Abstract
Study Design
Cross-sectional study with repeated measures design.
Objective
To compare the myosin heavy chain isoform distribution within and between paraspinal muscles and to test the theory that fiber type gradients exist as a function of paraspinal muscle depth.
Summary of Background Data
There is still uncertainty regarding the fiber type distributions within different paraspinal muscles. It has been previously proposed that deep fibers of the multifidus muscle may contain a higher ratio of type I to type II fibers, because, unlike superficial fibers, they primarily stabilize the spine, and may therefore have relatively higher endurance. Using a minimally invasive surgical approach, utilizing tubular retractors that are placed within anatomic inter-muscular planes, it was feasible to obtain biopsies from the multifidus, longissimus, iliocostalis and psoas muscles at specific predefined depths.
Methods
Under an IRB approved protocol, muscle biopsies were obtained from 15 patients who underwent minimally invasive spinal surgery, using the posterior paramedian (Wiltse) approach or the minimally invasive lateral approach. Myosin heavy chain (MyHC) isoform distribution was analyzed using SDS-PAGE electrophoresis. Since multiple biopsies were obtained from each patient, MyHC distribution was compared using both within- and between-muscle repeated measures analyses.
Results
The fiber type distribution was similar among the posterior paraspinal muscles and was composed of relatively high percentage of type I (63%), compared to type IIA (19%) and type IIX (18%) fibers. In contrast, the psoas muscle was found to contain a lower percentage of type I fibers (42%) and a higher percentage of type IIA (33%) and IIX fibers (26%; P<0.05). No significant difference was found for fiber type distribution among three different depths of the multifidus and psoas muscles.
Conclusion
Fiber type distribution between the posterior paraspinal muscles is consistent and is composed of relatively high percentage of type I fibers, consistent with a postural function. The psoas muscle, on the other hand, is composed of a higher percentage of type II fibers such as in the appendicular muscles. Our data do not support the idea of a fiber type gradient as a function of depth for any muscle studied.
Keywords: Lumbar multifidus, Muscle mechanics, Lumbar spine
Introduction
Fiber type distribution within a muscle may provide insights into its functional characteristics. Mammalian muscle fibers are divided into two general types according to their myosin heavy chain profile and oxidative activity. The type I fibers possess high oxidative activity, and express “slow” myosin, resulting in a prolonged twitch and low contraction velocity (hence, sometimes referred to as ‘slow twitch’).1 Type II fibers express “fast” myosin, resulting in a rapid twitch and high contraction velocity (hence, sometimes referred to as ‘fast twitch’).1 They are also better equipped with glycolytic enzymes that regenerate ATP by anaerobic mechanisms.2 Type II fibers can be further subdivided into two subtypes; the type IIX fiber (previously known as IIB) 3 and the type IIA fiber with type IIX being a faster isoform than type IIA. In fact, it has been demonstrated that each fiber type primarily expresses a specific isoform of the contractile protein myosin, which permits fiber types to be unambiguously identified on the basis of their differing myosin heavy chain isoforms (MyHC).4
Previous studies of fiber type distribution in paraspinal muscles consistently report higher type I to type II fiber ratios.5,6 This finding was purported to correlate with their primary role as stabilizers of the spine rather than movement generators.7 It has also been previously proposed, based on electromyography, that deep fibers of the multifidus (DM) muscle contain higher type I to type II fibers ratio compared to the superficial part (SM) of the muscle.8 This was interpreted to indicate that DM fibers act as a tonic stabilizer of the spine whereas SM fibers function as extensors and rotators of the spine similar to the rest of the erector spinae muscles. Unfortunately, this study only inferred fiber type from recruitment records obtained from electrophysiological studies.
Minimally invasive spine (MIS) surgery uses small diameter tubular retractors to gain access to the spinal elements, without disturbing the normal anatomy of the paraspinal muscles. The surgical retractors are placed and opened within intermuscular planes, enabling excellent visualization and access to the different muscles and to specific anatomic regions within each muscle. Using MIS, we were able to obtain muscle- and region-specific biopsies of different posterior and anterior paraspinal muscles, during surgery. Furthermore, for multifidus and psoas muscles, additional biopsies were taken from the deep, intermediate and superficial sections of the muscles from each patient.
This study has two main goals. The first goal was to compare fiber type distribution among posterior paraspinal and the psoas muscles, by measuring the MyHC isoform distribution. The second goal was to compare the gradient of MyHC isoforms within different depths of multifidus and psoas muscles.
Methods
Patient population
Under a University of California San Diego Human Subjects Protection Program approved protocol, multifidus, iliocostalis, longissimus, and psoas muscle specimens were obtained from patients undergoing spinal surgery.
Biopsy Technique
Muscle samples, from the posterior paraspinal muscles, were obtained from patients undergoing minimally invasive fusion with posterior instrumentation. A paramedian (Wiltse) approach to the posterior spine was utilized to access the posterior elements of the spine.9 The skin and dorsolumbar fascia were incised 4 cm lateral to the midline and the intermuscular plane between the multifidus and longissimus muscles was identified using blunt dissection down to the facet joint. Following the placement of an expandable tubular retractor between these muscles (QUADRANT –Medtronic, Sofamor-Danek Inc., USA) both multifidus and longissimus muscles could be approached at different muscle depths (Figure 1A). A small segment of the multifidus was collected from its superficial lateral border as well as its intermediate and deep segments, adjacent to the facet joint. Using the same skin incision, the tubular retractor was repositioned laterally, between longissimus and iliocostalis muscles, in order to obtain a biopsy from the superficial medial border of the iliocostalis muscle (Figure 1B). The psoas muscle biopsies were obtained from patients undergoing minimally invasive lateral approach, interbody fusion.10 The patients were positioned in the lateral decubitus position. Using a posterior retroperitoneal approach, the psoas muscle was identified and then gently separated with serial dilators using blunt dissection. Special care was taken to minimize muscle damage during this procedure. An expandable retractor was then inserted over the tubular dilators and placed within the muscle and on top of the disc annulus (DLIF –Medtronic, Sofamor-Danek Inc., USA). Once the retractor blades were expanded, the psoas muscle was approached and biopsied at superficial, intermediate and deep levels (Figure 1C).
Figure 1.
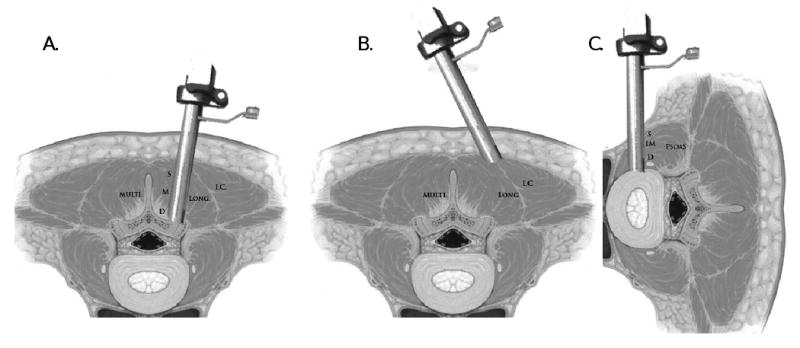
Anatomic illustration of the paraspinal biopsy technique. A. The surgical retractor is positioned between the multifidus and longissimus muscles. Biopsies were obtained from the superficial (S), middle (M), and deep (D) sections of the multifidus muscle. B. The surgical retractor is positioned between the longissimus and iliocostalis muscles. C. The surgical retractor is positioned inside the psoas muscle. Biopsies were obtained from the superficial (S), middle (M), and deep (D) sections of the psoas muscle. Figure adapted from Guiot et al. SPINE Volume 27, Number 4, pp 432–438
Fiber type analysis
Myosin heavy chain (MyHC) isoforms were defined by SDS-PAGE as previously described.11 A myofibril-rich fraction of individual biopsies (n=74 biopsies from 15 patients) was prepared and the final pellet was resuspended to 0.125 μg μl-1 protein (BCA protein assay, Pierce, Rockford, IL) in a sample buffer consisting of dithiothreitol (DTT, 100 mM), sodium dodecyl sulphate (SDS, 2%), Tris-base (80 mM) pH 6.8, glycerol (10%) and Bromophenol Blue (1.2% w/v). Samples were boiled (2 min) and stored at -80°C for up to two weeks prior to loading onto the gel. Total acrylamide concentration was 4% and 8% in the stacking and resolving gels, respectively (bis-acrylamide, 1:50). Gels (16 × 22 cm, 0.75 mm thick) were run at a constant current of 10 mA until voltage rose to 275 V, and thereafter at constant voltage for 21 h at 4-6°C. A volume of 1.25 μg total protein was loaded into a well and gels were silver stained. To be certain that adequate sensitivity was achieved for detecting minor MyHC bands, each sample was also run on separate gels that were silver-stained (BioRad, Hercules, CA). Because of the approximately 50-fold greater sensitivity of silver stain, samples were diluted 5-10-fold. MyHC bands were identified and quantified with densitometry (GS-800, BioRad).
Data Analysis
Between-muscle comparisons of MyHC content were made using one-way analyses of variance with repeated measures after screening data for normality and homogeneity of variances. For intramuscular comparison of MyHC, repeated measures one-way analyses of variance was used to evaluate trends in fiber type distribution within each patient. When significant differences were identified for each dependent variable, post-hoc LSD tests were used to identify differences between individual muscles and between muscle regions. All values are reported as mean ± standard error unless otherwise noted. Statistical tests were performed using SPSS (version 15.0, Chicago, IL, USA) with p-values set to 0.05.
Results
Muscle biopsies were collected from 15 patients, 4 males and 11 females, who underwent minimally invasive spinal surgery by a single surgeon. The average age of the patients was 68±12 (mean±SD) years. Nine patients underwent minimally invasive lateral approach interbody fusion with percutaneous pedicle screws instrumentation. The six remaining patients underwent a posterior only decompression with fusion. A total of 74 biopsies were collected and analyzed including 31 multifidus, 24 psoas, 8 longissimus and 8 Iliocostalis. Three biopsies were discarded due to technical problems.
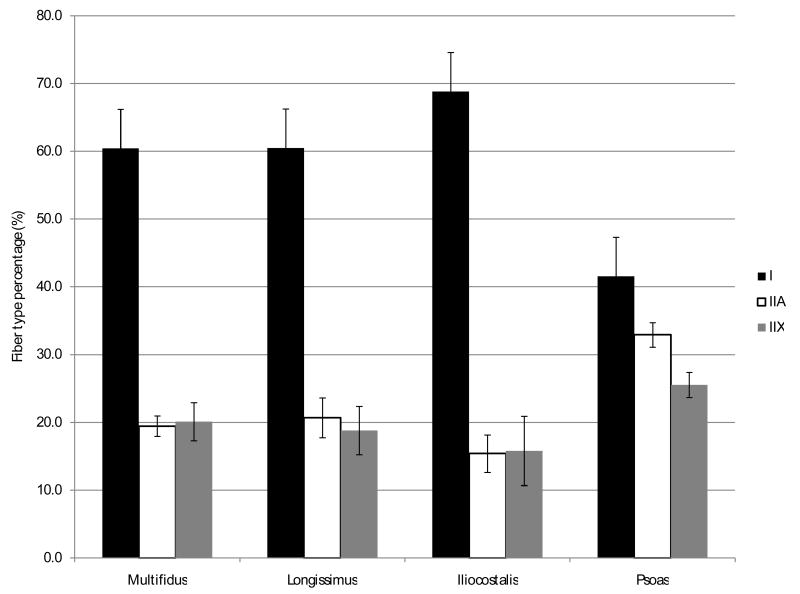
Fiber type distribution among posterior paraspinal muscles (multifidus, longissimus and iliocostalis) was similar (Figure 2). Consistent with previous studies, we found a predominance of type I fibers (63.3±4.7%) and an even distribution of type II fibers subtypes IIA (18.5±2.4%) and IIX (18.2±3.8%). However, the fiber type distribution in the psoas muscles consisted of predominantly type II fibers (58.5±1.8%) with a higher proportion of type IIA (32.9±1.8%) than type IIB (25.5±1.8%) fibers.
Figure 2.
Fiber type distribution in the different paraspinal muscles.
The fiber type distribution was also found to be similar when comparing the superficial, middle, and deep sections of the multifidus and psoas muscles (Figures 3 and 4). This demonstrates that there are no fiber type gradients within these muscles.
Figure 3.
Fiber type distribution among different depths of the psoas muscle.
Figure 4.
Fiber type distribution among different depths of the multifidus muscle.
Discussion
Extensive effort has been devoted to the study of fiber type characteristics in the paraspinal muscles, both in the healthy population and in low back pain (LBP) patients.7 Our goal was to determine the normal fiber type distribution of paraspinal muscles using modern methodology in order to better understand their function in the normal spine and to evaluate the alterations that might result due to spinal pathologies.
To date, fiber type analysis from living patients was performed during open posterior spine surgery or using percutaneous needle biopsy.12,13 Both of these biopsy techniques are limited in their anatomic accuracy since they rely on proximity to the spinous process for localization. As a result, studies that investigated regional distribution of fiber types among different paraspinal muscles were forced to use cadaveric specimens, whose spinal history was unknown. The use of a minimally invasive approach for obtaining biopsies has enabled us to obtain biopsies from different posterior paraspinal muscles as well as from the psoas muscle.14,15,10 By using the intermuscular plane between the multifidus and longissimus muscles we were also able to biopsy the multifidus muscle at different depths ranging from its deepest fibers that attach to the superior articular process through the intermediate and the superficial parts.9,16
The patient sample in this study included only patients who suffered from severe long-standing spinal degeneration. Nevertheless, when comparing the fiber type distribution in the muscle biopsies taken from these patients, we did not show a higher percentage of type II fibers compared to published data from the healthy population.5,24
There is consistent evidence to show that paraspinal muscles exhibit an array of histopathological features in patients with spinal degeneration. These changes include: fiber type specific atrophy, fatty infiltration, core target fibers as well as fiber type grouping.17 However there is conflicting evidence with regards to alteration in fiber type distribution after spinal degeneration. When comparing muscle biopsies from patients with low back pain undergoing spinal surgery to matching healthy individuals, Manion et al. found a higher percentage of type II fibers in the LBP population.12 They proposed that a genetic tendency for a higher type II to type I ratio in this population might result in easy fatigability of the paraspinal muscles. However, this statement fails to recognize that type IIA fibers have very high oxidative capacity and thus, are not easily fatigued. Previous functional and electrophysiological studies have reported that chronic LBP patients exhibit significantly reduced endurance compared to normal subjects.18,19 It was hypothesized that this easy fatigability in LBP patients might expose their spinal, osteo-ligamentous structures, to increased, recurrent trauma during normal, daily activities, which results in accelerated degeneration and back pain.20, 21 However, the results of our study, as well as previous studies, do not support that a change in fiber type is the source of this altered fatigability.6,22,23
Previous functional studies also suggested that the deep fibers of the multifidus muscle possess mainly a tonic stabilizing function whereas its superficial fibers have largely a phasic function acting as extensors and rotators of the spine similar to the function of erector spinae muscles.8,25,26 Therefore it was suggested that the deeper section of the multifidus should be composed of a relatively higher proportion of type I fibers compared to its superficial parts. However there is limited and confounding evidence to support this theory.27 Similarly, there are sparse and contradicting data regarding differences in fiber type distribution between the different paraspinal muscles. Jorgensen et al. reported a higher proportion of type I fibers in the longissimus than in the multifidus or the iliocostalis muscles.13 In contrast, two other studies found no difference in fiber type distribution between these muscles.28,24 All of these studies have failed to use a within-subject comparison of the samples. This is significant since it was shown that fiber type distribution is subject to relatively high degree variability among subjects. Moreover, histochemical staining techniques for ATPase, used to measure the fiber type distribution in these studies, are indirect and thus less accurate methods to determine the fiber type distribution compared to direct measurements of the MyHC distribution.29, 30
Our data demonstrate that there is no difference in fiber type distribution among the different posterior paraspinal muscles. Moreover, no differences were found comparing different regions of multifidus and psoas muscles. This was found when comparing both the entire sampling group and within each subject. A significant difference was found between fiber type distributions in the psoas muscle compared to the posterior paraspinal muscles. This probably results from the psoas functioning as a flexor of the hip joint in addition to it being a paraspinal muscle.
Although no difference in fiber type distribution was found between the posterior paraspinal muscles nor within the multifidus muscle, it is important to note that this similarity in MyHC distribution between these muscles does not necessarily imply that their functional role is identical. It is certainly possible that the extracellular rather than the intracellular muscle components are important in the determination of its function.31 Recent studies, published by our group, found that the multifidus muscle has a distinctively different architectural design and biomechanical properties compared to the other paraspinal muscles.32 It is composed of shorter fibers that are arranged in tightly packed bundles, creating a muscle with a relatively high physiological cross-sectional area (PCSA). This unique design enables it to produce large forces over a relatively short excursion, making it ideally suited to act as a stabilizer rather than a mover of the spine. Additionally, its elastic modulus was found to be greater compared to the other paraspinal muscles. Future studies should continue to characterize this muscle group by defining both intracellular and extracellular proteins and alterations to these systems with back disease.
Conclusion
Using a minimally invasive approach it was possible to obtain anatomically well-defined muscle biopsies from specific paraspinal muscles as well as from specific anatomic regions of these muscles, in patients undergoing spinal surgery. Our results demonstrate that the fiber type distribution among the different posterior paraspinal muscles as well as within different depths of the multifidus muscle are the same and are composed primarily of type I fibers. The psoas muscle on the other hand is composed of a higher proportion of type II fibers, similar to the other appendicular muscles.
Key points.
Obtaining muscle biopsies from different paraspinal muscles, of living spinal patient, is feasible using MIS technique.
Fiber type distribution was consistent among the posterior paraspinal muscles and at different depths of the multifidus and psoas muscles.
The psoas muscle is composed of a higher proportion of type II fibers, similar to the appendicular muscles.
Acknowledgments
This work was supported by NIH grants HD048501, and HD050837. Dr. Regev was supported by the fellowship grant from the American Physician Fellowship for Medicine in Israel.
Footnotes
IRB: Each author certifies that his or her institution has approved the protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Bibliography
- 1.Pette D, Spamer C. Metabolic properties of muscle fibers. Fed Proc. 1986;45(13):2910–4. [PubMed] [Google Scholar]
- 2.Pereira Sant'Ana JA, Ennion S, Sargeant AJ, Moorman AF, Goldspink G. Comparison of the molecular, antigenic and ATPase determinants of fast myosin heavy chains in rat and human: a single-fibre study. Pflugers Arch. 1997;435(1):151–63. doi: 10.1007/s004240050495. [DOI] [PubMed] [Google Scholar]
- 3.Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand L, Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol. 1994;267(6 Pt 1):C1723–8. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- 4.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76(2):371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 5.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18(1):111–29. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 6.Ford D, Bagnall KM, McFadden KD, Greenhill B, Raso J. Analysis of vertebral muscle obtained during surgery for correction of a lumbar disc disorder. Acta Anat (Basel) 1983;116(2):152–7. doi: 10.1159/000145737. [DOI] [PubMed] [Google Scholar]
- 7.Mannion AF. Fibre type characteristics and function of the human paraspinal muscles: normal values and changes in association with low back pain. J Electromyogr Kinesiol. 1999;9(6):363–77. doi: 10.1016/s1050-6411(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 8.Donisch EW, Basmajian JV. Electromyography of deep back muscles in man. Am J Anat. 1972;133(1):25–36. doi: 10.1002/aja.1001330103. [DOI] [PubMed] [Google Scholar]
- 9.Vialle R, Wicart P, Drain O, Dubousset J, Court C. The Wiltse paraspinal approach to the lumbar spine revisited: an anatomic study. Clin Orthop Relat Res. 2006;445:175–80. doi: 10.1097/01.blo.0000203466.20314.2a. [DOI] [PubMed] [Google Scholar]
- 10.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–43. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75(5):2337–40. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- 12.Mannion AF, Weber BR, Dvorak J, Grob D, Muntener M. Fibre type characteristics of the lumbar paraspinal muscles in normal healthy subjects and in patients with low back pain. J Orthop Res. 1997;15(6):881–7. doi: 10.1002/jor.1100150614. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen K, Nicholaisen T, Kato M. Muscle fiber distribution, capillary density, and enzymatic activities in the lumbar paravertebral muscles of young men. Significance for isometric endurance. Spine. 1993;18(11):1439–50. [PubMed] [Google Scholar]
- 14.Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28(15 Suppl):S26–35. doi: 10.1097/01.BRS.0000076895.52418.5E. [DOI] [PubMed] [Google Scholar]
- 15.Holly LT, Schwender JD, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus. 2006;20(3):E6. doi: 10.3171/foc.2006.20.3.7. [DOI] [PubMed] [Google Scholar]
- 16.Anand N, Baron EM, Bray RS. Benefits of the Paraspinal Muscle-Sparing Approach Versus the Conventional Midline Approach for Posterior Nonfusion Stabilization: Comparative Analysis of Clinical and Functional Outcomes. SAS Journal. 2007;1:63–67. doi: 10.1016/SASJ-2007-0101-RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattila M, Hurme M, Alaranta H, Paljarvi L, Kalimo H, Falck B, Lehto M, Einola S, Jarvinen M. The multifidus muscle in patients with lumbar disc herniation. A histochemical and morphometric analysis of intraoperative biopsies. Spine. 1986;11(7):732–8. doi: 10.1097/00007632-198609000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Alaranta H, Luoto S, Heliovaara M, Hurri H. Static back endurance and the risk of low-back pain. Clin Biomech (Bristol, Avon) 1995;10(6):323–324. doi: 10.1016/0268-0033(95)00002-3. [DOI] [PubMed] [Google Scholar]
- 19.Dolan P, Adams MA. Repetitive lifting tasks fatigue the back muscles and increase the bending moment acting on the lumbar spine. J Biomech. 1998;31(8):713–21. doi: 10.1016/s0021-9290(98)00086-4. [DOI] [PubMed] [Google Scholar]
- 20.Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5(4):390–6. doi: 10.1097/00002517-199212000-00002. discussion 397. [DOI] [PubMed] [Google Scholar]
- 21.Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5(4):383–9. doi: 10.1097/00002517-199212000-00001. discussion 397. [DOI] [PubMed] [Google Scholar]
- 22.Bajek S, Bobinac D, Bajek G, Vranic TS, Lah B, Dragojevic DM. Muscle fiber type distribution in multifidus muscle in cases of lumbar disc herniation. Acta Med Okayama. 2000;54(6):235–41. doi: 10.18926/AMO/32283. [DOI] [PubMed] [Google Scholar]
- 23.Crossman K, Mahon M, Watson PJ, Oldham JA, Cooper RG. Chronic low back pain-associated paraspinal muscle dysfunction is not the result of a constitutionally determined “adverse” fiber-type composition. Spine. 2004;29(6):628–34. doi: 10.1097/01.brs.0000115133.97216.ec. [DOI] [PubMed] [Google Scholar]
- 24.Thorstensson A, Carlson H. Fibre types in human lumbar back muscles. Acta Physiol Scand. 1987;131(2):195–202. doi: 10.1111/j.1748-1716.1987.tb08226.x. [DOI] [PubMed] [Google Scholar]
- 25.Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine. 2002;27(2):E29–36. doi: 10.1097/00007632-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 26.Lee LJ, Coppieters MW, Hodges PW. Differential activation of the thoracic multifidus and longissimus thoracis during trunk rotation. Spine. 2005;30(8):870–6. doi: 10.1097/01.brs.0000158956.77897.ec. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald DA, Moseley GL, Hodges PW. The lumbar multifidus: does the evidence support clinical beliefs? Man Ther. 2006;11(4):254–63. doi: 10.1016/j.math.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Rantanen J, Rissanen A, Kalimo H. Lumbar muscle fiber size and type distribution in normal subjects. Eur Spine J. 1994;3(6):331–5. doi: 10.1007/BF02200146. [DOI] [PubMed] [Google Scholar]
- 29.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50(6):500–9. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Weiss A, Schiaffino S, Leinwand LA. Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family: implications for functional diversity. J Mol Biol. 1999;290(1):61–75. doi: 10.1006/jmbi.1999.2865. [DOI] [PubMed] [Google Scholar]
- 31.Delp SL, Suryanarayanan S, Murray WM, Uhlir J, Triolo RJ. Architecture of the rectus abdominis, quadratus lumborum, and erector spinae. J Biomech. 2001;34(3):371–5. doi: 10.1016/s0021-9290(00)00202-5. [DOI] [PubMed] [Google Scholar]
- 32.Ward SR, Kim CW, Eng CM, Gottschalk LJt, Tomiya A, Garfin SR, Lieber RL. Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J Bone Joint Surg Am. 2009;91(1):176–85. doi: 10.2106/JBJS.G.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]