Abstract
Background
Osteosarcoma is a rare complication of Paget’s disease with a very poor prognosis. Treatment is controversial: the older age of the patients affected by Paget’s disease may limit the use of chemotherapy and axial involvement may limit the practicality of surgery.
Questions/purposes
The purposes of this study are (1) to report the survival in patients treated for osteosarcoma in Paget’s disease; (2) to identify correlations between type of treatment and survival comparing our data with those in the literature; (3) to determine if the extent of Paget’s disease and risk of malignant transformation are associated; (4) to assess if prognosis is related with site; and (5) to identify the variations of histologic subtypes of these osteosarcomas.
Methods
We retrospectively reviewed the medical records of 26 patients treated between 1961 and 2006 who had bone sarcoma arising from a site of Paget’s disease. Twenty two of the 26 patients had surgery. In six surgery only was performed; three had surgery, adjuvant chemotherapy, and radiotherapy; one surgery and radiotherapy; 12 underwent surgery and chemotherapy, adjuvant in 10 patients and neoadjuvant in two; two had only radiotherapy and two had only chemotherapy. We performed survival analyses between various combinations of treatment.
Results
At last followup four patients had no evidence of disease (NED) at a minimum followup of 42.6 months (mean, 139 months; range, 42.6–257.4 months) and 22 died with disease (DWD) at a minimum time of 1 month (mean, 20.2 months; range, 1–84 months). One of the six patients (11%) treated with surgery only had NED at 10 years; the other five died from disease at a mean of 30 months. Three of 12 patients (25%) treated with surgery and chemotherapy are NED at a mean followup of 12 years; nine died of disease at a mean of 24 months. All patients treated without surgery died at a mean of 7.5 months (range, 1–13.7 months).
Conclusions
Despite improvements in surgery and medical treatments the prognosis remains poor in patients with Paget’s sarcoma.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Paget’s disease of bone (Paget’s disease) or osteitis deformans was first described by Sir James Paget in 1877 [23]. It is characterized by an imbalance of normal bone remodeling processes with functional and morphologic abnormalities of the osteoclasts and osteoblasts. The bone is highly vascular with production of mature bony trabeculae and cortices that are mechanically weaker and more susceptible to various stresses than normal bone. The prevalence of Paget’s disease in the general population is estimated at 1% to 2% [30]. The etiology remains unknown although environmental (viral) and genetic factors in the pathogenesis of the disease are probable [1, 6]. The diagnosis of Paget’s disease is based on imaging examination of the skeleton, bone biopsy, and biochemical markers of bone formation and resorption [16].
Complications of Paget’s disease include skeletal deformities, fractures, left ventricular failure, and cranial nerve impairment [29, 32]. Bone sarcoma is a rare complication of Paget’s disease [2, 33, 35] reportedly occurring in 0.1% to 0.95% of patients [20, 27, 37]. It is more common in older patients. A male predominance of 1.5:1 to 2:1 has been reported [7, 10, 11, 17, 21, 31, 34]. Most of these tumors are osteosarcomas with an extremely poor prognosis that has not improved over the years [7, 18, 21]. Mangham et al. reported a median survival of 8 months from diagnosis, whereas Deyrup et al. described a 5-year survival rate of 10% [7, 18]. One reason for the low survival could be the older age of these patients and a consequent difficulty treating them with aggressive chemotherapy. Another possibility is that Paget sarcomas are usually high grade [7, 21]. Paget’s osteosarcoma has a different distribution of lesions compared with primary osteosarcomas of pediatric age. Sarcoma arising in Paget’s disease shows a predilection for multiple bone sites such as the femur, humerus, pelvis, and skull, whereas the distal femur alone represents the more common site of disease in adolescent osteosarcoma [12].
At onset, patients can have pain, palpable mass, neurologic symptoms, or they can be asymptomatic until a pathologic fracture occurs with consequent bone deformity. Aggressive osteolytic changes, bone destruction, and soft tissue mass are radiographic findings that signify malignant change (Fig. 1). Paget’s disease of bone can appear as a monostotic form or a polyostotic form. A review of the literature by Deyrup et al. [7] reported 377 cases of Paget’s sarcoma; 295 cases (78%) arose in polyostotic disease, whereas only 82 (22%) arose in monostotic disease [4, 8, 13–15, 19, 22, 26, 28].
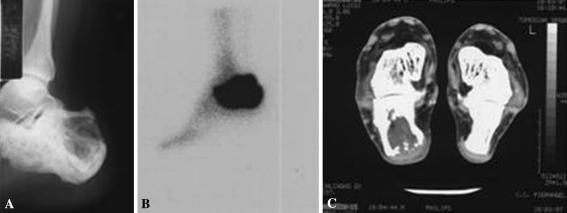
Fig. 1A–C.
Osteosarcoma involving the right calcaneus. (A) The first radiograph shows a lytic lesion of the bone. (B) Bone scan shows increased uptake of the calcaneus. (C) CT scan shows osteolysis of the Pagetic calcaneus with disruption of the posterior cortical.
The purposes of this study are (1) to report the survival in patients treated for osteosarcoma in Paget’s disease; (2) to identify correlations between type of treatment and survival comparing our data with those in the literature; (3) to determine if the extent of Paget’s disease and risk of malignant transformation are associated; (4) to assess if prognosis is related with site; and (5) to identify the variations of histologic subtypes of these osteosarcomas.
Patients and Methods
We retrospectively reviewed all 26 patients diagnosed with osteosarcoma arising in Paget’s disease between January 1961 and December 2006. Data and clinical followup information were obtained from medical charts; four patients were alive and were recalled specifically for this report. There were 21 males and five females with a mean age at diagnosis of sarcoma of 64 years (range, 38–80 years).
Osteosarcoma always arose in bones at the site of involvement with Paget’s disease and even on polyostotic forms had a unique localization. In 15 (58%) of the 26 patients, the diagnosis of Paget’s disease was made at a mean time of 107 months before the diagnosis of the osteosarcoma; in 11 patients, it was established at the time the sarcoma was diagnosed. The most frequent sites affected by tumor were the femur (eight patients) (Fig. 1), the humerus (seven patients), the tibia (four patients), the pelvis (two patients), the acromion, the fibula, the calcaneus (Fig. 2), the calvaria, and the spine (one patient each). To evaluate if the extent of Paget’s disease correlates with the risk of malignant transformation we divided our patients with a previous diagnosis of monostotic or polyostotic Paget’s disease. The data of all patients were evaluated at the latest examination for the purpose of this study.
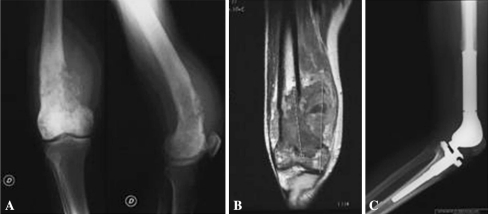
Fig. 2A–C.
Distal femur reconstruction after resection for osteosarcoma in Paget’s disease of bone. (A) Osteolytic disruption by osteosarcoma developed a pathologic fracture of the anterior femoral cortex. (B) Coronal MRI view shows intramedullary extension and involvement of the adjacent soft tissues. (C) Radiograph shows reconstruction with a GMRS® prosthesis (Stryker, Howmedica, Osteonics, Allendale, NJ).
The diagnosis of Paget’s disease was confirmed in all cases by radiographic reports and by a review of histopathologic samples. All tumors were high-grade osteosarcoma with histologic findings of proliferative atypical tumoral mesenchymal cells producing osteoid and abundant reactive giant cells. The osteosarcoma variants were osteoblastic (18 cases), fibroblastic (five cases), telangiectatic (two cases), and chondroblastic (one case).
In 22 of the 26 patients, surgery was performed. Six patients had surgery only, three amputations and three resections. In three cases, surgery (amputation), adjuvant chemotherapy, and radiotherapy were performed. One patient had surgery (amputation) and radiotherapy. Twelve patients had surgery and chemotherapy; adjuvant chemotherapy in 10 cases (eight amputations, two resections) and neoadjuvant chemotherapy in two cases (both amputations) were performed. Two patients had only radiotherapy and two had only chemotherapy. Resection and reconstruction were performed in only six cases; two patients with tumors at the proximal humerus were treated with allograft and a MRS Bioimpianti® prosthesis (Gruppo Bioimpianti S.r.l., Peschiera Borromeo, Milano, Italy). Three patients had tumors at the distal femur: two were treated with HMRS® prostheses (Stryker, Howmedica, Osteonics, Allendale, NJ) and one with GMRS® prosthesis (Stryker, Howmedica, Osteonics, Allendale, NJ). In one patient with a proximal tibia osteosarcoma, reconstruction using HMRS® (Stryker) prosthesis was performed.
Patients were periodically checked with CT and MRI of the involved site and CT scan of the chest. These checks were performed in our outpatient clinic every 3 months for the first 3 years, every 4 months in the fourth year, every 6 months in the fifth year, and then annually for a further 5 years. After 10 years from treatment, patients were considered free of disease and only rechecked for their reconstructions.
Survival analysis was performed using the Kaplan-Meier survival analysis [15]. The starting point was the date of surgery and the end point was patients’ death. We compared survival curves between subcohorts using the Log-rank statistical analysis. Data were recorded in a Microsoft® Excel® 2003 spreadsheet and analyzed using MedCalc® Software Version 11.1 (MedCalc Software, Mariakerke, Belgium).
Results
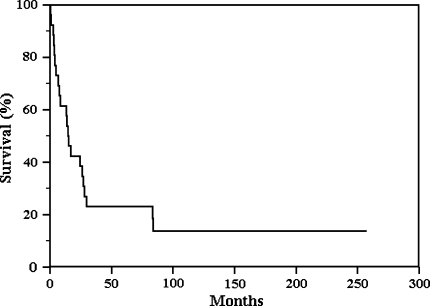
The overall 5-year survival rate was less than 25% with a higher mortality rate in the first 2 years after treatment (Fig. 3). Twenty-two of the 26 patients died with disease (DWD) at a mean time of 20.2 months (range, 1–84 months); one of them was initially treated with resection of the tumor and later (after 13 months) with amputation for local recurrence. The 17 patients with metastatic disease all died at a mean of 20 months from diagnosis (range, 1–84 months). Metastases were present at diagnosis in 13 patients, whereas four patients developed metastases later (one patient had both bilateral pulmonary metastases and local recurrence at 12 months). Five patients had no metastases but they died with disease at a mean of 16 months from diagnosis (range, 3–28 months).
Fig. 3.
Kaplan-Meier curve of all 26 patients shows a 5-year survival rate less than 25%. There is a higher mortality rate in the first 2 years after treatment.
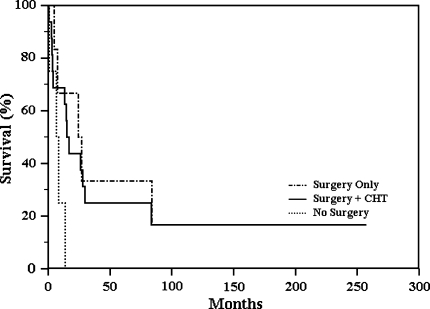
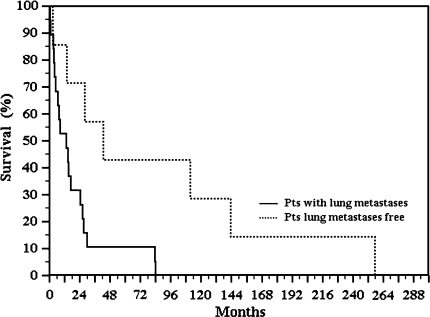
We observed a high mortality rate in patients treated without surgery and a trend (p = 0.1057) in patients treated with surgery only or with surgery plus chemotherapy (Fig. 4). Patients with lung metastases at the time of diagnosis had worse survival (p = 0.013) than those who were lung tumor-free (Fig. 5). Four patients had no evidence of disease (NED) at a minimum of 43 months (mean, 139 months; range, 43–257 months); one of them had resection only, one had amputation plus neoadjuvant chemotherapy, and two had amputation and adjuvant chemotherapy. These patients are NED at 111, 257, 144, and 43 months, respectively. None of the patients who are alive had evidence of lung or brain metastases (Table 1). Surgical margins were radical in eight patients, wide in 13, and marginal in one patient.
Fig. 4.
Kaplan-Meier curve reporting the type of treatment outlines the high mortality in patients treated without surgery. The curve shows a better trend (p = 0.1057) in patients treated with surgery only or with surgery plus chemotherapy (CHT).
Fig. 5.
Kaplan-Meier curve compares survival of patients with pulmonary metastatic disease with survival of patients who are lung tumor-free. Patients with pulmonary metastatic disease at the time of diagnosis had worse survival (p = 0.013) than those who did not.
Table 1.
Clinical data for 26 patients with osteosarcoma in Paget’s disease of bone
| Age (years) | Gender | Paget’s disease | Tumor location | Surgical treatment | Lung metastases | Chemotherapy and/or radiotherapy | Oncologic result |
|---|---|---|---|---|---|---|---|
| 58 | Male | Monostotic | Proximal humerus | Amputation | At diagnosis | CHT | Died |
| 47 | Male | Monostotic | Proximal humerus | Amputation | At diagnosis | CHT + Radio | Died |
| 75 | Male | Monostotic | Ilium | No surgery | At diagnosis | Radio | Died |
| 60 | Male | Monostotic | Proximal tibia | Amputation | At diagnosis | CHT | Died |
| 62 | Male | Monostotic | Proximal femur | Amputation | At diagnosis | – | Died |
| 74 | Male | Monostotic | Distal femur | Reconstruction | At diagnosis | CHT | Died |
| 57 | Female | Monostotic | Pelvis | Amputation | At diagnosis | – | Died |
| 62 | Male | Monostotic | Proximal humerus | Amputation | At diagnosis | Radio | Died |
| 75 | Male | Monostotic | Proximal femur | No surgery | At diagnosis | Radio | Died |
| 68 | Male | Monostotic | Proximal humerus | Reconstruction | At diagnosis | CHT | Died |
| 64 | Male | Monostotic | Proximal femur | Amputation | At diagnosis | CHT | Died |
| 54 | Male | Poliostotic | Proximal tibia | No surgery | At diagnosis | CHT | Died |
| 67 | Male | Monostotic | Proximal femur | Amputation | At diagnosis | CHT | Died |
| 76 | Female | Monostotic | Distal femur | Reconstruction | After 10 months | – | Died |
| 66 | Male | Poliostotic | Distal femur | Reconstruction* | After 6 months | CHT + Radio | Died |
| 47 | Male | Monostotic | Proximal fibula | Resection | After 35 months | CHT | Died |
| 66 | Male | Poliostotic | Proximal humerus | Amputation | After 72 months | CHT | Died |
| 65 | Male | Monostotic | Distal femur | Amputation | No | CHT | Died |
| 67 | Male | Monostotic | Proximal humerus | Reconstruction | No | – | Died |
| 80 | Male | Monostotic | Acromion | Amputation | No | – | Died |
| 53 | Male | Poliostotic | Calcaneus | Amputation | No | CHT | Alive |
| 58 | Female | Poliostotic | Calvaria | Amputation | No | CHT + Radio | Died |
| 75 | Female | Monostotic | Proximal tibia | Reconstruction | No | – | Alive |
| 52 | Male | Poliostotic | S1–S2 | No surgery | No | CHT | Died |
| 38 | Male | Poliostotic | Proximal humerus | Amputation | No | CHT | Alive |
| 68 | Female | Monostotic | Proximal tibia | Amputation | No | CHT | Alive |
*This patient developed a local recurrence after 13 months and underwent amputation; CHT = chemotherapy; Radio = radiotherapy.
Seven patients of our series had previous polyostotic Paget’s disease while 19 patients had previous monostotic disease. The four patients with axial disease (two pelvis, one vertebra, one calvaria) all died, whereas four of the patients with appendicular disease (two tibia, one humerus, one calcaneus) are NED.
Histologically, the osteoblastic variant was the most common type (18 patients [69%]) followed by the fibroblastic (two patients), the telangiectatic (two patients), and the chondroblastic variants (one patient).
Discussion
The occurrence of bone sarcoma is the most severe complication in Paget’s disease. Osteoblastic osteosarcoma is the most common Pagetic bone sarcoma [13, 25, 30, 34]. In the literature there is controversy regarding the optimal treatment for patients with osteosarcoma in Paget’s disease; the older age of the patients affected by the disease may limit the use of chemotherapy, and axial involvement may be a factor limiting the feasibility of surgery [7, 17, 18, 21, 31, 37]. We performed this study to evaluate the role of surgery and adjuvant treatments to the survival of patients with osteosarcoma in monostotic and polyostotic Paget’s disease.
We acknowledge several limitations of our study. First was the small number of patients. However, the disease is relatively rare, even in a center such as ours. Second, most of our patients had monostotic disease and therefore we cannot draw conclusions about the prognosis of polyostotic Paget’s disease. Monostotic disease is more prevalent in patients with Paget’s disease [5, 10], thus the proportion of patients with sarcomatous transformation necessarily may be increased in monostotic forms. Third, the different treatments of our patients are included in a period of more than 40 years. Fourth, the numbers of patients in the subcohorts is too small to generate reliable statistical differences. Fifth, we could not evaluate the effectiveness of bisphosphonates therapy in preventing sarcomatous transformation in Paget’s disease.
The poor prognosis of patients with Pagetic osteosarcoma has been previously confirmed [7, 17, 18, 21]. Survival of 5 days to 2.5 years in 22 patients with Paget’s sarcoma has been reported [21]. Others reported a survival rate of 14% at approximately 2.5 years and showed neoadjuvant chemotherapy and radiotherapy did not improve survival in these patients [18]. In a study, a median survival of 8 months from diagnosis was reported, with only four patients living longer than 2 years; survival was unrelated to age, metastases at diagnosis, or chemotherapy [17]. In our series, four patients were NED at a mean of 11.5 years and 22 patients were DWD at a mean of 20.2 months. Of the four patients NED, one patient was treated with surgery only (proximal tibia resection), one with neoadjuvant chemotherapy and amputation, and two with surgery (amputation) and adjuvant chemotherapy (Table 2). Why does this disorder have such a poor prognosis? First, Paget’s sarcoma probably is more malignant than other primary tumors of the connective tissue [18] and Paget’s sarcomas are more often high-grade tumors [18, 37]. Second, the older age of the patients with Paget’s disease may limit the use of chemotherapy. Moreover, the skeletal distribution of sarcomas seems to reflect the distribution of uncomplicated Paget’s disease. Obviously, axial involvement may be a further factor limiting the feasibility of surgery [37].
Table 2.
Comparison of data from major series of sarcomas in Paget disease reported in the literature and our data
| Study | Number of patients | Mean age (years) | DWD/time | NED/time | Metastases | Monostotic/polyostotic disease | Appendicular/axial involvement |
|---|---|---|---|---|---|---|---|
| Moore et al. [22] | 22 | 71 | 20/5 days to 2.5 years | 1/5 years | – | 7/15 | 16/5 (one patient had multifocal Paget osteosarcoma) |
| Mankin et al. [19] | 40 | 68 ± 13 | 5/2.5 years | – | 14 | – | 25/18 |
| Deyrup et al. [7] | 70 | 66 | 40/1 month to 20 years | 3 (3 months, 20 and 21 years) | 32 | 33/37 | 32/38 (two patients had multifocal osteosarcoma) |
| Mangham et al. [18] | 32 | 73.1 ± 7.9 | 31/9 days to 10 years | 1 (at 10 years) | – | 13/32 | 22/10 |
| Ruggieri et al. [current study] | 26 | 64 | 22/1 month to 7 years | 4 (3.6, 9.2, 12, and 21 years) | 17 | 19/7 | 21/5 |
DVD = dead of disease; NED = no evidence of disease.
Survival of patients with Pagetic osteosarcoma has been independent of palliative chemotherapy or other therapeutic modalities [7, 17] and limb-salvage surgery versus amputation [31]. The same studies showed no difference in the proportion of patients with monostotic or polyostotic Paget’s disease and Paget’s sarcoma [7, 17]. Surgery is always preferred, if feasible, and amputation is indicated in pathologic fractures or massively destroyed bones if wide margins cannot be obtained with resection. In the present series, resection and reconstruction were performed in only six patients; in the remaining 16 patients, amputation was performed. Surgery was the only common treatment for our four patients NED. Chemotherapy was administered in 17 patients; only three of these patients are NED. One patient was affected by Paget’s osteosarcoma of the humerus at age 38. He had a 2-year history of pain in his left arm before he sustained a pathologic fracture and was treated with a cast and calcitonin. Subsequently, he had a destructive lesion and nonunion of the pathologic fracture; a needle biopsy revealed a Grade 4 osteoblastic osteosarcoma. He had neoadjuvant chemotherapy with good response and forequarter amputation. He is NED at 21 years followup. Our series shows a high percentage of patients with osteosarcoma in monostotic Paget’s disease (73%). This might possibly contradict the relationship between disease extent and malignant transformation [4, 8, 13–15, 19, 22, 26, 28, 35].
The location of Pagetic osteosarcoma differs compared with primary osteosarcoma. In primary osteosarcoma, the distal femur and proximal tibia represent the predominant sites by far, whereas in our series, the femur and humerus are the most frequently involved sites (eight and seven cases, respectively). The literature shows a predilection of sarcomas for the femur, pelvis, and humerus in patients with Paget’s disease [7, 9, 17, 18]. The anatomic sites involved by Paget’s sarcoma closely correspond with those of skeletal involvement by Paget’s disease [3]. Like in our series, the humerus shows frequent involvement by Paget’s sarcoma [7, 17, 18, 25]. The large number of lesions occurring in this site makes the humerus a high-risk location when it is involved by uncomplicated Paget’s disease [25].
We found similar survival in patients with appendicular disease and axial disease (two pelves, one vertebra, one calvaria) but our four patients NED had a primary lesion involving extremities (tibia [two patients], humerus, calcaneus). This fact is probably related to the easier feasibility of surgical resection in the appendicular skeleton. A study reported lower survival in patients with axial compared to appendicular disease even if the difference was not significant [7].
Atypical, highly pleomorphic spindle cells forming osteoid and primitive bone and osteoclast-like giant cells are characteristic histopathologic features of Pagetic osteosarcoma [13, 25, 30, 34]. In their study Ueda et al. found a higher prevalence of MYC gene amplification in Pagetic osteosarcomas than in primary osteosarcomas [36]. MYC gene amplification is associated with poorer prognosis in musculoskeletal sarcomas [24]. Despite recent advances in imaging, chemotherapy, radiotherapy, and surgical techniques, Paget’s osteosarcoma continues to have a very poor prognosis, probably as a result of the older age of patients who do not want standard adjuvant chemotherapy.
Acknowledgments
We thank Dr. Marco Alberghini, MD, Director of the Surgical Pathology of Istituto Rizzoli for reviewing all slides.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Barker DJ, Clough PW, Guyer PB, Gardner MJ. Paget’s disease of bone in 14 British towns. BMJ. 1977;1:1181–1183. doi: 10.1136/bmj.1.6070.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry HC. Neoplastic changes. In: Barry HC, editor. Paget’s Disease of Bone. Edinburgh, Scotland: Livingstone; 1969. pp. 136–193. [Google Scholar]
- 3.Carter SR, Grimer RJ, Sneath RS. A review of 13-years experience of osteosarcoma. Clin Orthop Relat Res. 1991;270:45–51. [PubMed] [Google Scholar]
- 4.Choquette D, Haraoui B, Altman RD, Pelletier JP. Simultaneous multifocal sarcomatous degeneration in Paget’s disease of bone: a hypothesis. Clin Orthop Relat Res. 1983;179:308–311. doi: 10.1097/00003086-198310000-00046. [DOI] [PubMed] [Google Scholar]
- 5.Cundy HR, Gamble G, Wattie D, Rutland M, Cundy T. Paget’s disease of bone in New Zealand: continued decline in disease severity. Calcif Tissue Int. 2004;75:358–364. doi: 10.1007/s00223-004-0281-z. [DOI] [PubMed] [Google Scholar]
- 6.Detheridge FM, Guyer PB, Barker DJ. European distribution of Paget’s disease of bone. BMJ. 1982;285:1005–1008. doi: 10.1136/bmj.285.6347.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deyrup AT, Montag AG, Inwards CY, Xu Z, Swee RG, Unni KK. Sarcomas arising in Paget disease of bone (a clinicopathologic analysis of 70 cases) Arch Pathol Lab Med. 2007;131:942–946. doi: 10.5858/2007-131-942-SAIPDO. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich LA, Roodman GD. The role of immune cells and inflammatory cytokines in Paget’s disease and multiple myeloma. Immunol Rev. 2005;208:252–266. doi: 10.1111/j.0105-2896.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 9.Greditzer HG, III, McLeod RA, Unni KK, Beabout JW. Bone sarcomas in Paget disease. Radiology. 1983;146:327–333. doi: 10.1148/radiology.146.2.6571760. [DOI] [PubMed] [Google Scholar]
- 10.Haddaway MJ, Davie MW, McCall IW, Howdle S. Effect of age and gender on the number and distribution of sites in Paget’s disease of bone. Br J Radiol. 2007;80:532–536. doi: 10.1259/bjr/84718521. [DOI] [PubMed] [Google Scholar]
- 11.Hailbach H, Farrell C, Dittrich FJ. Neoplasms arising in Paget’s disease of bone: a study of 82 cases. Am J Clin Pathol. 1985;83:594–600. doi: 10.1093/ajcp/83.5.594. [DOI] [PubMed] [Google Scholar]
- 12.Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21:58–63. doi: 10.1359/jbmr.06s211. [DOI] [PubMed] [Google Scholar]
- 13.Huvos AG, Butler A, Bretsky SS. Osteogenic sarcoma associated with Paget’s disease of bone: a clinicopathologic study of 65 patients. Cancer. 1983;52:1489–1495. doi: 10.1002/1097-0142(19831015)52:8<1489::AID-CNCR2820520826>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Jattiot F, Goupille P, Azais I, Roulot B, Alcalay M, Jeannou J, Bontoux D, Valat JP. Fourteen cases of sarcomatous degeneration in Paget’s disease. J Rheumatol. 1999;26:150–155. [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 16.Lewis MM, Rudin BJ, Steiner GC. Multifocal Paget’s sarcoma. Bull Hosp Jt Dis Orthop Inst. 1985;45:162–168. [PubMed] [Google Scholar]
- 17.Lyles KW, Siris ES, Singer FR, Meunier PJ. A clinical approach to diagnosis and management of Paget’s disease of bone. J Bone Miner Res. 2001;16:1379–1387. doi: 10.1359/jbmr.2001.16.8.1379. [DOI] [PubMed] [Google Scholar]
- 18.Mangham DC, Davie MW, Grimer RJ. Sarcoma arising in Paget’s disease of bone: declining incidence and increasing age at presentation. Bone. 2009;44:431–436. doi: 10.1016/j.bone.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Mankin HJ, Hornicek FJ. Paget’s sarcoma: a historical and outcome review. Clin Orthop Relat Res. 2005;438:97–102. doi: 10.1097/01.blo.0000180053.99840.27. [DOI] [PubMed] [Google Scholar]
- 20.McKenna RJ, Schwinn CP, Soong KY, Higinbotham NL. Osteogenic sarcoma in Paget’s disease. Cancer. 1964;17:42–66. doi: 10.1002/1097-0142(196401)17:1<42::AID-CNCR2820170108>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 21.Merkow RL, Lane JM. Paget’s disease of bone. Orthop Clin North Am. 1990;21:171–189. [PubMed] [Google Scholar]
- 22.Moore TE, King AR, Kathol MH, El-Khoury GY, Palmer R, Downey PR. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199–1203. doi: 10.2214/ajr.156.6.2028867. [DOI] [PubMed] [Google Scholar]
- 23.Morlock G, Sanvy J, Serre H. Sarcomas associated with Paget’s disease. Rev Rhum Mal Osteoartic. 1975;42:669–679. [PubMed] [Google Scholar]
- 24.Ozaki T, Ikeda S, Kawai A, et al. Alterations of retinoblastoma susceptibility gene accompained by c-myc amplification in human bone and soft tissue tumors. Cell Mol Biol. 1993;39:235–242. [PubMed] [Google Scholar]
- 25.Paget J. On a form of chronic inflammation of bones (osteitis deformans) Clin Orthop Relat Res. 1966;49:3–16. doi: 10.1097/00003086-196611000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Poretta C, Dahlin DC, Janes JM. Sarcoma in Paget’s disease of bone. J Bone Joint Surg Am. 1957;39:1314–1329. [PubMed] [Google Scholar]
- 27.Price CH, Goldie W. Paget’s sarcoma of bone. A study of eighty cases from the Bristol and the Leeds bone tumour registries. J Bone Joint Surg Br. 1969;51:205–224. [PubMed] [Google Scholar]
- 28.Rosenmertz SK, Schare HJ. Osteogenic sarcoma arising in Paget’s disease of mandible: review of the literature and report of a case. Oral Surg Oral Med Oral Pathol. 1969;28:304–309. doi: 10.1016/0030-4220(69)90219-9. [DOI] [PubMed] [Google Scholar]
- 29.Schatzki S, Dudley HR. Bone sarcoma complicating Paget’s disease: a report of 3 cases with long survival. Cancer. 1961;14:517–523. doi: 10.1002/1097-0142(199005/06)14:3<517::AID-CNCR2820140311>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Schmorl G. About Pagetic osteitis deformans [in German] Virchows Arch Pathol Anat Physiol. 1932;283:694–751. doi: 10.1007/BF01887990. [DOI] [Google Scholar]
- 31.Seton M, Choi HK, Hansen MF, Sebaldt RJ, Cooper C. Analysis of environmental factors in familial versus sporadic Paget’s disease of bone—the New England Registry for Paget’s Disease of Bone. J Bone Miner Res. 2003;18:1519–1524. doi: 10.1359/jbmr.2003.18.8.1519. [DOI] [PubMed] [Google Scholar]
- 32.Shaylor PJ, Peake D, Grimer RJ, Carter SR, Tillman RM, Spooner D. Paget’s osteosarcoma—no cure in sight. Sarcoma. 1999;3:191–192. doi: 10.1080/13577149977631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverman SL. Paget disease of bone (Therapeutic Options) J Clin Rheumatol. 2008;14:299–305. doi: 10.1097/RHU.0b013e318188b1f3. [DOI] [PubMed] [Google Scholar]
- 34.Singer FR. Paget’s sarcoma. In: Singer FR, editor. Paget’s Disease of Bone. New York, NY: Plenum Publishing; 1977. pp. 93–102. [Google Scholar]
- 35.Smith J, Botet JF, Yeh SDJ. Bone sarcomas in Paget disease: a study of 85 patients. Radiology. 1984;152:583–590. doi: 10.1148/radiology.152.3.6235535. [DOI] [PubMed] [Google Scholar]
- 36.Ueda T, Healey JH, Huvos AG, Ladanyi M. Amplification of the MYC gene in osteosarcoma secondary to Paget’s disease of bone. Sarcoma. 1997;1:131–134. doi: 10.1080/13577149778209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wick MR, Siegal GP, Unni KK, McLeod RA, Greditzer HG., III Sarcomas of bone complicating osteitis deformans (Paget’s disease): fifty years’ experience. Am J Surg Pathol. 1981;5:47–59. doi: 10.1097/00000478-198101000-00008. [DOI] [PubMed] [Google Scholar]