Abstract
Background
The emergence of limb salvage surgery as an option for patients with osteosarcoma is attributable to preoperative chemotherapy and advancements in musculoskeletal imaging and surgical technique. While the indications for limb salvage have greatly expanded it is unclear whether limb salvage affects overall survival.
Questions/purposes
We asked whether over the past three decades limb-sparing procedures in high-grade osteosarcoma had increased, and whether this affected survival and ultimate amputation.
Methods
We retrospectively reviewed 251 patients with high-grade osteosarcoma treated from 1980 to 2004 with a multidisciplinary approach, including neoadjuvant chemotherapy. We compared survival rates, limb-salvage treatment, and amputation after limb-sparing procedure during three different periods of time. Fifty-three patients were treated from 1980 to 1989, 97 from 1990 to 1999, and 101 from 2000 to 2004. Thirty-seven patients were treated with primary amputations and 214 with primary limb salvage.
Results
The 5-year survival rate in the first period was 36%, whereas in the 1990s, it was 60% and 67% from 2000–2004. Limb salvage surgery rate in the 1980s was 53% (28 of 53), whereas in the 1990s, it was 91% (88 of 97) and 97% from 2000–2004 (98 of 101). In the limb salvage group, 22 of the 214 patients (10%) required secondary amputation; the final limb salvage rate in the first period was 36% (19 of 53), whereas in the 1990s, it was 81% (79 of 97) and 93% from 2000–2004 (94 of 101).
Conclusions
Patients with osteosarcoma treated in the last two periods had higher rates of limb salvage treatment and survival, with lower secondary amputation.
Level of Evidence
Level III, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteosarcomas are a highly malignant bone tumor and are the most common primary malignancy of bone in children and adolescents and the fifth most common malignancy among adolescents and young adults [15, 22]. Rosen et al. [19] initiated the concept of “neoadjuvant” chemotherapy to allow time for construction of custom-made endoprostheses for limb salvage. The prognosis of osteosarcoma has improved substantially as a result of different combinations of cisplatinum, ifosfamide, doxorubicin, and high-dose methotrexate combined with improvement in local tumor control [2–13]. With advances in preoperative chemotherapy, the surgeon can more readily perform limb preservation surgery as a result of reduction in tumor volume, and can from resected tissue evaluate the histologic effect of that chemotherapy [14, 16, 18, 23].
With modern therapy combined with limb-sparing surgery, at least two-thirds of patients with nonmetastatic extremity osteosarcomas will be long-term survivors with a limb preservation rate higher than 80% [2, 13, 20, 21]. Surgical management has evolved simultaneously with the emergence of effective chemotherapy. Although complete extirpation of the tumor remains the primary objective, the nature and scope of the approach taken to accomplish this goal has changed with an emphasis on more conservative surgery to maintain function [1]. However, a patient who has had a limb salvage procedure eventually may need a secondary amputation owing to local recurrence, infection, or functional orthopaedic complications (allograft massive resorption, hardware or prosthetic failure) [1]. Thus, along with adjuvant treatment, limb salvage procedures have increased in most centers in recent years. However, it is not known whether the more conservative surgery affects the survival rate or the incidence of complications leading to a secondary amputation. We presumed the more conservative surgeries performed during the last three decades would not have a negative influence on the survival at the same period of time.
We therefore determined (1) the differences in primary limb salvage surgery and amputation rates during each of the last three decades; (2) the differences in overall survival of patients treated during each of the analyzed periods; and (3) the secondary amputation rate resulting from postoperative complications or recurrences.
Patients and Methods
We retrospectively reviewed 327 consecutive patients with high-grade osteosarcomas treated from January 1980 to July 2004 in our Institution. We excluded from the study 76 patients with an osteosarcoma located in the axial skeleton (21 patients), with lung metastasis at initial diagnosis (46 patients) or secondary osteosarcomas (9 patients). This left 251 nonmetastatic high-grade osteosarcomas of the limb extremities available for this study; all 251 patients were treated surgically. Detailed clinical data were collected from the patient files and survival data were obtained using our clinical database. For comparison, we divided this series into three different groups according to the period when they were treated. All patients in this series were followed for at least 5 years or until they died of disease (DOD). Fifty-three patients were treated from January 1980 to December 1989 (first period), 97 from January 1990 to December 1999 (second period), and 101 from January 2000 to July 2004 (third period). In the first period (the 1980s), 33 of the 53 patients were male and 20 patients were female with a mean age of 23 years (range, 7–65 years). The primary lesions were located in the femur (29), tibia (15), humerus (7), and fibula (2). We followed patients for a minimum of 4 months (average, 72 months; range, 4–244 months) (Table 1). In the second period (the 1990s), 53 of the 97 patients were males and 44 patients were females with a mean age of 23 years (range, 4–64 years). The primary lesions were located in the femur (57), tibia (26), fibula (seven), humerus (four), radius (one), talus (one), and metatarsus (one) (Table 1). We followed patients for a minimum of 2 months (average, 67 months; range, 2–198 months). In the third period (from 2000–2004), 59 of the 101 patients were males and 42 patients were females with a mean age of 20 years (range, 4–82 years). The primary lesions were located in the femur (60), tibia (27), humerus (eight), fibula (four), radius (one), and metacarpus (one). We followed patients for a minimum of 6 months (average, 46 months; range, 6–104 months) (Table 1). The present study was approved by the local Institutional Review Board.
Table 1.
Demographic differences between groups according to the three decades
| Variables | First decade (1980s) | Second decade (1990s) | Third decade (2000s) |
|---|---|---|---|
| Number | 53 patients | 97 patients | 101 patients |
| Followup | 72 months (range, 4–244 months) | 67 months (range, 2–198 months) | 46 months (range, 6–104 months) |
| Age | 22 years (range, 7–65 years) | 23 years (range, 4–64 years) | 20 years (range, 4–82 years) |
| Gender | 20 female; 33 male | 44 female; 53 male | 42 female; 59 male |
| Location (number) | Femur (29) Tibia (15) Humerus (7) Fibula (2) |
Femur (57) Tibia (26) Fibula (7) Humerus (4) Radius (1) Talus (1) Metatarsus (1) |
Femur (60) Tibia (27) Humerus (8) Fibula (4) Radius (1) Metacarpus (1) |
Staging studies included AP and lateral radiographs of the affected bone, MRI of the affected segment (since 1986), CT of the limb (from 1980 to 1986) and lungs, and a total body bone scan. No patients included in this series had pulmonary metastases at first consultation. All patients had Enneking [9] Stage IIB disease.
From December 1983 to April 1990, patients were treated with a combination of cisplatinum (100 mg/m2, week 0-6-14), ifosfamide (3 g/m2 for 2 days, week 3-11-17), and adriamycin (25 mg/m2 for 3 days, week 3-6-11-14-17). Surgical treatment occurred in the 9th week of the protocol. From 1990 to the present time, chemotherapy consisted of a combination of ifosfamide (1.8 g/m2 for 5 days, week 0-5-10), adriamycin (25 mg/m2 for 3 days, week 0-5-10), and high-dose methotrexate (12 g/m2, week 3-4-8-9-13-14). Surgical treatment occurred in the 15th week of the protocol. Local tumor control was achieved either by amputation or by a limb salvage procedure. These procedures were performed by four different surgeons in the same Institution.
Limb salvage procedure was performed according to preoperative studies. When the osteosarcoma contaminated the joint, an extraarticular resection was performed (entire joint en bloc resection); if the joint was not involved a transarticular resection was performed. Joint contamination occurs infrequently owing to inappropriate biopsy placement, extension of tumor along the intraarticular structures or due to a pathologic fracture.
We determined the primary surgical treatment (amputation or limb salvage) and, if the primary surgical treatment was limb salvage surgery, we determined the percentage of secondary amputations resulting from infection, local recurrence or other reconstructive complication. The overall survival rates were estimated by the Kaplan-Meier method. Difference between survival curves for each decade was performed using the log rank test. We used SPSS 17.0 for Windows (Chicago, IL) for statistical analyses.
Results
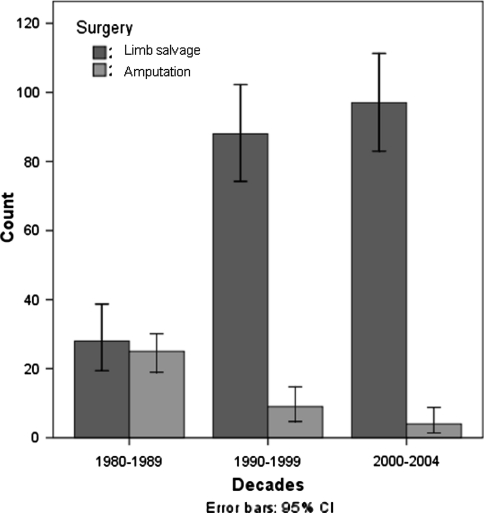
During the first period (the 1980s), 28 of 53 (53%) patients underwent primary limb salvage surgery and in the remaining 25 patients (47%), the amputation was the initial surgical treatment for local tumor control. In the 1990s, 88 of 97 patients (91%) underwent primary limb salvage for local tumor control, whereas in the remaining nine patients (9%), the amputation was the primary surgical treatment. In the most recent time frame (2000–2004), 98 of 101 patients (97%) underwent primary limb salvage for local tumor control with only three amputations (3%) for primary surgical treatment (Fig. 1). Regarding the limb salvage procedure, in the first period 14 were extraarticular resections and 14 intraarticular, in the second period 7 were extraarticular resections and 81 intraarticular, and in the last period 4 were extraarticular resections and 94 intraarticular.
Fig. 1.
This figure shows the total number of patients treated with amputation and limb salvage procedure in each period. Note the increasing number of limb salvage procedure in the last two periods. CI = confidence interval.
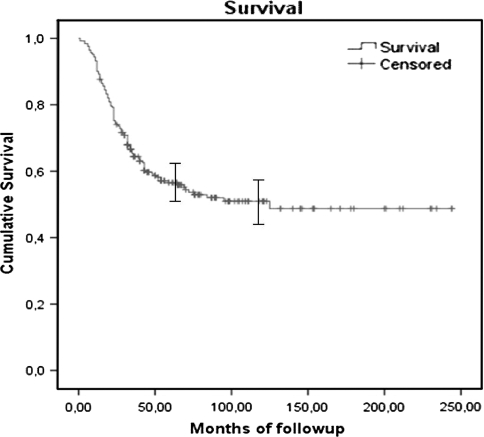
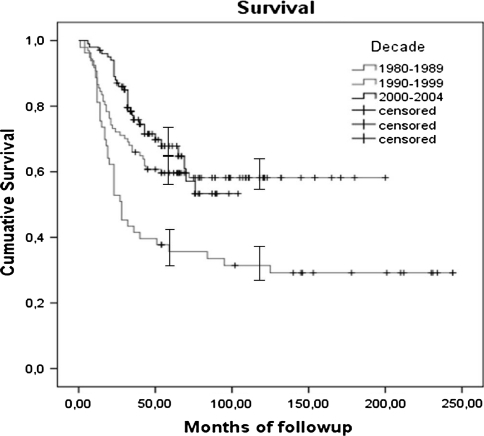
The overall survival rate for the whole study population was 56% (standard error [SE], 3.3%) at 5 years and 51% (SE, 3.6%) at 10 years (Fig. 2). The overall survival rate for the first period (1980s) was 36 % (SE, 6.7%) at 5 years and 31% (SE, 6.5%) at 10 years. For the second period (1990s), the overall survival was 60% (SE, 5%) at 5 years and 58% (SE, 5.1%) at 10 years. In the most recent time frame (2000–2004), the overall survival rate was 67% at 5 years (SE, 5.1%) (Fig. 3). We observed a lower survival rate in the group of patients treated during the 1980s compared with patients treated during the 1990s (p = 0.002) and from 2000–2004 (p = 0.000). Survival was similar (p = 0.44) in patients treated during the 1990s and 2000–2004.
Fig. 2.
Kaplan-Meier curve showing the overall survival of the entire series. The I bars indicate the 95% confidence intervals.
Fig. 3.
Kaplan-Meier curve showing the differences in survivorship according to each analyzed period. No difference was noted in the last two periods. The I bars indicate the 95% confidence intervals.
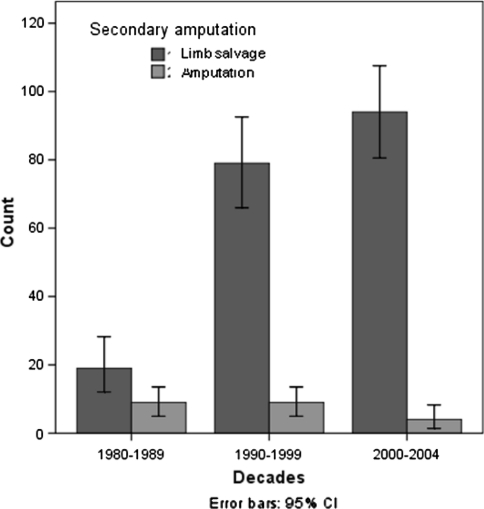
In the 214 patients who underwent a limb salvage procedure, 22 patients required a secondary amputation as a result of local complications. In 15 cases, the cause of secondary amputation was a local recurrence, and in the remaining seven, the secondary amputation was performed due to a deep persistent infection. The final limb salvage rate after secondary amputation, for patients treated during the first period, was 36% (19 of 53), whereas in patients treated during the 1990s and from 2000–2004, it was 81% (79 of 97) and 93% (94 of 101), respectively (Fig. 4).
Fig. 4.
This figure shows the final number of patients that preserved their limb and the number of secondary amputations in each study period. Note that the number of secondary amputations is lower in the last two periods had decreased. CI = confidence interval.
Regarding tumor localization, in the first period there were 24 limb salvage procedures and 22 primary amputations in the lower extremity, and four limb salvage procedures and three primary amputations in the upper extremity. In this period, the nine secondary amputations were in the lower extremity. In the second period there were 83 limb salvage procedures and nine primary amputations in the lower extremity, and five limb salvage procedures in the upper extremity with no primary amputation. Nine secondary amputations were performed in the lower extremity. In the last period, there were 89 limb salvage procedures and two primary amputations in the lower extremity, and nine limb salvage procedures and one primary amputation in the upper extremity. There were three secondary amputations in the lower extremity and one in the upper extremity.
Discussion
The prognosis of osteosarcoma has improved substantially in the last three decades. Surgical management has evolved simultaneously with the emergence of effective chemotherapy. Although complete extirpation of the tumor remains the primary objective, the nature and scope of the approach taken to accomplish this goal has changed with an emphasis on more conservative surgery to maintain function. Although limb salvage procedures have increased in most advanced centers in recent years, it has not been clearly determined whether this affects the survival rate and the incidence of complications leading to a secondary amputation. However, with the addition of adjunct therapy, we presumed the more conservative surgeries performed during the last three decades would not influence patient survival at the same period of time and that the rate of secondary amputations would not increase with the higher rate of limb salvage. We therefore determined (1) the differences in primary limb salvage surgery and amputation rates during each of the last three decades; (2) the differences in overall survival of patients treated during each of these decades; and (3) the secondary amputation rate resulting from postoperative complications or recurrences.
We acknowledge some limitations of this study. First, patients treated during the 1980s had a larger variety of systemic treatments. These variations could have negatively influenced survival and could in part explain our findings. Second, the learning curve in limb salvage surgical techniques improved in parallel with chemotherapy treatments. Surgeon experience could improve during the study period. The larger number of amputations in the earlier decade may be explained by the lack of experience as these techniques were introduced. Third, there were obvious variations in the staging (preoperative studies), type of diagnosis biopsies (open or needle, outside poorly planned biopsies), surgery, and the margins owing to the wide range of resections and the lack of ability to control for these sorts of variables in any subcohort analysis.
Before the emergence of limb salvage surgery in the 1970s, amputation of the affected limb was considered the definitive surgical intervention. Amputation remains the indicated treatment when resection to disease-free margins leaves a nonfunctional limb. Better local control may be achieved with amputation compared with limb salvage surgery when extensive contamination is found at the time of biopsy. The emergence of limb salvage surgery as an option for patients with osteosarcoma is attributable to the use of preoperative chemotherapy and to advancements in musculoskeletal imaging and surgical techniques [2]. Improvements in imaging, mainly in MRI, help the surgeon to reduce margins. The level of osteotomy to allow a safe margin is determined on the basis of the intramedullary spread of tumor as revealed by image methods. There is not complete accordance among orthopaedic surgeons about what is a safe tumor margin. In the late 70s adequate margins were defined in osteosarcoma as extracompartmental or extraarticular resections [19, 20]. In the 80s and beginning of the 90s most surgeons considered 5 cm above the tumor as an appropriate margin, and the articular cartilage was considered as a safe margin to prevent tumor spread [18, 23]. However, recently, 2 cm above the tumor is considered by many some surgeons as an adequate margin for tumor resection [1, 22]. The goal of limb salvage is to preserve a functioning limb without increasing the risk of tumor recurrence. However, there is evidence that closer resection margins used by most surgeons, in good responder patients to chemotherapy, does not increase the local recurrence rate [20, 21].
The survival of patients with malignant bone sarcomas has improved dramatically over the past 30 years, largely as a result of chemotherapeutic advances. Before the use of systemic chemotherapy, 80% to 90% of patients developed metastases despite achieving local tumor control and died of their disease [7]. Because of subclinical metastatic disease present at the time of diagnosis [6], chemotherapy can eradicate these micrometastases if initiated at a time when the disease burden is low. This benefit can improve survival in patients presenting with localized high-grade osteosarcoma compared with surgery alone [8, 17].
Using modern protocols of treatment over the last three decades, we observed improved survival (Fig. 3) with a parallel increase in limb-sparing procedures and a decrease in amputation rate (Fig. 1). Our survival rates are similar to those in the literature [3–5, 10, 14, 21] (Table 2). Sluga et al. [21] reported no differences in overall survival between amputations (60%) or limb salvage procedures (71%) in 130 patients treated with neoadjuvant chemotherapy. Bacci et al. [3] reported 43 patients treated with preoperative and postoperative chemotherapy with a survival rate of 68% at seven years with a 91% of limb salvage rate. Although a recent report [12] showed no difference in survival whether the surgery is performed before or after neo-adjuvant chemotherapy, this series had a higher amputation rate (50%) than other reports.
Table 2.
Comparison of results for overall survival, limb salvage rate, and secondary amputation from the literature
| Study | Number of cases | Primary amputation | Overall survival | Secondary amputation |
|---|---|---|---|---|
| Bacci et al. [3] | 43 | 9% | 68% | 9% |
| Ferrari et al. [11] | 182 | 8% | 77% | 4% |
| Honegger et al. [14] | 37 | 24% | 75% | 17% |
| Sluga et al. [21] | 130 | 35% | 67% | 1.2% |
| Current study (1980s) | 53 | 47% | 31% | 32% |
| Current study (1990s) | 97 | 9% | 58% | 10% |
| Current study (2000s) | 101 | 3% | 67% | 4% |
The goal of limb salvage is to preserve a functioning limb without increasing the risk of tumor recurrence. The secondary amputation is a crucial factor to evaluate local control of the disease or functional success after limb-sparing surgery. In our series of 214 patients who underwent limb preservation, the secondary amputation rate was 10%, and even in the last two decades, we performed a higher number of limb salvage procedures with a lower rate of secondary amputations (Fig. 4). Local recurrence and deep infection were the two causes of secondary amputation in our series. Aksnes et al. [1] reported four patients with secondary amputation in a group of 67 patients (6%) who initially had limb-sparing surgery. Two resulted from an infection, one for a local recurrence and the last for poor function. The authors observed no differences in quality of life between amputees and those undergoing limb-sparing surgery except in physical functioning (Table 2).
We observed patients treated in the last two decades have a higher rate of limb salvage treatment without compromising survival rate and the indication for secondary amputation resulting from postoperative complications or recurrences decreased at the same period of time. These observations were similar to the literature according to the reports of different decades, in which worst results in limb salvage and overall survival were observed during the 1980s or prior to that time [7–9, 14]. This improvement in treatment of nonmetastatic high-grade osteosarcoma of the limb must be related to the advances in chemotherapy and limb salvage techniques.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution has approved the reporting of this study and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aksnes LH, Bauer HC, Jebsen NL, Follerås G, Allert C, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br. 2008;90:786–794. doi: 10.1302/0301-620X.90B6.19805. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Lari S, Mercuri M, Donati D, Longhi A, Forni C, Bertoni F, Versari M, Pignotti E. Osteosarcoma of the limb. J Bone Joint Surg Br. 2002;84:88–92. doi: 10.1302/0301-620X.84B1.12211. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Ferrari S, Longhi A, Forni C, Bertoni F, Fabbri N, Zavatta M, Versari M. Neoadjuvant chemotherapy for high grade osteosarcoma of the extremities: long-term results for patients treated according to the Rizzoli IOR/OS-3b protocol. J Chemother. 2001;13:93–99. doi: 10.1179/joc.2001.13.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Forni C, Longhi A, Ferrari S, Mercuri M, Bertoni F, Serra M, Briccoli A, Balladelli A, Picci P. Local recurrence and local control of non-metastatic osteosarcoma of the extremities. A 27-year experience in a single institution. J Surg Oncol. 2007;96:118–123. doi: 10.1002/jso.20628. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Fifteen year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 6.Bruland OS, Høifødt H, Saeter G, Smeland S, Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res. 2005;11:4666–4673. doi: 10.1158/1078-0432.CCR-05-0165. [DOI] [PubMed] [Google Scholar]
- 7.Dahlin DC, Coventry MB. Osteogenic sarcoma. A study of six hundred cases. J Bone Joint Surg Am. 1967;49:101–110. [PubMed] [Google Scholar]
- 8.Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5:21–26. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- 9.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9–24. [PubMed] [Google Scholar]
- 10.Ferrari S, Bertoni F, Mercuri M, Picci P, Giacomini S, Longhi A, Bacci G. Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity. An analysis of 300 patients treated at the Rizzoli Institute. Ann Oncol. 2001;12:1145–1150. doi: 10.1023/A:1011636912674. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, Müller C, Tienghi A, Wiebe T, Comandone A, Böhling T, Del Prever AB, Brosjö O, Bacci G, Saeter G, Italian and Scandinavian Sarcoma Groups Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity. A joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 12.Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP, Pediatric Oncology Group Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins D, Arndt C. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98:2447–2456. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 14.Honegger HP, Cserhati MD, Exner GU, Hochstetter A, Groscurth P. Zürich experience with preoperative, high dose methotrexate-containing chemotherapy in patients with extremity osteosarcomas (OSA) Ann Oncol. 1991;2:489–494. doi: 10.1093/oxfordjournals.annonc.a057998. [DOI] [PubMed] [Google Scholar]
- 15.Huvos AG. Osteogenic Sarcoma. Bone Tumors. Diagnosis, Treatment and Prognosis. 2. Philadelphia, PA: WB Saunders; 1991. pp. 85–276. [Google Scholar]
- 16.Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, Glabbeke M, Kirkpatrick A, Hauben EI, Craft AW, Taminiau AH, MRC BO06 and EORTC 80931 collaborators; European Osteosarcoma Intergroup Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy. A randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 17.Link MP, Goorin AM, Horowitz M, Meyer WH, Belasco J, Baker A, Ayala A, Shuster J. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991;270:8–14. [PubMed] [Google Scholar]
- 18.Mercuri M, Capanna R, Manfrini M, Bacci G, Picci P, Ruggieri P, Ferruzzi A, Ferraro A, Donati D, Biagini R, Maio M, Cazzola A, Campanacci M. The management of malignant bone tumors in children and adolescents. Clin Orthop Relat Res. 1991;264:156–168. [PubMed] [Google Scholar]
- 19.Rosen G, Marcove RC, Caparros B, Nirenberg A, Kosloff C, Huvos AG. Primary osteogenic sarcoma—the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2192. doi: 10.1002/1097-0142(197906)43:6<2163::AID-CNCR2820430602>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331–1337. [PubMed] [Google Scholar]
- 21.Sluga M, Windhager R, Lang S, Heinzl H, Bielack S, Kotz R. Local and systemic control after ablative and limb sparing surgery in patients with osteosarcoma. Clin Orthop Relat Res. 1999;358:120–127. doi: 10.1097/00003086-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2124–2135. doi: 10.1016/j.ejca.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Uchida A, Myoui A, Araki N, Yoshikawa H, Shinto Y, Ueda T. Neoadjuvant chemotherapy for pediatric osteosarcoma patients. Cancer. 1997;79:411–415. doi: 10.1002/(SICI)1097-0142(19970115)79:2<411::AID-CNCR26>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]