Abstract
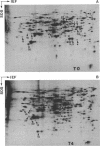
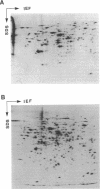
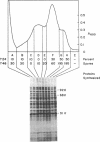
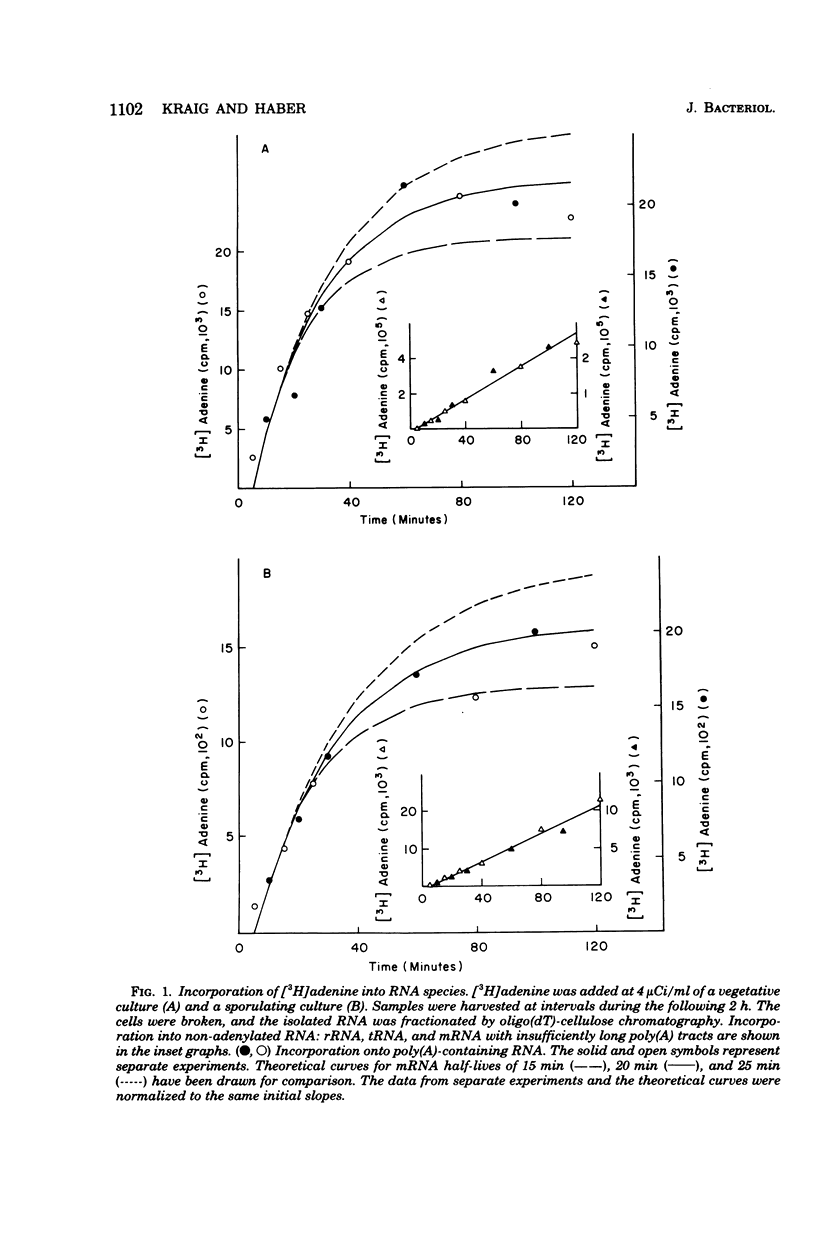
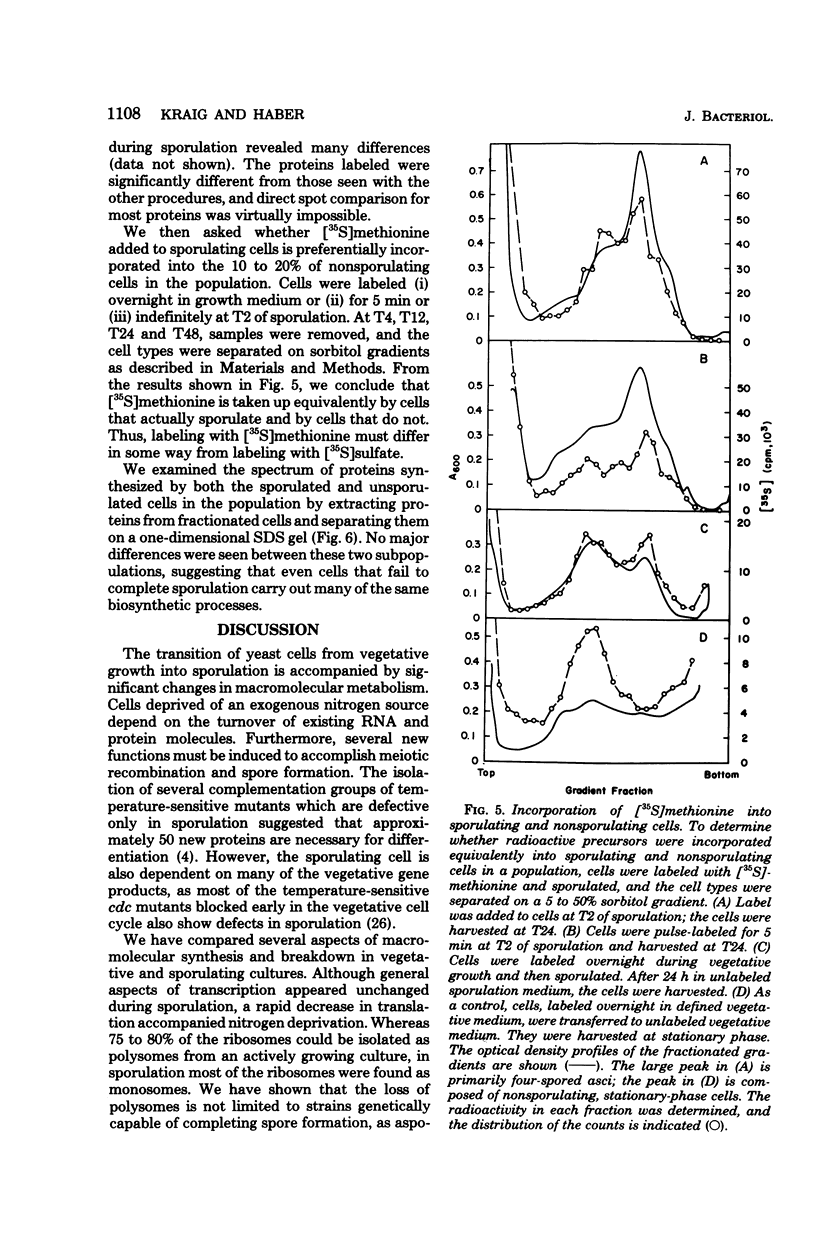
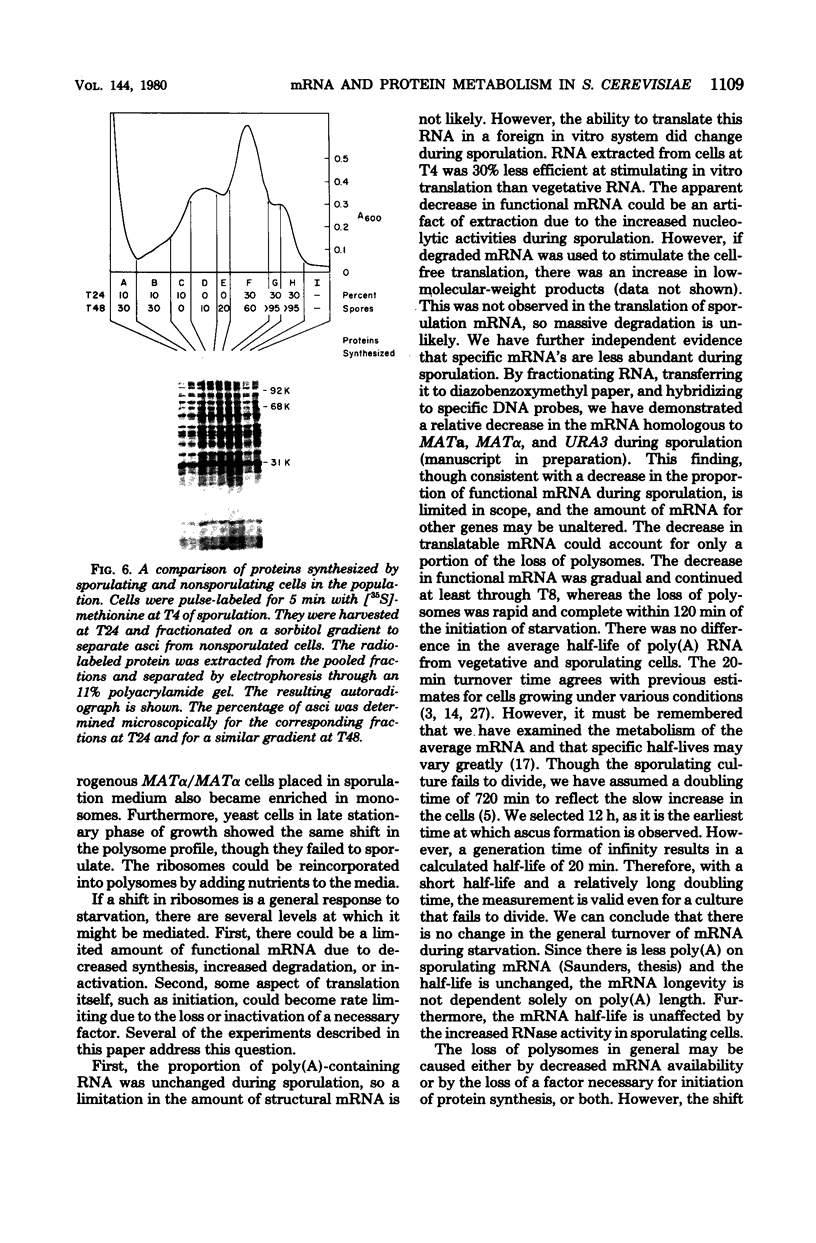
To investigate differences between growing yeasts and those undergoing sporulation, we compared several parameters of messenger ribonucleic acid (RNA) transcription and translation. The general properties of messenger RNA metabolism were not significantly altered by the starvation conditions accompanying sporulation. The average messenger RNA half-life, calculated from the kinetics of incorporation of [3H]adenine into polyadenylic acid-containing RNA, was 20 min on both cell populations. Furthermore, 1.3 to 1.4% of the total RNA was adenylated in both growing and sporulating cells. However, the proportion of RNA that could be translated in a wheat germ system slowly decreased during sporulation. Within 8 h after the induction of sporulation, isolated RNA stimulated half as much protein synthesis as the equivalent amount of vegetative RNA. There were significant differences in protein synthesis. The percentage of ribosomes in polysomes decreased threefold as the cells entered sporulation. This decrease began within 5 min of the initiation of sporulation, and the steady-state pattern was attained within 120 min. However, the ribosomes were not irreversibly inactivated; they could be reincorporated into polysomes by returning the sporulating cells to growth medium. Though unable to sporulate, strains homozygous for mating type, MAT alpha/MAT alpha, showed a similar decrease in the number of polysomes when placed in sporulation medium. Furthermore, the same shift toward monosomes was observed during stationary phase of growth. We conclude that the redistribution of ribosomes represents a general metabolic response to starvation. Our data indicate that the loss of polysomes is most likely caused by a decrease in the initiation of translation rather than a severe limitation in the amount of messenger RNA. Furthermore, the loss of polysomes is not due to the decreased synthesis of a major class of abundant proteins. Of the 400 vegetative proteins resolved by two-dimensional gel electrophoresis, only 19 were not synthesized by sporulating cells. Approximately 10 to 20% of the cells in a sporulating culture failed to complete ascus formation. We have shown that [35S]methionine is incorporated equivalently into cells committed to sporulation and cells that fail to form asci. Furthermore, the proteins synthesized by these two populations were indistinguishable, on one-dimensional gels. We compared proteins labeled by various protocols, including long-term and pulse-labeling during sporulation and prelabeling during vegetative growth before transfer to sporulation medium. The resulting two-dimensional gel patterns differed significantly. Many spots labeled by the long-term techniques may have arisen by protein processing. We suggest that pulse-labeling produces the most accurate reflection of instantaneous synthesis of proteins.
Full text
PDF



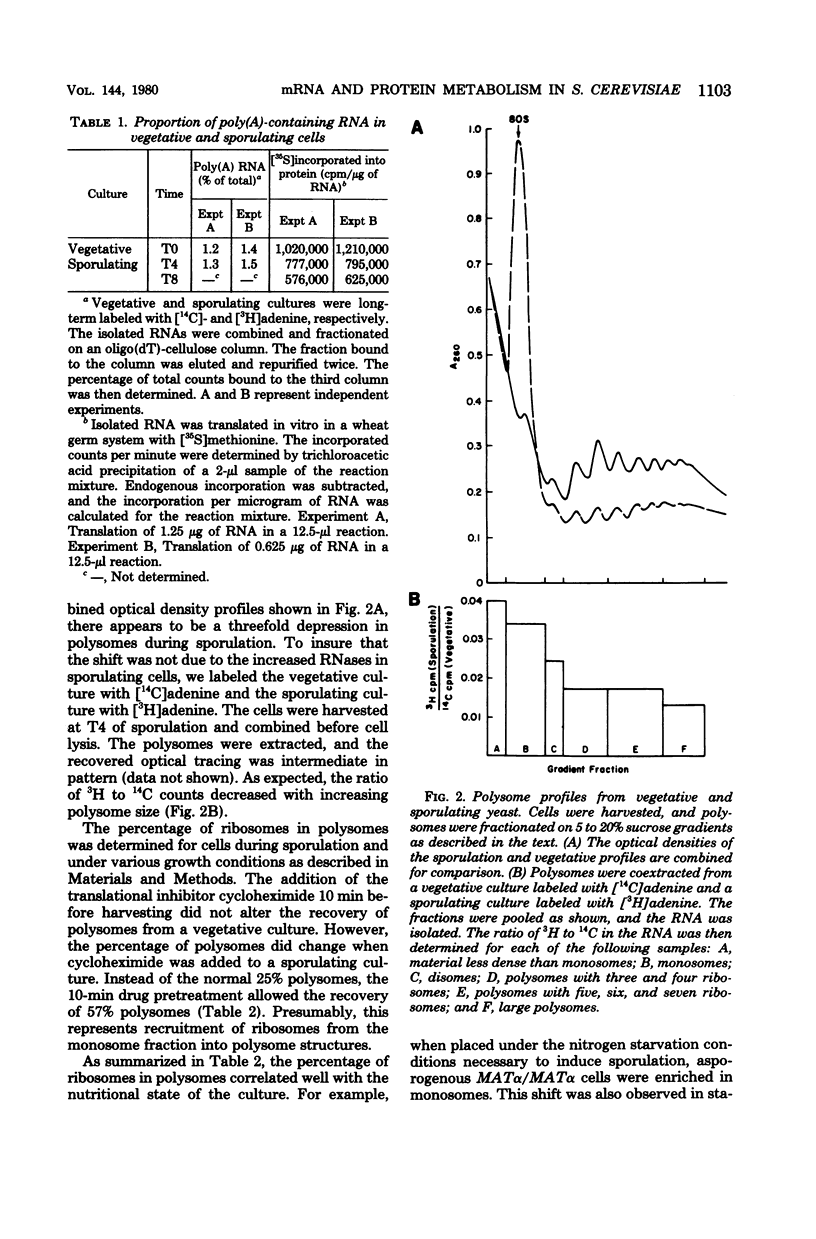

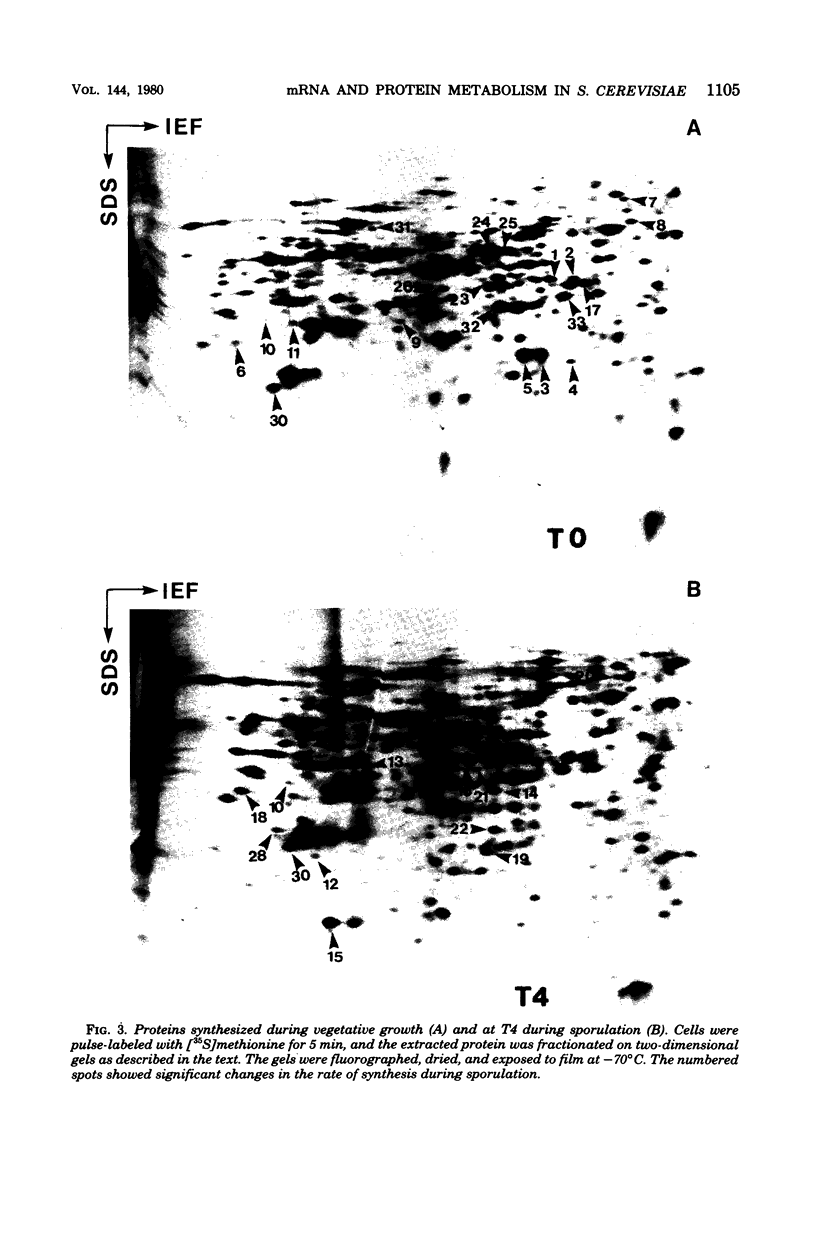
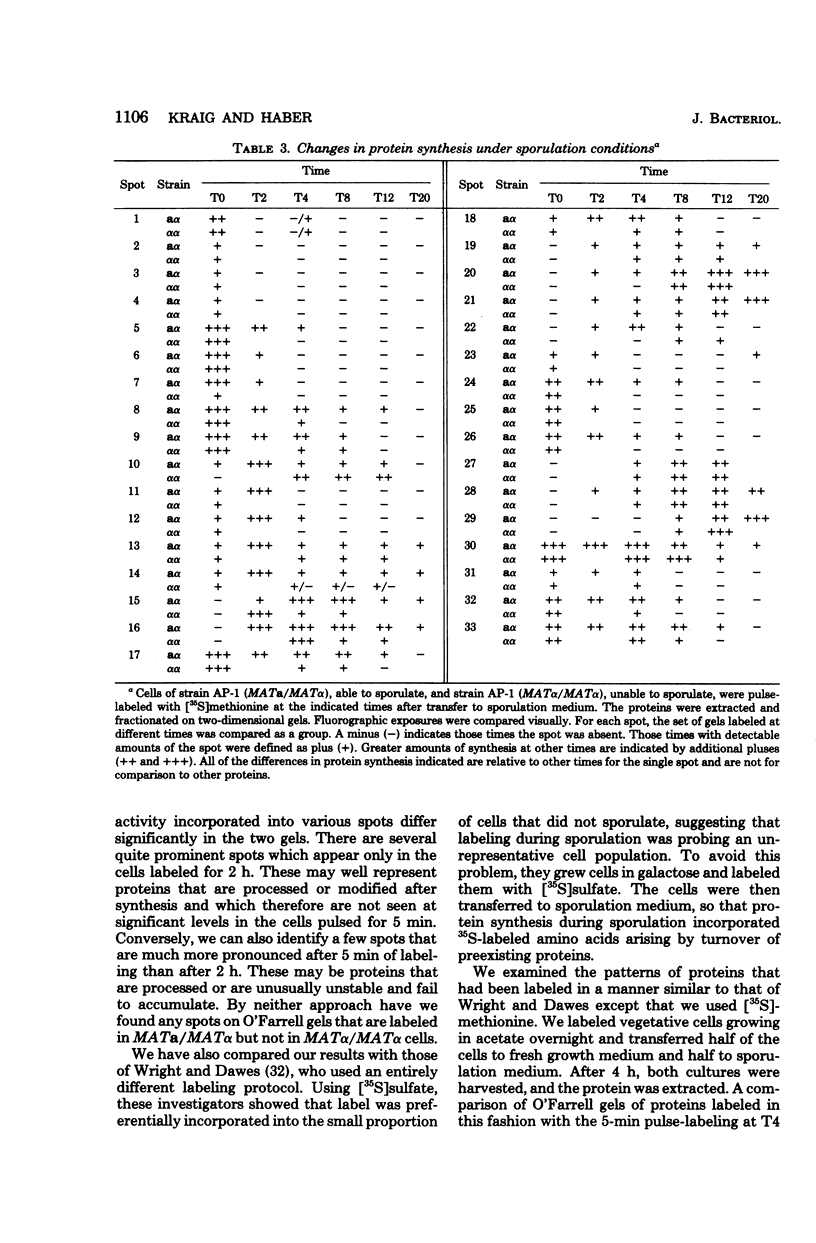
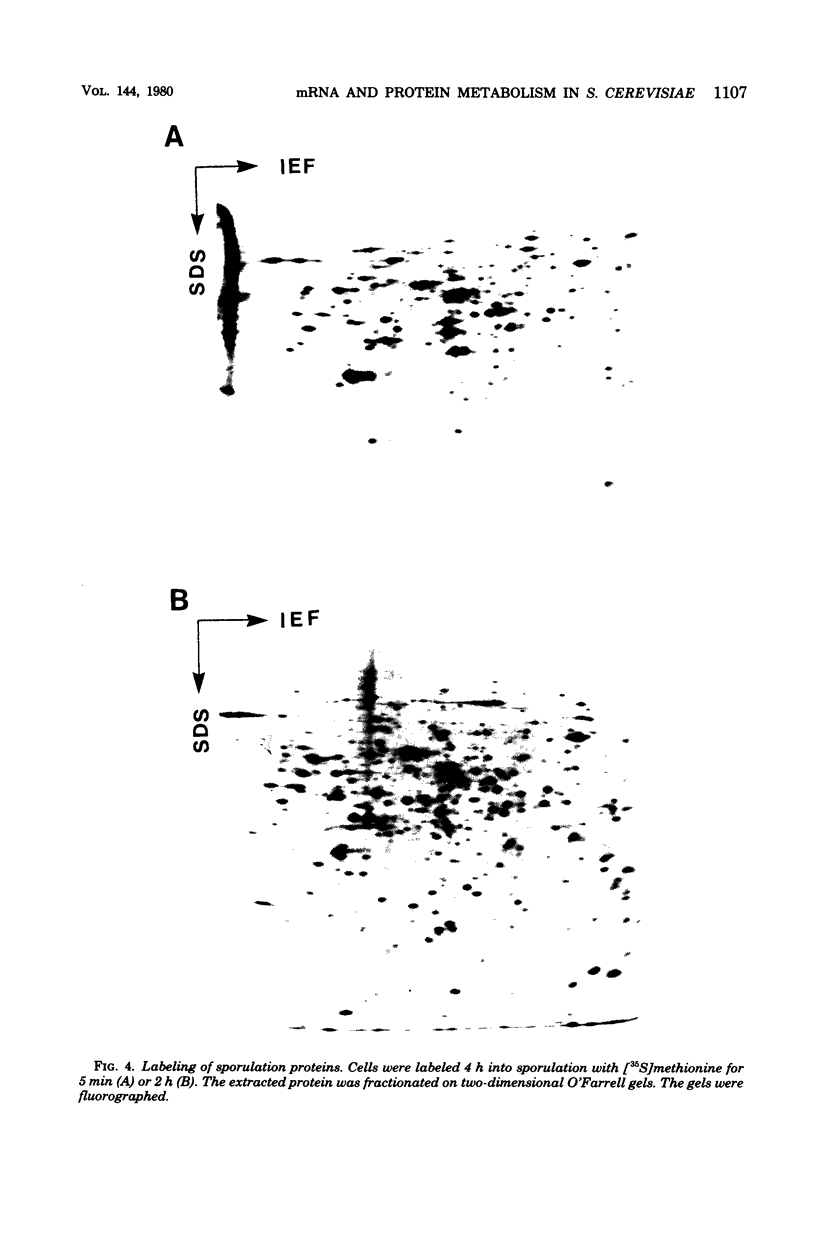



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Translational control of protein synthesis during the early stages of differentiation of the slime mold Dictyostelium discoideum. Cell. 1977 Sep;12(1):301–310. doi: 10.1016/0092-8674(77)90208-2. [DOI] [PubMed] [Google Scholar]
- Barnes W. M., Siegel R. B., Reznikoff W. S. The construction of lambda transducing phages containing deletions defining regulatory elements of the lac and trp operons in E. coli. Mol Gen Genet. 1974 Mar 27;129(3):201–215. doi: 10.1007/BF00267913. [DOI] [PubMed] [Google Scholar]
- Cannon M., Davies J. E., Jimenez A. Inhibition by lomofungin of nucleic acid and protein synthesis in Saccharomyces cerevisiae. FEBS Lett. 1973 Jun 1;32(2):277–280. doi: 10.1016/0014-5793(73)80852-x. [DOI] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E. Genes controlling meiosis and spore formation in yeast. Genetics. 1974 Sep;78(1):215–225. doi: 10.1093/genetics/78.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Frank K. R., Mills D. Ribosome activity and degradation in meiotic cells of Saccharomyces cerevisiae. Mol Gen Genet. 1978 Mar 20;160(1):59–65. doi: 10.1007/BF00275119. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Hutchison H. T., Holland T. M., McLaughlin C. S. The effect of cycloheximide upon polyribosome stability in two yeast mutants defective respectively in the initiation of polypeptide chains and in messenger RNA synthesis. Mol Gen Genet. 1970;106(4):347–361. doi: 10.1007/BF00324052. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Rosbash M. Number and distribution of polyadenylated RNA sequences in yeast. Cell. 1977 Mar;10(3):453–462. doi: 10.1016/0092-8674(77)90032-0. [DOI] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Hynes N. E., Phillips S. L. Turnover of polyadenylate-containing ribonucleic acid in Saccharomyces cerevisiae. J Bacteriol. 1976 Feb;125(2):595–600. doi: 10.1128/jb.125.2.595-600.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Lodish H. F. Genetic control of development of the cellular slime mold, Dictyostelium discoideum. Annu Rev Genet. 1975;9:145–185. doi: 10.1146/annurev.ge.09.120175.001045. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Halvorson H. O. Proteinase activities of Saccharomyces cerevisiae during sporulation. J Bacteriol. 1975 Nov;124(2):863–869. doi: 10.1128/jb.124.2.863-869.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Cooper T. G. Kinetics of induced and repressed enzyme synthesis in Saccharomyces cerevisiae. J Bacteriol. 1975 Mar;121(3):1064–1073. doi: 10.1128/jb.121.3.1064-1073.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Hopper A. K. Protein synthesis in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Sep;119(3):952–960. doi: 10.1128/jb.119.3.952-960.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager W. H., Planta R. J. Yeast ribosomal proteins are synthesized on small polysomes. Eur J Biochem. 1976 Feb 2;62(1):193–197. doi: 10.1111/j.1432-1033.1976.tb10113.x. [DOI] [PubMed] [Google Scholar]
- McCusker J. H., Haber J. E. Efficient sporulation of yeast in media buffered near pH6. J Bacteriol. 1977 Oct;132(1):180–185. doi: 10.1128/jb.132.1.180-185.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. Effect of pH on adenine and amino acid uptake during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Oct;112(1):519–526. doi: 10.1128/jb.112.1.519-526.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. Isolation of polyribosomes from yeast during sporulation and vegetative growth. Appl Microbiol. 1974 May;27(5):944–948. doi: 10.1128/am.27.5.944-948.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pearson N. J., Haber J. E. Changes in regulation of ribosomal protein synthesis during vegetative growth and sporulation of Saccharomyces cerevisiae. J Bacteriol. 1980 Sep;143(3):1411–1419. doi: 10.1128/jb.143.3.1411-1419.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo V., Simchen G., Shilo B. Initiation of meiosis in cell cycle initiation mutants of Saccharomyces cerevisiae. Exp Cell Res. 1978 Mar 15;112(2):241–248. doi: 10.1016/0014-4827(78)90206-9. [DOI] [PubMed] [Google Scholar]
- Tonnesen T., Friesen J. D. Inhibitors of ribonucleic acid synthesis in Saccharomyces cerevisiae: decay rate of messenger ribonucleic acid. J Bacteriol. 1973 Sep;115(3):889–896. doi: 10.1128/jb.115.3.889-896.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trew B. J., Friesen J. D., Moens P. B. Two-dimensional protein patterns during growth and sporulation in Saccharomyces cerevisiae. J Bacteriol. 1979 Apr;138(1):60–69. doi: 10.1128/jb.138.1.60-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C., Fulton C. Transcription during cell differentiation in Naegleria gruberi. Preferential synthesis of messenger RNA. Biochim Biophys Acta. 1973 Jun 8;312(1):52–71. doi: 10.1016/0005-2787(73)90052-x. [DOI] [PubMed] [Google Scholar]
- Wejksnora P. J., Haber J. E. Ribonucleoprotein particle appearing during sporulation in yeast. J Bacteriol. 1978 Apr;134(1):246–260. doi: 10.1128/jb.134.1.246-260.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitomer R. S., Montgomery D. L., Nichols D. L., Hall B. D. Transcriptional regulation of the yeast cytochrome c gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3627–3631. doi: 10.1073/pnas.76.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko G. S., Mitchell A. P., Jones E. W. Septum formation, cell division, and sporulation in mutants of yeast deficient in proteinase B. Proc Natl Acad Sci U S A. 1979 May;76(5):2395–2399. doi: 10.1073/pnas.76.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]