Abstract
Background/rationale
Image-guided needle biopsies are commonly used to diagnose musculoskeletal tumors, but nondiagnostic (ND) results can delay diagnosis and treatment. It is important to understand which factors or diagnoses predispose to a ND result so that appropriate patient education or a possible change in the clinical plan can be made. Currently it is unclear which factors or specific lesions are more likely to lead to a ND result after image-guided needle biopsy.
Questions/purposes
We therefore identified specific factors and diagnoses most likely to yield ND results. We also asked whether an image-guided needle biopsy of bone and soft tissue lesions is an accurate and clinically useful tool.
Methods
We retrospectively reviewed data from a prospectively collected database for a case-control study of 508 image-guided needle biopsies of patients with suspected musculoskeletal tumors between 2003 and 2008.
Results
The interpretations of 453 of the 508 (89%) needle biopsies were accurate and clinically useful. Forty-five biopsies (9%) were ND and 10 (2%) were incorrect (IC). Bone lesions had a higher ND rate than soft tissue lesions (13% vs. 4%). The specific diagnosis with the highest ND rate was histiocytosis. Elbow and forearm locations had higher ND rates than average. Malignant tumors had a higher IC rate than benign tumors (5% vs. 0%); fibromyxoid sarcoma and rare subtypes of osteosarcoma had higher IC rates than other diagnoses. Repeat needle or open biopsies were performed in 71 (14%) patients. Bone lesions were more likely than soft tissue lesions to require repeat biopsies (18% vs. 9%).
Conclusions
A high rate of accuracy and clinical usefulness is possible with image-guided needle biopsies of musculoskeletal lesions. We believe these biopsies appropriate in selected circumstances but a key factor for appropriate use is an experienced musculoskeletal tumor team with frequent communication to correlate clinical, radiographic, and histologic information for each patient.
Level of Evidence
Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The image-guided needle biopsy is an established method used to diagnose musculoskeletal tumors [9, 17]. There has been a shift away from open incisional biopsies at major cancer centers as interventional radiologists and musculoskeletal pathologists gain skills in procuring and processing core biopsies and fine-needle aspirates [2, 4, 5, 21]. In addition, core biopsy devices that reduce crush artifact are now available. Advantages of an outpatient needle biopsy include decreased cost, decreased procedural complication rates, and decreased contamination of surrounding tissues [4, 8, 9, 11–14, 16, 21–24].
On review of the literature regarding needle biopsy, the terms ‘diagnostic’ and ‘accuracy’ are not clearly defined or consistently used. In addition, the use of image guidance, fine-needle aspiration (FNA) vs. core biopsy, and bone vs. soft tissue lesions is not uniform. This makes study comparisons difficult. It is clear, however, that nondiagnostic (ND) needle biopsies are a challenge to clinicians, pathologists, and patients as they potentially delay diagnosis and treatment and increase patient anxiety [20]. Published series have documented that overall ND rates for image-guided needle biopsies range between 5% and 29% [1, 8, 15, 17]. Specific diagnoses rarely are reported to have an effect on the ND rates [14, 15], although sclerotic or necrotic lesions are more difficult to diagnose [3, 7, 8, 14]. The literature suggests a range in accuracy from 74% to 99% with factors such as tissue type, lesion location, and type of biopsy affecting the result [19, 23, 24]. Some authors do not clearly state the rate of repeat needle or open biopsies, but the range is 3% to 25% [17, 21, 24]. We present a large series that confirms earlier studies and examines factors and specific diagnoses predisposing to ND results.
We therefore asked the following questions: (1) What are the rates and predisposing factors (ie, tissue type, specific diagnoses, location, imaging modality) for ND results? (2) What are the rates and predisposing factors for IC results? (3) What are the rates and predisposing factors for repeat biopsies?
Patients and Methods
We retrospectively reviewed our prospective database and identified 538 patients who had undergone 548 biopsies for suspected musculoskeletal tumors between May 2003 and March 2008. Included were 10 patients who had undergone two biopsies at different anatomic locations and separated by at least 1 year. Forty patients (40 biopsies) were excluded, as they did not meet the inclusion criteria of having an initial image-guided needle biopsy. Of these 40 patients, two had percutaneous biopsies without image guidance to confirm a soft tissue sarcoma recurrence, and 38 had only open, incisional biopsies. The final study group included 498 patients with 508 biopsies. There were 254 women and 244 men with a mean age at the time of biopsy of 58 (± 21) years.
The image-guided biopsies were performed by experienced musculoskeletal radiologists, and specimens were analyzed by expert musculoskeletal pathologists. A weekly indications conference and regular discussion between the orthopaedic oncologists, musculoskeletal radiologists, and musculoskeletal pathologists occur so that everyone understands the clinical presentation and radiographic appearance before performing the biopsy. Needle biopsies were performed either to establish a diagnosis when the clinical and radiographic scenario was not clear or to confirm a clinically and radiographically likely diagnosis.
In general, soft tissue masses or bone lesions with soft tissue extension were biopsied with either ultrasound (US) or CT guidance, whereas CT was used for intraosseous lesions. The final determination of modality is made by consensus between the orthopaedic oncologist and musculoskeletal radiologist with additional consideration given to the most expedient scheduling to minimize patient anxiety. There were 339 CT-guided biopsies and 169 US-guided biopsies. The procedure consisted of two to four separate FNA passes and one to six 20-gauge (US) or 16-gauge (CT) core biopsies. Radiographically classic lesions, such as giant cell tumors, often resulted in fewer core biopsies than indeterminate bone or soft tissue lesions. We have an IRB-approved protocol that allows up to four additional core biopsy specimens to be removed from consenting patients for research and tissue banking purposes. The decision regarding how and where to biopsy a specific lesion was individualized and left to the discretion of the highly experienced radiology team. In general, the imaging modality was used to identify solid, nonnecrotic areas of the lesion. If a bone tumor has soft tissue extension, the leading edge of soft tissue was biopsied rather than the bone.
The cytopathology team directed handling of the tissue immediately after the biopsy. The FNAs were processed at the point-of-care as direct smears, one air-dried and evaluated with the Three-Step stain (Richard-Allan Scientific, Kalamazoo, MI), the other ethanol-fixed and Papanicolaou stained. The smears were reviewed on site for adequacy by a board-certified cytopathologist. All biopsy specimens were reviewed by experienced, board-certified cytopathologists with input from experienced bone/soft tissue surgical pathologists. When indicated, immunohistochemical and/or molecular studies were performed on the core biopsy specimens. The time to final diagnosis was recorded from the actual biopsy to when the electronic record was finalized. In actual practice, the surgeon often has the final diagnosis several days earlier after direct communication with the musculoskeletal pathologist.
Lesions were classified and analyzed based on their tissue of origin (soft tissue vs. bone), anatomic site, and final histologic diagnosis. The final diagnostic categories included malignant bone tumors, malignant soft tissue tumors, benign bone tumors, benign soft tissue tumors, metastatic tumors, and nonneoplastic lesions. The malignant bone tumor group represented primary malignant tumors of bone and plasmacytoma/myeloma and lymphoma. The malignant soft tissue tumor group represented primary malignant tumors of soft tissue. The nonneoplastic group included bone and soft tissue lesions without evidence of tumor, such as fractures, hematoma, fibrous lesions, tissue necrosis, gout, chronic inflammation, osteomyelitis, or soft tissue abscesses.
One of the difficulties when assessing the literature regarding needle biopsy is the lack of clear definitions and terms related to diagnostic and/or accurate results. Results of our study were categorized as ND if classified using this term on the pathology report and if the histologic specimen did not allow the orthopaedic oncologist to formulate a definitive plan for treatment. Biopsy specimens strongly suggestive of a particular diagnosis that allowed the orthopaedic oncologist to use his/her judgment based on the overall clinical, radiographic, and histologic pictures to initiate the best treatment were considered clinically useful rather than ND, which has been described [8, 16, 17]. Common examples are bone lesions that clinically and radiographically suggest a benign appearance (Fig. 1). Serial clinical and radiographic followups of the 18 lesions in this category revealed a 0% incorrect rate. Results were classified as IC if subsequent repeat open or needle biopsies, the eventual surgical specimen, or serial radiographic/clinical followups did not confirm the initial needle biopsy diagnosis. A total of 212 lesions in 202 patients were treated with definitive surgery, and 296 lesions in 296 patients were followed without surgery (ie, metastatic disease, lymphoma, inoperable desmoid) or allowed to followup as needed (ie, hemangioma, benign bone or soft tissue lesions). Formally, accuracy is defined as true positives plus true negatives/true positives plus true negatives plus false positives plus false negatives. Tumor subtype (ie, well-differentiated vs. myxoid liposarcoma) was required to be accurate in this analysis. The total number of clinically useful biopsies was comprised of all diagnostic and accurate results.
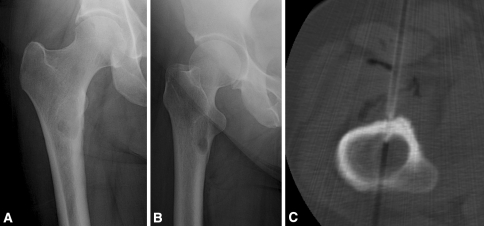
Fig. 1A–C.
(A) AP and (B) lateral radiographs are shown of the right hip of a 52-year-old woman with progressive, localized right thigh pain occurring at rest and at night . She has no history of cancer but is a long-time cigarette smoker, and a CT scan of the chest revealed a lung nodule and a thyroid mass. Although the plain radiographic appearance is suggestive of fibrous dysplasia, an alternative cause for her pain was not identified, therefore, a CT-guided needle biopsy was performed. (C) The biopsy revealed “bone and fibrous tissue with reactive changes” per the formal pathology report. There was “no malignancy in the specimen”. In this case a definitive diagnosis of fibrous dysplasia was not provided by the pathologist, but the surgeon chose to counsel the patient and continue to follow her with serial radiographs. In the biopsy database, this lesion was categorized as fibrous dysplasia.
To determine factors predisposing to ND results, IC results, or repeat biopsies, each biopsy record was characterized with respect to patient demographics (age and gender) and clinical characteristics. In addition, the type of imaging modality (CT or US) was collected. Finally, the final diagnostic category was recorded. The tissue and tumor types were considered key variables, and we performed a power analysis with reference to the results of previous studies [3, 8, 17]. A power analysis suggested we needed 56 patients in each group to detect one standard deviation difference at 80% power in the key variables with an alpha of .05.
Comparisons between the clinically useful rate and IC rate of each diagnostic category and the remaining biopsy records were made using Fisher’s Exact and chi square tests. Univariate analysis was performed to compare demographics and categorical (malignant bone, malignant soft tissue, benign bone, benign soft tissue, metastatic, and nonneoplastic) ND rates with the average ND rate.
To determine whether specific diagnoses or categories were more likely to be ND, we used a multivariate logistic regression model with biopsy outcome as the dependent measure (diagnostic versus ND). Independent variables included patient demographics, clinical characteristics, and type of imaging modality. Odds ratios and 95% confidence intervals (CI) were calculated for each independent variable. All analyses were conducted using SPSS statistical software package (SPSS, Inc, Chicago, IL).
Results
The overall ND rate of the initial needle biopsy was 9%, the IC rate was 2%, and the clinically useful rate was 89% (Table 1). Primary malignant and benign bone tumors had higher ND rates than their soft tissue counterparts (p = 0.001) (Table 2). Bone lesions had a higher (p < 0.001) ND rate than soft tissue lesions. Nonneoplastic lesions had higher (p = 0.04) clinically useful rates than neoplastic lesions. For single diagnoses, histiocytosis had a higher (p = 0.003) ND rate than average (Table 3). Lymphoma (p = 0.054) and osteomyelitis (p = 0.081) were the next most likely ND diagnoses. Lung metastasis had the lowest (0%) ND rate in the metastasis group.
Table 1.
Diagnostic rates for categorical diagnosis
| Category | Clinically useful | Nondiagnostic | Incorrect | Total |
|---|---|---|---|---|
| Malignant bone tumors | 70 (82%) | 12 (14%) | 3 (4%) | 85 |
| Benign bone tumors | 63 (83%) | 13 (17%) | 0 (0%) | 76 |
| Malignant soft tissue tumors | 56 (85%) | 4 (6%) | 6 (9%) | 66 |
| Benign soft tissue tumors | 102 (96%) | 4 (4%) | 0 (0%) | 106 |
| Metastasis | 48 (89%) | 5 (9%) | 1 (2%) | 54 |
| Nonneoplastic lesions | 114 (94%) | 7 (6%) | 0 (0%) | 121 |
| All biopsies | 453 (89%) | 45 (9%) | 10 (2%) | 508 |
Table 2.
Diagnostic rates for pairings of diagnoses
| Diagnostic pairings | Clinically useful | Nondiagnostic | Incorrect | Open biopsy | Total |
|---|---|---|---|---|---|
| Bone lesions | 233 (86%) | 35 (13%) | 4 (1%) | 48 (18%) | 272 |
| Soft tissue lesions | 220 (93%) | 10 (4%) | 6 (3%) | 22 (9%) | 236 |
| p Value | 0.007 | 0.0007 | 0.52 | 0.007 | |
| Primary bone tumors | 133 (83%) | 25 (15%) | 3 (2%) | 37 (23%) | 161 |
| Primary soft tissue tumors | 158 (92%) | 8 (5%) | 6 (3%) | 14 (8%) | 172 |
| p Value | 0.01 | 0.001 | 0.50 | 0.0002 | |
| Malignant tumors | 174 (86%) | 21 (10%) | 10 (5%) | 34 (17%) | 205 |
| Benign tumors | 165 (90%) | 17 (9%) | 0 (0%) | 22 (12%) | 182 |
| p Value | 0.15 | 0.74 | 0.02 | 0.19 | |
| Metastatic tumors | 48 (89%) | 5 (9%) | 1 (2%) | 5 (10%) | 54 |
| Nonmetastatic tumors | 291 (87%) | 33 (10%) | 9 (3%) | 51 (15%) | 333 |
| p Value | 0.76 | 0.88 | 0.71 | 0.24 | |
| Neoplastic | 339 (88%) | 38 (10%) | 10 (2%) | 55 (14%) | 387 |
| Nonneoplastic | 114 (94%) | 7 (6%) | 0 (0%) | 15 (13%) | 121 |
| p Value | 0.04 | 0.17 | 0.12 | 0.61 |
Table 3.
Diagnosis rate for specific diagnosis
| Diagnosis | Clinically useful | Nondiagnostic | Incorrect | Repeat biopsy | Total |
|---|---|---|---|---|---|
| Giant cell tumor | 27 (96%) | 1 (4%) | 0 (0%) | 2 (8%) | 28 |
| Lymphoma | 20 (77%) | 5 (19%) | 1 (4%) | 10 (38%) | 26 |
| Liposarcoma | 15 (83%) | 1 (6%) | 2 (11%) | 2 (11%) | 18 |
| Myeloma | 16 (94%) | 1 (6%) | 0 (0%) | 3 (18%) | 17 |
| Osteosarcoma | 13 (81%) | 1 (6%) | 2 (13%) | 3 (19%) | 16 |
| Pigmented villonodular synovitis | 14 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 14 |
| Myxoma | 13 (93%) | 1 (7%) | 0 (0%) | 1 (7%) | 14 |
| Lung metastasis | 13 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 13 |
| Schwannoma | 12 (92%) | 1 (8%) | 0 (0%) | 1 (8%) | 13 |
| Lipoma (atypical, spindle cell) | 12 (100%) | 0 (0%) | 0 (0%) | 1 (8%) | 12 |
| Chondrosarcoma | 10 (91%) | 1 (9%) | 0 (0%) | 2 (18%) | 11 |
| Aneurysmal bone cyst | 9 (89%) | 2 (11%) | 0 (0%) | 4 (33%) | 11 |
| Undifferentiated pleomorphic sarcoma (soft tissue) | 9 (82%) | 2 (18%) | 0 (0%) | 3 (25%) | 11 |
| Osteomyelitis* | 8 (73%) | 3 (27%) | 0 (0%) | 3 (27%) | 11 |
| Renal metastasis | 7 (78%) | 1 (11%) | 1 (11%) | 2 (22%) | 9 |
| Fibromyxoid sarcoma | 5 (62%) | 0 (0%) | 3 (38%) | 1 (13%) | 8 |
| Histiocytosis | 4 (50%) | 4 (50%) | 0 (0%) | 3 (38%) | 8 |
| Ganglion cyst | 7 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 |
| Breast metastasis | 6 (86%) | 1 (14%) | 0 (0%) | 1 (14%) | 7 |
| Ewing sarcoma | 6 (86%) | 1 (14%) | 0 (0%) | 1 (14%) | 7 |
| Desmoid tumor | 6 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 |
| Stress fracture | 5 (83%) | 1 (17%) | 0 (0%) | 1 (17%) | 6 |
| Prostate metastasis | 4 (67%) | 2 (33%) | 0 (0%) | 2 (33%) | 6 |
| Hemangioma | 4 (80%) | 1 (20%) | 0 (0%) | 1 (20%) | 5 |
| Hemangioendothelioma (bone) | 3 (60%) | 2 (40%) | 0 (0%) | 2 (40%) | 5 |
* Only two patients with osteomyelitis had positive microbiologic results on needle biopsy despite none previously having taken antibiotics; diagnoses not listed with sample size less than five patients owing to limitations of statistical testing (most diagnoses with fewer than five patients were nonneoplastic lesions).
Elbow and forearm lesions had higher (p = 0.01) ND rates than average, whereas those located in the pelvis and spine had comparable ND rates to other sites (Table 4). CT-guided biopsies had higher (p = 0.012) ND rates than US-guided biopsies (Table 5). The mean time to final diagnosis for ND biopsies was longer (p < 0.001; 10 days; range, 3-28 days) than clinically useful biopsies (4.2 days).
Table 4.
Diagnosis rate by anatomic site
| Site | Clinically useful | Nondiagnostic | Incorrect | Repeat biopsy | Total |
|---|---|---|---|---|---|
| Elbow | 6 (75%) | 2 (25%) | 0 (0%) | 3 (38%) | 8 |
| Forearm | 23 (77%) | 6 (20%) | 1 (3%) | 6 (20%) | 30 |
| Leg | 45 (85%) | 7 (13%) | 1 (2%) | 10 (19%) | 53 |
| Spine | 7 (88%) | 1 (13%) | 0 (0%) | 2 (25%) | 8 |
| Pelvis | 89 (90%) | 10 (10%) | 0 (0%) | 12 (12%) | 99 |
| Knee | 37 (90%) | 3 (7%) | 1 (2%) | 4 (10%) | 41 |
| Thigh | 113 (90%) | 8 (6%) | 5 (4%) | 25 (20%) | 126 |
| Chest | 16 (94%) | 1 (6%) | 0 (0%) | 3 (18%) | 17 |
| Arm | 51 (86%) | 6 (3%) | 2 (10%) | 3 (5%) | 59 |
| Shoulder | 30 (97%) | 1 (3%) | 0 (0%) | 2 (6%) | 31 |
| Foot/ankle | 29 (100%) | 0 (0%) | 0 (0%) | 1 (3%) | 29 |
| Hand/wrist | 7 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 |
Table 5.
Diagnostic rates by radiographic method
| Radiographic method | Clinically useful | Nondiagnostic* | Incorrect | Repeat biopsy | Total |
|---|---|---|---|---|---|
| CT | 296 (87%) | 37 (11%) | 6 (2%) | 57 (17%) | 339 |
| Ultrasound | 157 (93%) | 8 (5%) | 4 (2%) | 14 (8%) | 169 |
* Difference was not significant when controlled by tissue type, as bone lesions are more commonly biopsied via CT.
Bone lesions were 2.5 (95% CI: 1.35–4.85) times more likely than soft tissue lesions to yield a ND result (Table 6). Histiocytosis (95% CI: 1.938–44.258) and lymphoma (95% CI: 1.022–9.113) had higher odds ratios for ND results than other diagnoses.
Table 6.
Multivariate analysis of factors affecting ND rates
| Factors | p Value | Odds ratio | 95% CI for odds ratio | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | .520 | 1.000 | .998 | 1.001 |
| Female | .458 | 1.279 | .667 | 2.451 |
| Male | 1.000 | |||
| CT | .195 | 1.763 | .748 | 4.158 |
| US | 1.000 | |||
| Bone | .004 | 2.553 | 1.345 | 4.848 |
| Soft tissue | 1.000 | |||
| Arm | .599 | 1.361 | .431 | 4.301 |
| Chest | .822 | .779 | .089 | 6.850 |
| Elbow | .027 | 7.977 | 1.273 | 49.979 |
| Foot/ankle | .998 | .252 | .002 | 10.244 |
| Forearm | .035 | 3.661 | 1.093 | 12.263 |
| Hand/wrist | .999 | .424 | .001 | 15.859 |
| Knee | .334 | 2.056 | .477 | 8.854 |
| Leg | .243 | 1.930 | .641 | 5.815 |
| Pelvis | .442 | 1.483 | .543 | 4.050 |
| Shoulder | .589 | .554 | .065 | 4.711 |
| Spine | .537 | 2.044 | .211 | 19.796 |
| *Thigh | 1.000 | |||
| BB | .065 | 2.792 | .938 | 8.313 |
| BST | .530 | .656 | .177 | 2.441 |
| MB | .059 | 2.686 | .962 | 7.501 |
| Met | .286 | 1.996 | .561 | 7.104 |
| MST | .646 | 1.364 | .363 | 5.130 |
| Nonneoplastic* | 1.000 | |||
| Histiocytosis* | .005 | 9.261 | 1.938 | 44.258 |
| Lymphoma* | .046 | 3.051 | 1.022 | 9.113 |
| Osteomyelitis* | .092 | 3.710 | .808 | 17.027 |
* Thigh is the reference category for all other diagnoses; BB = benign bone tumors; BST = benign soft tissue tumors; MB = malignant bone tumors; Met = metastasis; MST = malignant soft tissue tumors.
The rate of IC biopsies was 2% (Table 7). Notably, benign bone and soft tissue tumors, and nonneoplastic lesions all were diagnosed accurately (Table 1). Malignant tumors had a higher (p = 0.02) IC rate than benign tumors (Table 2). Low- and intermediate-grade fibromyxoid sarcoma had a higher (p = 0.0001) IC rate than average. Osteosarcoma (telangiectatic and low-grade intraosseous subtypes) had a higher (13% vs. 2%; p = 0.05) IC rate than average (Table 3). Six of the 10 IC diagnoses had a repeat biopsy that revealed the error before definitive care. Nine of the 10 IC diagnoses were treated with definitive surgery and one was treated with chemotherapy (lymphoma). Of the 66 patients with a malignant soft tissue tumor, four had an increase and one had a decrease in grade between the needle biopsy and final resection diagnosis. Treatment of one patient with an increase in grade from intermediate- to high-grade sarcoma potentially was affected. The patient had preoperative radiation, postoperative (vs. preoperative) chemotherapy, and remains disease-free after 30 months.
Table 7.
Incorrect diagnoses
| Patient | Needle biopsy | Repeat biopsy* | Treatment | Final diagnosis | Change in treatment |
|---|---|---|---|---|---|
| 1 | No neoplasm | Metastatic renal carcinoma (n) | Right femoral nail | Metastatic renal carcinoma | |
| 2 | Intramuscular myxoma | None | Surgical resection | Dedifferentiated liposarcoma Grade 2 | |
| 3 | No neoplasm | Telangiectatic osteosarcoma (n) | Chemotherapy and surgery | Telangiectatic osteosarcoma | |
| 4 | Benign nerve sheath tumor | Neurofibrosarcoma (o) | Preoperative chemotherapy and radiation, surgical resection | Neurofibrosarcoma Grade 3 | |
| 5 | Perineuroma | Low grade sarcoma (o) | Surgical resection | Fibromxyoid sarcoma | |
| 6 | Spindle cell neoplasm/ solitary fibrous tumor | None | Surgical resection | Malignant fibrosarcoma | Add postoperative radiation therapy |
| 7 | Osteonecrosis | Diffuse large B cell lymphoma (o) | Chemotherapy | Diffuse large B cell lymphoma Grade 2 | |
| 8 | Nodular fasciitis | None | Surgical resection | Fibrosarcoma, intermediate grade | Postoperative radiation |
| 9 | Osteofibrous dysplasia | Osteosarcoma Grade 1 (o) | Surgical resection | Fibroblastic osteosarcoma Grade 1 | |
| 10 | High-grade cancer (metastatic prostate carcinoma) | None | Surgical resection | Pleomorphic liposarcoma | Add postoperative radiation therapy |
* n = needle biopsy; o = open biopsy.
The overall rate for repeat biopsies was 14% (71/508) (Table 8). Lymphoma had a higher (p = 0.002) repeat biopsy rate than average; histiocytosis and aneurysmal bone cyst trended toward higher than average (Table 3). Of the 71 repeat biopsies, 31 were considered clinically useful. Nine of 31 cases were performed to clarify the grade of a known tumor diagnosis: six of 31 as a confirmative frozen section at definitive surgery and 16 of 31 to verify the accuracy of the initial biopsy (ie, initial biopsy was diagnostic but not always accurate). The remaining 40 of 71 repeat biopsies were attributable to initial ND results. These included 35 subsequent open biopsies with 94% accuracy and five needle biopsies with 100% accuracy. Of the five patients with initial ND results without a repeat biopsy, three died of metastatic cancer and two refused repeat biopsy and requested clinical followup. The diagnoses in the latter two patients ultimately were established as a resolved benign bone lesion and a healed stress fracture.
Table 8.
Repeat biopsy rates for categorical diagnosis
| Category | Repeat needle | Repeat open | Repeat biopsy | Category denominator |
|---|---|---|---|---|
| Malignant bone tumors | 9 (11%) | 13 (15%) | 22 (26%) | 85 |
| Benign bone tumors | 0 (0%) | 15 (20%) | 15 (20%) | 76 |
| Malignant soft tissue tumors | 2 (3%) | 5 (8%) | 7 (11%) | 56 |
| Benign soft tissue tumors | 2 (2%) | 5 (5%) | 7 (7%) | 106 |
| Metastasis | 2 (4%) | 3 (6%) | 5 (10%) | 54 |
| Nonneoplastic lesions | 1 (1%) | 14 (12%) | 15 (13%) | 121 |
| All biopsies | 16 (3%) | 55 (11%) | 71 (14%) | 508 |
Discussion
Needle biopsies of musculoskeletal lesions are performed commonly in major cancer centers. This method of diagnosis is lower in cost and can be performed in a radiology suite or orthopaedic clinic rather than the operating room. The goal, however, is to avoid trading diagnostic accuracy for convenience and cost containment. In the literature, there is no consensus regarding which factors predict a ND or IC result. Our goal was to evaluate a large series of image-guided FNA and core biopsies of bone and soft tissue to answer the following questions: (1)What are the rates and predisposing factors (ie, tissue type, specific diagnoses, location, imaging modality) for ND results? (2) What are the rates and predisposing factors for IC results? (3) What are the rates and predisposing factors for repeat biopsies?
There are several limitations of this study. First is the nonrandomized selection of a specific imaging modality (CT vs. US) to guide the biopsy. Although this creates selection bias, the statistical methods incorporated a regression analysis of the results while controlling for the imaging modality. Multiple modalities are effective, and the choice is influenced by equipment availability, patient anxiety, and radiologist expertise/preference [2–5, 9, 18, 24]. Second, the study was not fashioned to prospectively identify which radiographic appearances are most likely to yield a ND result (prebiopsy probability), but rather to analyze the results after a team approach to care was instituted. Third, descriptive histology results that correlated with clinical and radiographic impressions were classified as clinically useful rather than ND. We think this terminology best recreates actual clinical decision-making, but if we categorized the descriptive biopsy results used for 18 patients as ND, it would result in a 14% (vs. 9%) ND rate. Finally, we did not document complications.
The available needle biopsy studies are inconsistent in term definition, use of image guidance, tissue type, and biopsy type (Table 9). Our 9% ND rate compares favorably with published results [1, 8]. In studies in which only FNA was performed, the ND and IC rates are falsely low [1, 11, 15, 19, 23]. Our study included FNA and core biopsies as we think this allows the highest chance of diagnosis. Known factors that predispose to a ND result include necrotic or sclerotic lesions [3, 7, 8, 14] with one study reporting soft tissue lesions [17]. The bone lesions in our series had a higher ND rate than soft tissue lesions. Liposarcoma, hemangioma, and osteomyelitis are mentioned as specific diagnoses that lead to ND results [9, 14, 15]. The most common diagnoses of our patients (metastatic disease, myeloma, giant cell tumor) have predictably low ND rates. Lymphoma was also common but had a higher ND rate and likelihood of repeat biopsy. Histiocytosis and osteomyelitis also were more likely to have a ND result. Patients should be counseled about a possible ND result if their radiographs are suggestive of these diagnoses. Unfortunately, lymphoma and histiocytosis do not always present with a classic radiographic appearance. The root cause of ND biopsies is likely multifactorial and includes a physical ‘miss’ of the lesion. Highly vascular lesions may produce only blood in the aspirate and core biopsy specimen. Extensively necrotic bone or soft tissue lesions can produce no viable tissue. Similarly, lymphomas often have crush artifact on the core biopsy specimen, precluding a definitive diagnosis. The use of MRI-directed biopsies may better identify and avoid areas of necrosis [5]. The current literature includes studies of nonimage-guided biopsies which may decrease diagnostic accuracy [1, 6, 16, 19, 21–23]. We think the likelihood of a diagnostic result is improved with image guidance. Finally, the location of the lesion can predispose to a ND result. In contrast to other series, the ND rates of our pelvic and spine lesions were comparable to rates from other locations, but forearm and elbow lesions had increased ND rates [9, 18]. Although we found a substantial delay in time to final diagnosis in the subset of ND biopsies, we suspect that delay would not translate to a worse prognosis. Minimizing the delay in diagnosis, however, may decrease anxiety for a patient with possible cancer. The challenge for the orthopaedic oncologist is to recognize the radiographic appearance of lesions with a high chance of a ND result. The information gained in our study would suggest that patients with clinical and radiographic presentations consistent with histiocytosis, lymphoma, or osteomyelitis might be better having the biopsy in the operating room.
Table 9.
Comparison of musculoskeletal needle biopsy study results
| Study | Year | Number of biopsies | Tissue type | Image guided? | Biopsy type | ND rate | IC rate | Repeat biopsy rate | Factors predisposing to ND | Diagnosis predisposing to ND | Factors predisposing to IC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ayala et al. [3] | 1983 | 222 | Bone | Fluoroscopic | Core | NS | 21% | NS | Necrosis, sclerotic lesion | NS | NS |
| Akerman et al. [1] | 1985 | 365 | ST | None | FNA | 5% | NS | NS | NS | NS | NS |
| Fraser-Hill & Renfrew [8] | 1992 | 102 | Bone > ST | NS | FNA/core | 29% | 16% | 13% | Sclerotic lesion | NS | Sclerotic lesion |
| Skrzynski et al. [22] | 1996 | 62 | Bone/ST | None | Core | 13% | 16% | 5% | NS | NS | ST tumors |
| Schweitzer et al. [21] | 1996 | 138 | Bone (no osteoblastic lesions) | None | FNA/core | 14% | 2% | 3% | NS | NS | NS |
| Yao et al. [24] | 1999 | 141 | Bone/ST | CT | Core | 21% | 26% | 18% | NS | NS | NS |
| Wakely & Kneisl [23] | 2000 | 82 | ST | None | FNA | 7% | 1% | 4% | NS | NS | NS |
| Duda et al. [7] | 2001 | 100 | Bone | CT | FNA/core | 11% | 9% | NS | Osteoblastic lesions | Sclerotic lesions, benign bone tumors | |
| Kilpatrick et al. [11] | 2001 | 145 | Bone/ST | None in majority | FNA | 16% | 3% | NS | NS | NS | NS |
| Hau et al. [9] | 2002 | 359 | Bone/ST | CT | FNA/core | 14% | 15% | NS | NS | Osteomyelitis | NS |
| Nagira et al. [15] | 2002 | 301 | ST | None | FNA | 7% | 10% | NS | NS | Hemangioma (ST) | NS |
| Domanski et al. [6] | 2005 | 130 | Bone/ST | None | FNA/core | 9% | 2% | 5% | NS | NS | NS |
| Altuntas et al. [2] | 2005 | 127 | Bone/ST | CT | Core | 8% | 12% | NS | NS | NS | NS |
| Mitsuyoshi et al. [14] | 2006 | 163 | Bone/ST | CT | Core | 12% | 12% | NS | Sclerotic lesions | Liposarcoma | NS |
| Ogilvie et al. [17] | 2006 | 120 | Bone/ST | Yes—type not specified | FNA/core | 23% | 3% | 25% | Soft tissue lesions | NS | Myxoid lesions |
| Puri et al. [18] | 2006 | 136 | Bone > ST | CT | Core | 21% | 5% | NS | NS | NS | Osteomyelitis |
| Rekhi et al. [19] | 2007 | 127 | ST | None | FNA | 11% | 2% | NS | NS | NS | NS |
| Oetgen et al. [16] | 2008 | 82 | Bone/ST | None | Core | NS | 13% | NS | NS | NS | NS |
| Current study | 2010 | 508 | Bone/ST | CT/US | FNA/core | 9% | 2% | 14% | Bone lesions; forearm/elbow | Histiocytosis, lymphoma, osteomyelitis | Malignant lesions, fibromyxoid sarcoma, rare osteosarcoma subtypes |
ND = nondiagnostic; IC = incorrect; NS = not stated in article; ST = soft tissue; FNA = fine needle aspirate; for studies using FNA only, the IC and ND rates often are misleading. FNA can differentiate between benign and malignant but often does not subtype as accurately.
The IC rate after needle biopsy of musculoskeletal lesions ranges from 1% to 26%, and our 2% IC rate compares favorably [23, 24]. Some reports suggest benign tumors have higher IC rates than malignant tumors [3, 4, 7, 9]. Conversely, others report malignant lesions with higher IC rates than benign or nonneoplastic lesions [17]. Some studies show bone lesions with higher IC rates than soft tissue lesions [7, 8, 17], others suggest the opposite [18, 22], and some report no difference [2, 10]. In our series, malignant tumors had the highest IC rates, as all benign bone and soft tissue tumors and nonneoplastic lesions were diagnosed accurately. The few lesions predisposed to an IC result that are mentioned in the literature include myxoid tumors and osteomyelitis [17, 18]. In our study, patients with unusual osteosarcoma subtypes or a diagnosis of benign versus low-grade malignant soft tissue lesions had higher IC rates. Patients with borderline soft tissue lesions (cellular intramuscular myxoma vs. low-grade myxoid sarcoma) based on a needle biopsy diagnosis should have either an open biopsy or a surgical resection that will not compromise their oncologic outcome.
The percentage of patients who required a repeat biopsy in this series was 14% which compares favorably with the 3% to 25% reported ranges [17, 21, 24]. The most common reason to perform a repeat biopsy was a ND result.
The favorable diagnostic and accuracy rates in our study are likely partly attributable to the expertise of our musculoskeletal radiologists and pathologists and the effective communication between all members of the musculoskeletal oncology team before and after the biopsy. These data underscore the importance and benefit of referring patients with a possible musculoskeletal tumor to a specialized referral center.
Acknowledgements
We thank Vaishali Parikh, BS, CCRP, Research Program Assistant, for maintenance of the needle biopsy database and Richard Skolasky PhD, for assistance with the statistical methods and analysis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Akerman M, Rydholm A, Persson BM. Aspiration cytology of soft-tissue tumors: the 10-year experience at an orthopedic oncology center. Acta Orthop Scand. 1985;56:407–412. doi: 10.3109/17453678508994359. [DOI] [PubMed] [Google Scholar]
- 2.Altuntas AO, Slavin J, Smith PJ, Schlict SM, Powell GJ, Ngan S, Toner G, Choong PF. Accuracy of computed tomography guided core needle biopsy of musculoskeletal tumours. ANZ J Surg. 2005;75:187–191. doi: 10.1111/j.1445-2197.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 3.Ayala AG, Zornosa J. Primary bone tumors: percutaneous needle biopsy. Radiologic-pathologic study of 222 biopsies. Radiology. 1983;149:675–679. doi: 10.1148/radiology.149.3.6580673. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco CH, Wallace S, Richli WR. Percutaneous skeletal biopsy. Cardiovasc Intervent Radiol. 1991;14:69–72. doi: 10.1007/BF02635534. [DOI] [PubMed] [Google Scholar]
- 5.Carrino JA, Khurana B, Ready JE, Silverman SG, Winalski CS. Magnetic resonance imaging-guided percutaneous biopsy of musculoskeletal lesions. J Bone Joint Surg Am. 2007;89:2179–2187. doi: 10.2106/JBJS.F.01230. [DOI] [PubMed] [Google Scholar]
- 6.Domanski HA, Akerman M, Carlén B, Engellau J, Gustafson P, Jonsson K, Mertens F, Rydholm A. Core-needle biopsy performed by the cytopathologist: a technique to complement fine-needle aspiration of soft tissue and bone lesions. Cancer. 2005;105:229–239. doi: 10.1002/cncr.21154. [DOI] [PubMed] [Google Scholar]
- 7.Duda SH, Johst U, Krahmer K, Pereira P, Konig C, Schafer J, Huppert P, Schott U, Bohm P. [Claussen CD [Technique and results of CT-guided percutaneous bone biopsy][in German] Orthopade. 2001;30:545–550. doi: 10.1007/s001320170064. [DOI] [PubMed] [Google Scholar]
- 8.Fraser-Hill MA, Renfrew DL. Percutaneous needle biopsy of musculoskeletal lesions: 1 Effective accuracy and diagnostic utility. AJR Am J Roentgenol. 1992;158:809–812. doi: 10.2214/ajr.158.4.1546597. [DOI] [PubMed] [Google Scholar]
- 9.Hau A, Kim I, Kattapuram S, Hornicek FJ, Rosenberg AE, Gebhardt MC, Mankin HJ. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol. 2002;31:349–353. doi: 10.1007/s00256-002-0474-3. [DOI] [PubMed] [Google Scholar]
- 10.Hodge JC. Percutaneous biopsy of the musculoskeletal system: a review of 77 cases. Can Assoc Radiol J. 1999;50:121–125. [PubMed] [Google Scholar]
- 11.Kilpatrick SE, Cappellari JO, Bos GD, Gold SH, Ward WG. Is fine-needle aspiration biopsy a practical alternative to open biopsy for the primary diagnosis of sarcoma? Experience with 140 patients. Am J Clin Pathol. 2001;115:59–68. doi: 10.1309/YN14-K8U4-5FLJ-DGJE. [DOI] [PubMed] [Google Scholar]
- 12.Layfield LJ, Dodd LG, Hirschowitz S, Crabtree SN. Fine-needle aspiration of primary osseous lesions: a cost effectiveness study. Diagn Cytopathol. 2010;38:239–243. doi: 10.1002/dc.21172. [DOI] [PubMed] [Google Scholar]
- 13.Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78:656–663. doi: 10.2106/00004623-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Mitsuyoshi G, Naito N, Kawai A, Kunisada T, Yoshida A, Yanai H, Dendo S, Yoshino T, Kanazawa S, Ozaki T. Accurate diagnosis of musculoskeletal lesions by core needle biopsy. J Surg Oncol. 2006;94:21–27. doi: 10.1002/jso.20504. [DOI] [PubMed] [Google Scholar]
- 15.Nagira K, Yamamoto T, Akisue T, Marui T, Hitora T, Nakatani T, Kurosaka M, Ohbayashi C. Reliability of fine-needle aspiration biopsy in the initial diagnosis of soft-tissue lesions. Diagn Cytopathol. 2002;27:354–361. doi: 10.1002/dc.10200. [DOI] [PubMed] [Google Scholar]
- 16.Oetgen ME, Grosser DM, Friedlaender GE, Lindskog DM. Core needle biopsies of musculoskeletal tumors: potential pitfalls. Orthopedics. 2008;31:pii:orthosupersite.com/view.asp?rlD = 32927. [DOI] [PubMed]
- 17.Ogilvie CM, Torbert JT, Finstein JL, Fox EJ, Lackman RD. Clinical utility of percutaneous biopsies of musculoskeletal tumors. Clin Orthop Relat Res. 2006;450:95–100. doi: 10.1097/01.blo.0000229302.52147.c7. [DOI] [PubMed] [Google Scholar]
- 18.Puri A, Shingade VU, Agarwal MG, Anchan C, Juvekar S, Desai S, Jambhekar NA. CT-guided percutaneous core needle biopsy in deep seated musculoskeletal lesions: a prospective study of 128 cases. Skeletal Radiol. 2006;35:138–143. doi: 10.1007/s00256-005-0038-4. [DOI] [PubMed] [Google Scholar]
- 19.Rekhi B, Gorad BD, Kakade AC, Chinoy R. Scope of FNAC in the diagnosis of soft-tissue tumors: a study from a tertiary cancer referral center in India. Cytojournal. 2007;4:20. doi: 10.1186/1742-6413-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roh JL, Lee YW, Kim JM. Clinical utility of fine-needle aspiration for diagnosis of head and neck lymphoma. Eur J Surg Oncol. 2007;34:817–821. doi: 10.1016/j.ejso.2007.07.200. [DOI] [PubMed] [Google Scholar]
- 21.Schweitzer ME, Gannon FH, Deely DM, O’Hara BJ, Juneja V. Percutaneous skeletal aspiration and core biopsy: complementary techniques. AJR Am J Roentgenol. 1996;166:415–418. doi: 10.2214/ajr.166.2.8553958. [DOI] [PubMed] [Google Scholar]
- 22.Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644–649. doi: 10.2106/00004623-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Wakely PE, Jr, Kneisl JS. Soft tissue aspiration cytopathology. Cancer. 2000;90:292–298. doi: 10.1002/1097-0142(20001025)90:5<292::AID-CNCR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Yao L, Nelson SD, Seeger LL, Eckardt JJ, Eilber FR. Primary musculoskeletal neoplasms: effectiveness of core-needle biopsy. Radiology. 1999;212:682–686. doi: 10.1148/radiology.212.3.r99se19682. [DOI] [PubMed] [Google Scholar]