly high signal mass with a thin, peripheral low-signal-intensity rim and marked surrounding soft tissue edema.
Differential Diagnosis
Undifferentiated pleomorphic sarcoma
Skin cancer
Fibrosarcoma
Abscess
Angiosarcoma
A core biopsy was performed and the histologic specimen was analyzed (Figs. 4–6).
Fig. 4.
A photomicrograph shows frequent round to polygonal tumor cells with ample light cytoplasm and well-defined cell borders (Stain, hematoxylin and eosin; original magnification, ×60).
Fig. 6.
Extensive lymphatic permeation by the lesion is shown (Stain, hematoxylin and eosin; original magnification, ×20).
Based on the history, physical examination, laboratory studies, imaging studies, and histology, what is the diagnosis and how should this lesion be treated?
Histology Interpretation
Microscopic pathologic analysis showed a hypercellular neoplasm composed primarily of large polygonal cells with abundant light eosinophilic cytoplasm and round to ovoid vesicular nuclei with discernible nucleoli (Fig. 4). The cells were arranged in solid sheets and formed rare slitlike spaces or poorly formed lumina. There was high cellularity with more than 20 mitoses per 10 high-power fields. There were no areas of necrosis. Extensive vascular permeation by tumor emboli also was seen (Figs. 5, 6). Immunohistochemical analysis was performed, which revealed the tumor to be strong and diffusely positive for vimentin, and endothelial marker CD31, focally positive for CD34, and negative for CK7, CK20, CD45, S100, estrogen, progesterone, desmin, and smooth muscle actin.
Fig. 5.
Conspicuous vascular invasion by the lesion is shown (Stain, hematoxylin and eosin; original magnification, ×4).
Diagnosis
Epithelioid angiosarcoma
Discussion and Treatment
Angiosarcoma is a rare high-grade sarcoma that occasionally arises in association with chronic lymphedema and/or radiation therapy [15, 32]. Many patients treated for breast cancer have chronic upper extremity lymphedema; this treatment has included radical lymph node dissections followed by radiation therapy to the axillary region [25, 27]. Angiosarcomas are aggressive neoplasms and are associated with a high rate of metastases [28]. Most high-grade soft tissue sarcomas that are less than 5 cm in dimension and confined to the subcutaneous tissue are curative with surgery alone or in combination with radiation therapy [3, 7, 8, 21]. Despite being small and confined to the subcutaneous tissues, this patient had metastatic disease develop in a relatively short time after surgery. One poor prognostic feature identified on pathologic analysis was that tumor emboli were identified in adjacent vascular channels [24].
The differential diagnosis based on clinical examination and radiologic imaging included various skin cancers (squamous cell carcinoma, basal cell carcinoma, amelanotic melanoma), fibrosarcoma, abscess, and undifferentiated pleomorphic sarcoma [9]. Various types of skin cancer can have similar clinical and radiologic appearances [9]. These cancers can appear nodular, reddened, indurated, and inflamed; they also can penetrate the subcutaneous tissues. The aforementioned skin cancers do not stain positive for vimentin and have characteristic histologic features, distinctly different from those of an angiosarcoma [2, 10–12, 26, 31]. Histologically, amelanotic melanoma is characterized by increased numbers of intraepithelial melanocytes arranged irregularly around the dermoepidermal junction with associated invasive nests of highly atypical cells with prominent nucleoli and numerous mitoses [12, 31]. It also has a characteristic immunohistochemical profile, staining positive for S-100 protein, HMB-45, and Melan A [12, 31]. T1-weighted MR images of melanoma give a high signal intensity for melanoma deposits although this should not be the case for amelanotic melanoma [4, 20]. Undifferentiated pleomorphic sarcoma (MFH) and fibrosarcoma are two of the most common types of sarcomas to arise from the subcutaneous tissue [30]. They can present in a clinically similar manner as the tumor in our patient. These entities usually are firm, immobile, and may appear diffuse and nodular [13]. They may appear inflamed and usually are nontender [30]. The MRI appearance of our patient’s tumor was not specific for any particular entity although possibly suggested a high-grade sarcoma. Most high-grade sarcomas are intermediate signal on T1-weighted images and heterogeneous on T2-weighted and gadolinium-enhanced images [4, 20, 23]. In many instances, the deep fascia usually provides a boundary to tumor extension and protects the underlying musculature [23]. Therefore, undifferentiated pleomorphic sarcoma (MFH), fibrosarcoma, and angiosarcoma can have a similar appearance on MRI [20]. Histologically, fibrosarcomas are composed of predominantly spindled fibroblasts that stain positive for vimentin, arranged in a herringbone fascicular pattern and surrounded by a collagen matrix of birefringent wavy fibers [13, 17, 30]. The appearance of a fibrosarcoma is dependent on grade with higher-grade tumors showing more cellularity, pleomorphism, and mitotic figures. Histologically, undifferentiated pleomorphic sarcomas are characterized by storiform to haphazard growth pattern, presence of multinucleated bizarre giant cells, and a mixture of pleomorphic fibroblasts, histocyte-like cells, and primitive mesenchymal cells [30]. Undifferentiated pleomorphic sarcomas also stain positive for vimentin [30]. High-grade fibrosarcomas can have a similar histologic appearance as an undifferentiated pleomorphic sarcoma. Undifferentiated pleomorphic sarcoma has been included in earlier reports of poorly differentiated fibrosarcoma [30]. Transition between the two has been described suggesting a form of tumor progression in some cases [30]. The dividing line between a high-grade fibrosarcoma and an undifferentiated pleomorphic sarcoma is quite subjective [30]. In either case, the histologic appearance of a fibrosarcoma and an undifferentiated pleomorphic sarcoma and the immunohistochemical profile of both entites are distinctly different from those of an epithelioid angiosarcoma [30]. Patients with chronic lymphedema also are predisposed to infection and abscess formation. [16]. The clinically indolent course, minimal tenderness to palpation, lack of fever, and normal laboratory results mitigated against an infection in this patient. Histologically, an abscess consists of neutrophils. An abscess typically would appear on MRI as either a homogeneous or heterogeneous mass with low signal intensity on short TR images and slightly higher signal intensity on the longer TR images [29]. Abscesses usually are surrounded by a capsular wall and do not enhance internally with contrast [29].
Soft tissue angiosarcomas are rare malignant tumors composed of rapidly dividing anaplastic cells that recapitulate many of the functional and morphologic features of normal endothelial cells [30]. The most common sites for soft tissue angiosarcoma are the lower extremity, followed by the upper extremity, trunk, and neck [22]. The peak incidence is during the seventh decade [28]. The risk for angiosarcoma is increased in women who have had radical mastectomy with axillary lymph node dissection and radiation treatment for breast cancer [6]. Most of these women also have chronic lymphedema in the treated extremity related to their surgery (Stuart-Treves syndrome) [32]. At 10 years, 0.1% to 0.2% of women who have received radiation treatment for breast cancer have a soft tissue angiosarcoma develop [13]. It has not been determined whether chronic lymphedema, radiation, or both lead to an increased risk of angiosarcoma [6, 32].
Histopathologically, angiosarcomas are composed of vascular spaces lined by atypical tumor cells. Mitoses occur at rates greater than three per high-powered field and are frequently abnormal [11]. Cells typically organize into irregular sheets. The gold standard for diagnosis is centered on immunochemistry for select vascular markers including CD31, CD34, and FVIII-Rag [21]. CD31 is the most specific marker for endothelial cells [26, 30]. Folpe et al. reported Fli-1 originally for Ewing’s sarcoma, expressed in the nucleus of vascular endothelium of normal vessels and benign and malignant vascular tumors [10]. D2-40 (Podoplanin) is mostly specific for lymphatic endothelium but is coexpressed in angiosarcoma [2]. Angiosarcomas, like all mesenchymal tumors, are vimentin positive [30].
The prognosis and long-term survival for patients with a soft tissue angiosarcoma depends on size, depth, and grade [7, 8]. Sarcomas that are high grade, deep to the fascia, and greater than 5 cm are considered high-risk tumors and are associated with approximately 50% to 65% survival at 5 years [8]. High-grade sarcomas less than 5 cm and superficial to the fascia are associated with a better prognosis, as much as 85% to 90% 5-year survival [7, 8]. Lymphovascular invasion, as identified in this patient, with tumor thrombi in adjacent veins is a negative prognostic factor that provokes major concern for further disseminated disease [8, 24]. In the case of angiosarcomas, there is no difference in survival between superficial and deep tumors in patients with a history of breast cancer, mastectomy, or radiation [7].
Surgery is the mainstay of treatment for angiosarcomas. Current recommended surgical treatment for a soft tissue angiosarcoma is to perform a wide surgical excision [14]. Occasionally an amputation may be required. Adjuvant radiation therapy typically is recommended for high-grade tumors that are deep to the fascia and greater than 5 cm in diameter to improve local control [3]. In this case it was recommended because of the close margins, extensive surrounding erythema-altered anatomy, and lymphovascular invasion. Surgical resections with positive margins and patients older than 50 years are associated with high rates of local recurrence, even with adjuvant radiation therapy [7, 8]. Treatment is controversial when patients already have received radiation therapy to the affected area. Some argue exposing an area to additional radiation increases the risk for local recurrence of not only the same cancer but also other types of malignancies [33]. Amputation has been suggested as a possible treatment when radiation exposure is a concern [14].
Chemotherapy has not been definitively shown to improve survival in patients with high-risk soft tissue sarcomas [5]. There is less support for administering chemotherapy for soft tissue sarcomas that are subcutaneous and relatively small as 85% to 90% of patients are alive at 5 years with surgery alone or with surgery combined with radiation [5, 33]. There is no conclusive evidence in the literature documenting improved survival for patients with angiosarcoma treated with any chemotherapy regimens; however, because of the high risk of metastatic disease developing, further investigation of various regimens including antiangiogenic therapy is needed.
Our patient was treated with a wide surgical excision that included skin, subcutaneous tissue, underlying fascia, and a thin layer of muscle for at least 2 cm beyond all borders of any erythema and 3.5 cm from any palpable tumor. The tumor was removed en bloc with the surrounding tissues. The final surgical pathology slides showed a high-grade epithelioid angiosarcoma with a maximum tumor size of 5 cm. The tumor extended into several large-caliber blood vessels and was associated with conspicuous lymphovascular invasion. Intralymphatic tumor emboli also were seen 0.5 mm from the deep aspect of the specimen. The margins were free of disease. This tumor was classified as a Stage III (T2b, N0, M0) high-grade epithelioid angiosarcoma. The patient was treated postoperatively with radiation using 185 cGy per fraction with high-energy photons. The beam was modified to accommodate for the chronic lymphedema. She did not have any unusual complications from the radiation.
It was controversial regarding whether chemotherapy should be administered given the size and superficial location of the tumor and whether the chronic lymphedema, altered anatomy, and lymphovascular invasion would increase the risk for metastatic spread. Some medical experts would consider our patient at greater risk for metastatic spread compared with other individuals with subcutaneous sarcomas of similar size and grade because of the altered anatomy, extensive lymphovascular invasion, and presence of tumor in an adjacent large vein.
After discussion with the patient, she was started on adjuvant Avastin® (bevacizumab; Genentech, Inc, South San Francisco, CA, USA) treatments while receiving local radiation. Avastin® works as a humanized monoclonal antibody to VEGF, which is secreted by angiosarcoma tumor cells, and to promote angiogenesis in these and other tumors. Serum levels of VEGF are higher in patients with angiosarcomas than in control subjects [1]. There have been two case reports involving two patients in whom Avastin® was administered preoperatively for angiosarcomas of the nose. There was a complete response in both patients [18]. Although the role of adjuvant chemotherapy in the treatment of angiosarcoma remains unclear, we believe there is some role for antiangiogenic treatments. In one case report, the authors reported a complete response with single-agent metronomic trofosfamide, a nitrogen mustard analog that works by disrupting DNA in actively dividing angiogenic cells [19]. These authors also suggested possible efficacy of other antiangiogenic agents such as cyclooxygenase II inhibitors and peroxisome proliferator-activated receptor-gamma agonists [19].
Our patient had a history of chronic congenital lymphedema/overgrowth of the affected extremity. She never received any form of radiation to the extremity. Therefore it is plausible to assume chronic lymphedema alone without radiation can predispose a person to having angiosarcoma develop. Despite the tumor size (5 cm), superficial location, patient age, clear margins, absence of metastases at presentation, and aggressive surgical and medical management, the patient had metastatic disease develop in the proximal femur and lungs 7 months after the surgery. After these lesions appeared, her femur was treated with 5040 cGy of radiotherapy and she began systemic chemotherapy consisting of a combination of Taxol® (Bristol-Myers Squibb, New York, NY, USA), Doxil® (Centocor Ortho Biotech Products LP, Horsham, PA, USA), and Avastin®. The patient died 15 months after surgery owing to progression of metastatic disease indicative of the aggressive nature of this type of cancer.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the reporting of this case report, that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Mount Sinai Medical Center.
References
- 1.Amo Y, Masuzawa M, Hamada Y, Katsuoka K. Serum concentrations of vascular endothelial growth factor-D in angiosarcoma patients. Br J Dermatol. 2004;150:160–161. doi: 10.1111/j.1365-2133.2004.05751.x. [DOI] [PubMed] [Google Scholar]
- 2.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budd GT. Management of angiosarcoma. Curr Oncol Rep. 2002;4:515–519. doi: 10.1007/s11912-002-0066-3. [DOI] [PubMed] [Google Scholar]
- 4.Dancey AL, Mahon BS, Rayatt SS. A review of diagnostic imaging in melanoma. J Plast Reconstr Aesthet Surg. 2008;61:1275–1283. doi: 10.1016/j.bjps.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 5.DeMartelaere SL, Roberts D, Burgess MA, Morrison WH, Pisters PW, Sturgis EM, Ho V, Esmaeli B. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639–646. doi: 10.1002/hed.20757. [DOI] [PubMed] [Google Scholar]
- 6.Di Tommaso L, Fabbri A. Cutaneous angiosarcoma arising after radiotherapy treatment of a breast carcinoma: description of a case and review of the literature][in Italian. Pathologica. 2003;95:196–202. [PubMed] [Google Scholar]
- 7.Eeles RA, Fisher C, A’Hern RP, Robinson M, Rhys-Evans P, Henk JM, Archer D, Harmer CL. Head and neck sarcomas: prognostic factors and implications for treatment. Br J Cancer. 1993;68:201–207. doi: 10.1038/bjc.1993.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espat NJ, Lewis JJ, Woodruff JM, Antonescu C, Xia J, Leung D, Brennan MF. Confirmed angiosarcoma: prognostic factors and outcome in 50 prospectively followed patients. Sarcoma. 2000;4:173–177. doi: 10.1155/2000/575781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher CD, Beham A, Bekir S, Clarke AM, Marley NJ. Epithelioid angiosarcoma of deep soft tissue: a distinctive tumor readily mistaken for an epithelial neoplasm. Am J Surg Pathol. 1991;15:915–924. doi: 10.1097/00000478-199110000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Folpe AL, Chand EM, Goldblum JR, Weiss SW. Expession of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasm from potential mimics. Am J Surg Pathol. 2001;25:1061–1066. doi: 10.1097/00000478-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Gao Z, Kahn LB. The application of immunohistochemistry in the diagnosis of bone tumors and tumor-like lesions. Skeletal Radiol. 2005;34:755–770. doi: 10.1007/s00256-005-0001-4. [DOI] [PubMed] [Google Scholar]
- 12.Geller AC, Miller DR, Annas GD, Demierre MF, Gilchrest BA, Koh HK. Melanoma incidence and mortality among US whites, 1969–1999. JAMA. 2002;288:1719–1720. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs JF, Huang PP, Lee RJ, McGrath B, Brooks J, McKinley B, Driscoll D, Kraybill WG. Malignant fibrous histiocytoma: an institutional review. Cancer Invest. 2001;19:23–27. doi: 10.1081/cnv-100000071. [DOI] [PubMed] [Google Scholar]
- 14.Herbert SH, Corn BW, Solin LJ, Lanciano RM, Schultz DJ, McKenna WG, Coia LR. Limb-preserving treatment for soft tissue sarcomas of the extremities: the significance of surgical margins. Cancer. 1993;72:1230–1238. doi: 10.1002/1097-0142(19930815)72:4<1230::aid-cncr2820720416>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer. 2001;92:172–180. doi: 10.1002/1097-0142(20010701)92:1<172::aid-cncr1306>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Jennings TA, Peterson L, Axiotis CA, Friedlaender GE, Cooke RA, Rosai J. Angiosarcoma associated with foreign body material: a report of three cases. Cancer. 1988;62:2436–2444. doi: 10.1002/1097-0142(19881201)62:11<2436::aid-cncr2820621132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Kearney MM, Soule EH, Ivins JC. Malignant fibrous histiocytoma: a retrospective study of 167 cases. Cancer. 1980;45:167–178. doi: 10.1002/1097-0142(19800101)45:1<167::aid-cncr2820450127>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Koontz BF, Miles EF, Rubio MA, Madden JF, Fisher SR, Scher RL, Brizel DM. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck. 2008;30:262–266. doi: 10.1002/hed.20674. [DOI] [PubMed] [Google Scholar]
- 19.Kopp HG, Kanz L, Hartmann JT. Complete remission of relapsing high-grade angiosarcoma with single-agent metronomic trofosfamide. Anticancer Drugs. 2006;17:997–998. doi: 10.1097/01.cad.0000224453.39200.d7. [DOI] [PubMed] [Google Scholar]
- 20.Kransdorf MJ, Murphey MD. The use of gadolinium in the MR evaluation of soft tissue tumors. Semin Ultrasound CT MR. 1997;18:251–268. doi: 10.1016/s0887-2171(97)80016-9. [DOI] [PubMed] [Google Scholar]
- 21.Malawer MM, Sugarbaker PH. Musculoskeletal Cancer Surgery: Treatment of Sarcomas and Allied Diseases. Norwell, MA: Kluwer Academic Publishing; 2001. [Google Scholar]
- 22.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683–697. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Miyake M, Tateishi U, Maeda T, Arai Y, Hasegawa T, Sugimura K. MR features of angiosarcoma in a patient with Maffucci’s syndrome. Radiat Med. 2005;23:508–512. [PubMed] [Google Scholar]
- 24.Morgan MB, Swann M, Somach S, Eng W, Smoller B. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867–874. doi: 10.1016/j.jaad.2003.10.671. [DOI] [PubMed] [Google Scholar]
- 25.Nakazono T, Kudo S, Matsuo Y, Matsubayashi R, Ehara S, Narisawa H, Yonemitsu N. Angiosarcoma associated with chronic lymphedema (Stewart-Treves syndrome) of the leg: MR imaging. Skeletal Radiol. 2000;29:413–416. doi: 10.1007/s002560000225. [DOI] [PubMed] [Google Scholar]
- 26.Orchard GE, Zelger B, Jones EW, Jones RR. An immunocytochemical assessment of 19 cases of cutaneous angiosarcoma. Histopathology. 1996;28:235–240. doi: 10.1046/j.1365-2559.1996.d01-411.x. [DOI] [PubMed] [Google Scholar]
- 27.Schindera ST, Streit M, Kaelin U, Stauffer E, Steinbach L, Anderson SE. Stewart-Treves syndrome: MR imaging of a postmastectomy upper-limb chronic lymphedema with angiosarcoma. Skeletal Radiol. 2005;34:156–160. doi: 10.1007/s00256-004-0807-5. [DOI] [PubMed] [Google Scholar]
- 28.Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: an analysis of 26, 758 cases. Int J Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 29.Wall SD, Fisher MR, Amparo EG, Hricak H, Higgins CB. Magnetic resonance imaging in the evaluation of abscesses. AJR Am J Roentgenol. 1985;144:1217–1221. doi: 10.2214/ajr.144.6.1217. [DOI] [PubMed] [Google Scholar]
- 30.Weiss S, Goldblum JR. Soft Tissue Tumors. 5. St Louis, MO: Mosby/Elsevier; 2008. pp. 716–717. [Google Scholar]
- 31.Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Fitzpatricks Dermatology in General Medicine. 7. New York, NY: McGraw-Hill Company Inc; 2009. [Google Scholar]
- 32.Wysocki WM, Komorowski A. Stewart-Treves syndrome. J Am Coll Surg. 2007;205:194–195. doi: 10.1016/j.jamcollsurg.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys. 2003;56:482–488. doi: 10.1016/s0360-3016(02)04510-8. [DOI] [PubMed] [Google Scholar]