Abstract
Estrogen receptor α (ERα) expression in breast cancer is predictive of response to endocrine therapy; however, resistance is common in ERα-positive tumors that overexpress the growth factor receptor ERBB2. Even in the absence of estrogen, ERα can be activated by growth factors, including the epidermal growth factor (EGF). EGF induces a transcriptional program distinct from estrogen; however, the mechanism of the stimulus-specific response is unknown. Here we show that the EGF-induced ERα genomic targets, its cistromes, are distinct from those induced by estrogen in a process dependent on the transcription factor AP-1. The EGF-induced ERα cistrome specifically regulates genes found overexpressed in ERBB2-positive human breast cancers. This provides a potential molecular explanation for the endocrine therapy resistance seen in ERα-positive breast cancers that overexpress ERBB2. These results suggest a central role for ERα in hormone-refractory breast tumors dependent on growth factor pathway activation and favors the development of therapeutic strategies completely antagonizing ERα, as opposed to blocking its estrogen responsiveness alone.
Keywords: ERBB2, breast cancer, cistrome, estrogen receptor, growth factors, transcription
More than two-thirds of human breast cancers overexpress the estrogen receptor α (ERα), where it is both a target of endocrine therapy and a predictor of response (Sorlie et al. 2001, 2003). Upon activation by estrogen, ERα is recruited to thousands of sites across the genome of human breast cancer cells, defining its cistrome (Carroll et al. 2005, 2006; Lin et al. 2007; Hua et al. 2008; Hurtado et al. 2008; Liu et al. 2008; Lupien et al. 2008; Fullwood et al. 2009). This process is highly organized through specific epigenetic events that restrict the recruitment of the receptor to a subset of its potential binding sites (Lupien et al. 2008). Accordingly, this ERα cistrome guides the response to estrogen in breast cancer cells by favoring the implementation of an ERα-positive breast tumor-specific transcriptional program (Carroll et al. 2006). The importance of the cistrome in defining a specific transcriptional program is further supported by work in osteosarcoma cells. Indeed, estrogen stimulation in these cells results in a distinct expression profile (Monroe et al. 2003; Krum et al. 2008) that is directly related to a unique ERα cistrome (Krum et al. 2008). Such lineage-specific recruitment patterns were also reported recently for other factors. Indeed, cell type-specific transcriptional programs associated with FoxA1 and p300 are linked to their lineage-specific cistromes (Lupien et al. 2008; Visel et al. 2009). Thus, the contribution of a given transcription factor to the execution of a specific transcriptional program is highly dependent on its cell type-specific cistrome.
In addition to cell type-specific transcription programs, ERα can also respond to a variety of stimuli in a given cell type. In breast cancer cells, ERα can be stimulated even in the absence of estrogen by a variety of growth factors, including epidermal growth factor (EGF) (Kato et al. 1995; Bunone et al. 1996; Joel et al. 1998; Smith 1998; Kato 2001; Kurokawa and Arteaga 2003). Indeed, growth factor-stimulated breast cancer proliferation as well as normal uterine growth is dependent on ERα (Ignar-Trowbridge et al. 1992; Kato et al. 1995; Lee and Yee 1995; Curtis et al. 1996; Lupu et al. 1996; Knowlden et al. 2003; Schiff et al. 2005). However, the transcriptional response induced by growth factor pathway stimulation in breast cancer cells differs from that of estrogen (Cunliffe et al. 2003; Dudek and Picard 2008). Furthermore, ERα-positive breast cancers that overexpress the EGF receptor-2 (ERBB2/HER2) are resistant to endocrine therapies that disrupt the estrogen-dependent ERα program. In the present study, we addressed the role of growth factor-specific ERα-dependent transcriptional responses in breast cancer cells.
Results
Growth factor-induced unique ERα-dependent transcriptional program
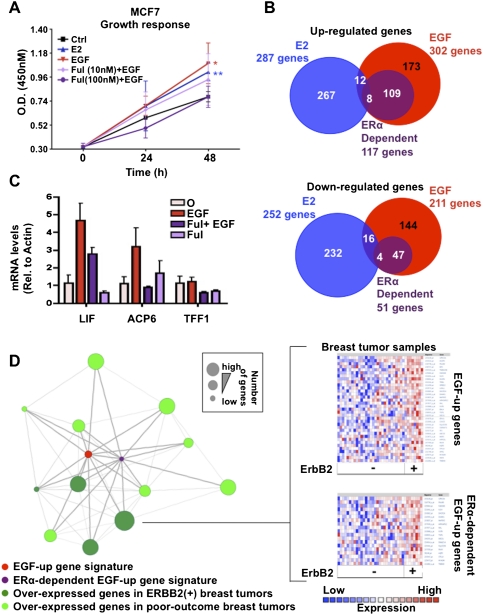
Given that growth factor pathway stimulation activates a number of transcription factors (Moasser 2007), we first assessed the contribution of ERα in EGF-mediated breast cancer cell proliferation. ERα depletion using the full antagonist fulvestrant (100 nM) or siRNA against ERα (Lupien et al. 2007, 2008) abrogated the EGF-mediated proliferation of MCF7 breast cancer cells (Fig. 1A; Supplemental Fig. S1A,B). Partial depletion using a lower dose of fulvestrant had reduced effects (Fig. 1A). ERα depletion also completely abrogated cellular proliferation triggered by activation of ERBB2 by heregulin in the ERα-positive BT474 breast cancer cell line (Supplemental Fig. S1C). Hence, ERα contributes significantly to growth factor pathway-mediated proliferation of breast cancer cells, including those overexpressing ERBB2.
Figure 1.
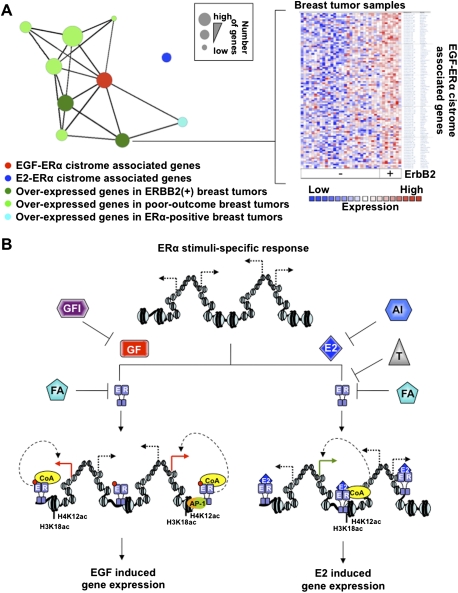
ERα is required for growth factor-mediated breast cancer cell proliferation. (A) Proliferation of MCF7 breast cancer cells pretreated or not with the full anti-estrogen fulvestrant (Ful) was measured following EGF stimulation. (*) P ≤ 0.05; (**) P ≤ 0.01; (***) P ≤ 0.001. (B) Comparison of estrogen-up (E2), EGF-up, and ERα-dependent EGF up-regulated as well as down-regulated transcriptional programs in MCF7 breast cancer cells. (C) mRNA levels derived from RT-qPCR of the ERα-dependent EGF target genes LIF and ACP6 are presented under EGF stimulation in MCF7 cells pretreated or not with Ful (100 nM). TFF1 is used as a negative control. (D) Oncomine Concepts Map analysis (Compendia Biosciences, Inc.; https://www.oncomine.com) was used to compare the EGF-induced gene signature in MCF7 breast cancer cells against all published gene signatures from primary breast tumors. This revealed significant correlations between EGF-up as well as ERα-dependent EGF-up gene signatures from MCF7 cells with gene signatures from poor-outcome (metastasis, recurrence, death, and high grade) as well as ERBB2-positive breast tumors (P ≤ 1e-2, odds ratio [O.R.] ≥2). No significant correlations were revealed between EGF-up or ERα-dependent EGF-up gene signatures and expression signatures from ERα-positive primary breast tumors. Each green circle in the left figure corresponds to the gene signature from primary breast tumors established in an independent study. The red circle corresponds to the EGF-up gene signature in MCF7 breast cancer cells. The purple circle corresponds to the ERα-dependent EGF-up gene signature from MCF7 breast cancer cells. Genes signatures significantly correlated with each other are linked to each other by a straight line. The right figure presents an example of how significant correlation between EGF-up and ERα-dependent EGF-up gene signatures with the ERBB2-positive breast cancer gene signature were established in one published study (Richardson et al. 2006). Specifically, the expression profile established in primary breast tumors from different patients (vertical axis) is presented for the genes found in the EGF-up and ERα-dependent EGF-up gene signature from MCF7 breast cancer cells (horizontal axis).
While EGF induced proliferation of MCF7 breast cancer cells to the same extent as estrogen, this involved a distinct transcriptional program (Fig. 1A,B; Cunliffe et al. 2003; Dudek and Picard 2008). ERα depletion using fulvestrant in EGF-treated cells revealed that >39% and 24% of EGF up-regulated and down-regulated genes in MCF7 cells, respectively, were fully or partially dependent on ERα (Fig. 1B,C). Similar results were obtained when silencing ERα expression using siRNA (Supplemental Fig. S1D,E). Noteworthy, by defining the EGF up-regulated transcriptional program in MCF7 cells, we found, using Oncomine Concepts Map analysis (Rhodes et al. 2007), that this program was correlated with the most highly expressed genes in ERBB2-positive breast tumors (odds ratio ≥2, P ≤ 1e-2) (Fig. 1D). Conversely the EGF down-regulated genes in MCF7 cells correlated with the most repressed genes from ERBB2-positive breast tumors (Supplemental Fig. S2). Importantly, these associations were also observed for the ERα-dependent EGF up-regulated or down-regulated transcriptional program (Fig. 1D; Supplemental Fig. S2). In addition, both the EGF-up and ERα-dependent EGF-up transcriptional programs were associated with poor-outcome expression signatures such as relapse, death, metastasis, and high tumor grade (Fig. 1D). Overall, these new results reveal the capacity of ERα to elicit stimuli-specific transcriptional programs in breast cancer cells. Furthermore, the association of the ERα-dependent EGF-up gene expression signature with ERBB2-positive and poor-outcome breast tumors suggests a role for ERα in these tumors and with endocrine therapy-resistant breast cancers dependent on growth factor pathway activation.
Stimulus-specific ERα cistrome leads to unique transcriptional program
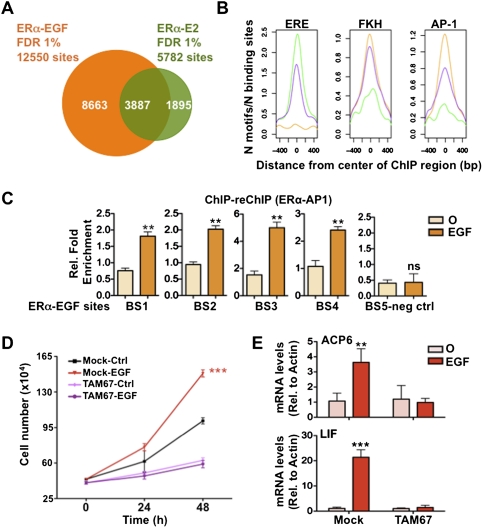
To address whether differential ERα recruitment to the genome mediates the stimulus-specific transcriptional responses under growth factor stimulation, we compared EGF and estrogen-induced ERα cistromes from MCF7 breast cancer cells (Fig. 2A; Supplemental Figs. S3–S5; Carroll et al. 2005, 2006; Lupien et al. 2008). Although both estrogen and EGF induced ERα recruitment to shared sites (Fig. 2A), growth factor pathway activation induced ERα recruitment to a significant number of unique sites (Fig. 2A; Supplemental Fig. S5A). In keeping with the central role of FoxA1 in ERα-positive breast cancer, the Forkhead (FKH) motif was highly enriched in both the estrogen and EGF-induced ERα cistromes (Fig. 2B). In fact, >45% of the EGF-unique ERα cistromes overlapped with the previously characterized FoxA1 cistromes in MCF7 cells (Supplemental Fig. S6; Lupien et al. 2008). However, the EGF-unique sites were more highly enriched for the AP-1 as opposed to estrogen-responsive element (ERE) motif (Fig. 2B). This suggests that recruitment of ERα following growth factor stimulation is occurring preferentially through an indirect tethering mechanism involving AP-1 family members. Chromatin immunoprecipitation (ChIP)–reChIP assays directed against ERα and AP-1 demonstrate that these two transcription factors are corecruited upon EGF stimulation to ERα-binding sites (Fig. 2C). Proliferation assays in the presence of a dominant-negative AP-1 mutant (TAM67) (Dhar et al. 2004) reveal the central role of AP-1 in EGF-mediated MCF7 breast cancer cell proliferation and in ERα-dependent EGF target gene regulation (Fig. 2D,E). Taken together, these results suggest that AP-1 is a critical partner in ERα signaling favorable to the growth of breast cancer cells under growth factor stimulation.
Figure 2.
AP-1 is central to the growth factor-induced ERα cistromes. (A) Genome-wide ChIP-on-chip analysis following EGF stimulation in MCF7 breast cancer cells reveals 12,550 ERα-binding sites (false discovery rate [FDR] 1%), 31% overlapping with the estrogen (E2)-induced ERα cistrome. (B) Sequence analysis of EGF-unique (orange), shared (purple), or E2-unique (green) ERα-binding sites reveals the preferential enrichment of EREs in the center of the E2-unique and shared binding sites, while the FKH and AP-1 motifs are preferentially enriched in the center of the shared and EGF-unique ERα cistromes. (C) ChIP–reChIP assays directed against ERα and AP-1 were performed to reveal the corecruitment of these factors following EGF stimulation on ERα-binding sites. (*) P ≤ 0.05; (**) P ≤ 0.01; (***) P ≤ 0.001. (D) Proliferation of MCF7 breast cancer cells transfected with the mock (pcDNA3.1) or AP-1 dominant-negative (TAM67) vectors was measured following EGF stimulation (*) P ≤ 0.05; (**) P ≤ 0.01; (***) P ≤ 0.001. (E) mRNA levels of EGF target genes were measured following EGF stimulation in MCF7 breast cancer cells transfected with the mock (pcDNA3.1) or AP-1 dominant-negative (TAM67) vectors. (*) P ≤ 0.05; (**) P ≤ 0.01; (***) P ≤ 0.001.
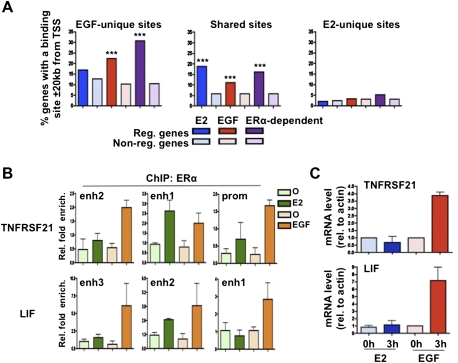
Of interest, EGF up-regulated genes were significantly associated with EGF-unique but not estrogen-unique ERα-binding sites (Fig. 3A). In fact, the estrogen-unique ERα-binding sites did not associate with the estrogen-responsive genes (Fig. 3A). This is in agreement with their poor overlap with FoxA1-binding regions (Supplemental Fig. S6), which we previously showed is typical of sites not driving an estrogen response in breast cancer cells (Lupien et al. 2008). The role of EGF-unique ERα-binding sites on the EGF-induced transcriptional response is exemplified by analyzing ERα recruitment in MCF7 breast cancer cells near the EGF-responsive TNFRSF21 and LIF genes (Fig. 3B,C; Supplemental Fig. S7A). Indeed, ERα was recruited to all three regulatory elements associated with TNFRSF21 and LIF following EGF stimulation, while estrogen treatment induced only ERα recruitment to TNFRSF21 enh1 and LIF enh2 (Fig. 3B; Supplemental Fig. S7A). These results suggest that the stimuli-specific transcriptional response in a given cell is in part dependent on a unique ERα cistrome. Furthermore, the increased number of ERα-binding sites following EGF stimulation near EGF target genes (Supplemental Fig. S7A) is in agreement with the notion that genes are more likely regulated when ERα-binding sites cluster near them (Krum et al. 2008).
Figure 3.
Growth factor-induced transcriptional response relates to stimuli-specific ERα cistromes. (A) Correlation between E2, EGF-up, or ERα-dependent EGF-up target genes with EGF-unique, shared, or E2-unique ERα-binding sites from MCF7 breast cancer cells. The occurrence of ERα-binding sites within 20 kb of the TSS of regulated genes was compared with that on nonregulated genes. (*) P ≤ 0.05; (**) P ≤ 0.01; (***) P ≤ 0.001. (B) ChIP-qPCR results against ERα performed under EGF or E2 stimulation in MCF7 cells on the regulatory element associated with the TNFRSF21 and LIF genes. (C) RT-qPCR results measuring expression of the EGF-specific responsive genes TNFRSF21 and LIF following 3 h of E2 or EGF stimulation.
Activation of ERα by growth factor pathway stimulation has been shown to be dependent on its phosphorylation at specific N-terminal residues, including, predominantly, Ser 118 (S118phos) (Kato et al. 1995; Bunone et al. 1996; Joel et al. 1998; Smith 1998; Kato 2001; Kurokawa and Arteaga 2003). In contrast, estrogen-mediated activation of ERα is dependent on an activation domain overlapping its ligand-binding domain (LBD) located in its C-terminal region that also leads to S118 phosphorylation (Chen et al. 2002). We found that recruitment of S118-phosphorylated ERα to the genome was detected following EGF stimulation at EGF-unique as well as EGF–estrogen shared ERα-binding sites (Supplemental Fig. S7B,C). This suggests that ERα phosphorylation alone is not sufficient to explain its stimulus-specific cistromes.
Stimulus-specific ERα coactivation
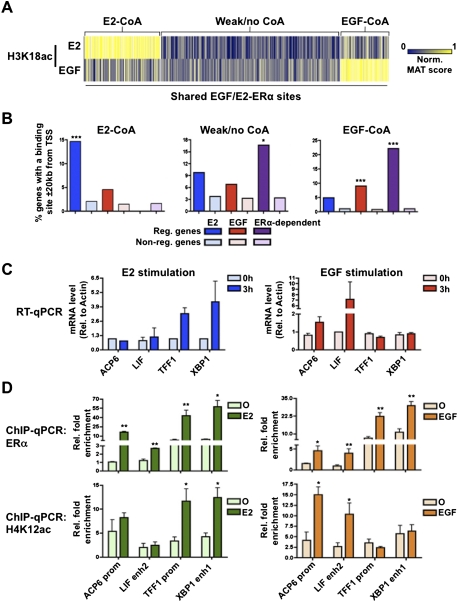
We demonstrated previously that FoxA1-binding sites shared across cells of different lineages can exhibit cell type-specific activities (Eeckhoute et al. 2009). Here, although a significant proportion of the EGF-induced ERα cistrome is unique, close to 4000 sites are shared with the estrogen-induced ERα cistrome (Fig. 2A). Furthermore, EGF as well as estrogen-regulated genes are enriched around these shared binding sites (Fig. 3A). Hence, this suggests that different subsets of the shared ERα-binding sites are active under EGF or estrogen stimulation. We demonstrated recently that only a fraction of the ERα cistrome undergoes coactivation and associates with regulated genes following estrogen stimulation in breast cancer cells (Lupien et al. 2009). Hence, a number of estrogen-induced ERα-binding events appear to be futile for the establishment of the estrogen-induced transcriptional response. Furthermore, coactivators were reported previously to undergo specific regulation following growth factor stimulation (Font De Mora and Brown 2000; Lopez et al. 2001). We therefore addressed the contribution of stimuli-specific coactivation of the shared ERα-binding sites in the distinct transcriptional response generated in MCF7 breast cancer cells following EGF or estrogen treatment. In order to address coactivation instead of coactivator recruitment, we decided to measure a post-translational modification induced by CBP/p300, a well-established ERα coactivator (Hanstein et al. 1996). This was accomplished by measuring the level of histone H3 acetylation on Lys 18 (H3K18ac) following either EGF or estrogen treatment in MCF7 breast cancer cells. Comparison of H3K18ac levels with those in control untreated cells revealed the stimuli-specific coactivation of shared ERα-binding sites (Fig. 4A). Indeed, a fraction of shared ERα-binding sites associated with strong induction of H3K18ac following EGF treatment, while a distinct fraction associated with the estrogen-induced H3K18ac (Fig. 4A). Importantly, EGF-regulated genes were specifically enriched near shared ERα-binding sites preferentially coactivated under that same treatment (Fig. 4B). Conversely, estrogen-regulated genes were significantly enriched near shared ERα-binding sites specifically coactivated following estrogen treatment (Fig. 4B). This was exemplified by the ACP6 and LIF EGF-regulated genes and the TFF1 and XBP1 estrogen-regulated genes (Fig. 4C). Both EGF and estrogen could induce ERα recruitment to the series of regulatory elements near these genes in MCF7 breast cancer cells (Fig. 4D; Supplemental Fig. S8); however, their coactivation was stimuli-specific. Indeed, histone acetylation (both H3K18ac and H4K12ac) could be induced only on the ERα-binding sites near ACP6 and LIF following EGF treatment (Fig. 4D; Supplemental Fig. S8). Similarly, estrogen could induce histone acetylation only on TFF1- and XBP1-associated ERα regulatory elements (Fig. 4D; Supplemental Fig. S8). Hence, this suggests that growth factor pathway activation leads to the coactivation of a different set of ERα-binding sites shared with other stimuli.
Figure 4.
Selective coactivation of ERα-binding sites under growth factor stimulation. (A) Coactivation as measured by H3K18ac levels established following either EGF or estrogen (E2) stimulation by ChIP-on-chip of chromosomes 8, 11, and 12 in MCF7 breast cancer cells. The MAT score for H3K18ac (EGF/control and E2/control) was established on all ERα-binding sites shared under E2 and EGF stimulation found on chromosomes 8, 11, and 12. K-means cluster analysis was used to identify ERα-binding sites preferentially associated with induced H3K18ac under E2 or EGF treatment. (B) Correlation between gene expression and the three subsets of shared ERα-binding sites based on H3K18ac levels was performed as described for Figure 2D. (C) Specific examples validating the concept of stimuli-specific coactivation at ERα-binding sites common to estrogen and EGF stimulation. RT-qPCR studies validate the stimuli-specific expression of ACP6, LIF, TFF1, and XBP1 in MCF7 breast cancer cells following E2 or EGF treatment in MCF7 cells. (D) ChIP-qPCR analysis demonstrates E2 and EGF equivalent induction of ERα recruitment to regulatory regions associated with ACP6, LIF, TFF1, and XBP1. Coactivation measured through acetylation of H4K12ac or H3K18ac (Supplemental Material) is specific to ERα-binding sites near genes induced under the same treatment in MCF7 cells.
ERα-dependent growth factor response relates to poor-outcome breast cancers
To address the relevance of growth factor-dependent ERα cistrome in breast tumors, we established the correlation between the genes with growth factor pathway-specific ERα-binding sites within 20 kb of their transcription start sites (TSSs) and breast tumor expression signatures using Oncomine Concepts Map analysis. Significant correlations were revealed between genes associated with EGF–ERα-specific sites and gene expression signatures found in poor-outcome (relapse, death, metastasis, and high tumor grade) as well as ERBB2-positive breast tumors (Fig. 5A). Genes with estrogen-associated ERα-binding sites within 20 kb of their TSSs were not associated with these gene expression signatures, but were associated with ERα-positive overexpressed gene signatures (Supplemental Fig. S9). Therefore, the growth factor pathway-specific ERα cistrome supports its role in the transcriptional response associated with breast tumors overexpressing ERBB2 and with poor outcomes.
Figure 5.
Growth factor ERα cistrome relates to poor-outcome expression signatures in breast tumors. (A) Oncomine Concepts Map analysis reveals significant association between genes specifically associated with an EGF-specific ERα-binding site within 20 kb of their TSS and gene expression signatures from ERBB2-positive, poor-outcome (metastasis, recurrence, death, and high grade), or ERα-positive breast tumors characterized in eight independent studies (each represented by individual circles). Only significantly associated gene lists are linked by a straight line (P ≤ 1e-4, O.R. ≥ 1.35). The gene list associated with estrogen (E2)-induced ERα-binding sites within 20 kb of their TSS does not significantly associate with any of the gene expression signatures linked to EGF–ERα-associated genes. The right panel presents an example of the expression profile for EGF–ERα-associated genes differentially expressed in ERBB2-positive breast tumors according to one independent study. Only genes significantly differentially expressed in ERBB2-positive versus -negative breast tumors are presented (P ≤ 5e-2). (B) Schematic representation of mechanisms involved in stimuli-specific transcriptional response acting through a shared transcription factor. E2 and the growth factor pathway (GF) induce a unique transcriptional response dependent on the ERα. This involves stimuli-specific ERα cistromes and coactivation. Current therapies for ERα-positive primary breast tumors, including aromatase inhibitors (AI) or selective ERα modulators such as tamoxifen (T), block only E2-mediated activation of the receptor. Full antiestrogens (FA) such as fulvestrant lead to ERα degradation and can therefore block E2- as well as GF-mediated activation of ERα. Growth factor inhibitors (GFI) should also block ERα activation following growth factor pathway activation.
Discussion
Taken together, our results demonstrate that differential recruitment and coactivation of ERα is a fundamental mechanism allowing for stimulus-specific transcriptional programs (Fig. 5B). Furthermore, as growth factor pathway activation is commonly associated with the development of hormone-refractory breast tumors (Dowsett 2001), our results suggest that ERα can play a fundamental role in their proliferation and involves the transcription factor AP-1. Indeed, hormone-refractory tumors are typically dependent on the overexpression of the EGFR or ERBB2 (Benz et al. 1992; Pietras et al. 1995; Kurokawa et al. 2000; Nicholson et al. 2001; Shou et al. 2004). In addition, a subset of hormone-refractory breast tumors are responsive to the full ERα antagonist fulvestrant (Howell et al. 1995; Martin et al. 2005). Furthermore, fulvestrant results in clinical benefits in ERBB2-overexpressing advanced breast cancers (Robertson et al. 2010). Additionally, in model systems, treatment of breast cancer cells with the combination of fulvestrant and growth factor pathway inhibitors more significantly represses growth than either treatment alone, and prevents the development of endocrine resistance (Kunisue et al. 2000; Gee et al. 2003; Pietras et al. 2003; Macedo et al. 2008). Considering that ERα- and ERBB2-positive breast cancers are resistant to endocrine therapies targeting estrogen stimulation of ERα, such as aromatase inhibitors or selective ER modulators, our results provide a mechanistic understanding for this clinical observation. Our results suggest that complete ERα antagonists that would block both its estrogen- and growth factor-stimulated activities would overcome this problem. Hence, the EGF-induced ERα cistrome reveals key features to be considered in the development of therapeutic strategies for hormone-refractory ERα-positive breast tumors.
Materials and methods
ChIP-on-chip and ChIP-qPCR and ChIP–reChIP-qPCR
Prior to stimulation, MCF7 cells were maintained for 3 d in phenol red-free medium (Invitrogen) supplemented with 10% charcoal dextran-treated fetal bovine serum (CDT-FBS) as described previously (Lupien et al. 2008). Cells were stimulated with the EGF (100 ng/mL) for 90 min and crosslinked using 1% formaldehyde (Kato et al. 1995; Cunliffe et al. 2003). Samples were sonicated (Fisher Sonic Desmembrator, model 500) and immunoprecipitated as described previously (Carroll et al. 2005, 2006) using antibodies against ERα (Santa Cruz Biotechnology, Inc., HC-20; Neomarkers, Ab-10), and H3K18ac (Millipore, 07-354). Three independent assays were performed. Purified samples were labeled and hybridized to microarrays (Affymetrix GeneChip Human Tiling 2.0R array sets). Genome-wide ChIP-on-Chip analysis was conducted using the model-based analysis of tiling arrays program (MAT) based on the latest human genomic sequence (Hg18) (Johnson et al. 2006). All ChIP-on-chip data used in this study can be accessed at http://research.dfci.harvard.edu/brownlab/datasets. ChIP-qPCR experiments were performed as in Carroll et al. (2005). Antibodies against ERα (Santa Cruz Boitechnology, Inc., HC-20; Neomarkers, Ab-10); ERα S118P (Millipore, 07-487), H3K18ac (Upstate Biotechnologies, Inc., 07-354), and H4K12ac (Upstate Biotechnologies, Inc., 07-595) were used for this assay. ChIP–reChIP was performed as described previously (Ross-Innes et al. 2010). AP-1 was re-ChIPed using a mix of anti-AP-1 antibodies (Santa Cruz Biotechnology, Inc., SC-44 and SC-253). Statistically significant differences were established using a Student's t-test comparison for unpaired data. Primer sequences used in this assay are found in Supplemental Table 1.
Gene expression profiling
Prior to stimulation, MCF7 cells were maintained for 3 d in phenol red-free medium (Invitrogen) supplemented with 10% CDT-FBS as described previously (Lupien et al. 2008). Cells were pretreated with fulvestrant (100 nM; ICI182,780) or control vehicle for 3 h and then stimulated with the EGF (100 μg/mL) for 3 h before RNA extraction using Qiagen RNeasy kit (Qiagen). Triplicate experiments were performed using Affymetrix U133Plus2.0 expression microarrays. The Robust Multichip Average (RMA) algorithm was used to analyze the data as described previously (Carroll et al. 2006), and level of differential expression for each time point relative to 0 h was established as in Lupien et al. (2008). Gene lists are found in Supplemental Table 2A–E. Statistically differentially expressed genes (t-test, P ≥ 1e-3) were defined as EGF-up genes. ERα-dependent EGF-up genes correspond to the EGF-up genes that were no longer significantly expressed when cells were treated with fulvestrant prior to EGF stimulation (EGF-responsive genes, t-test P ≥ 1e-3, minus fulvestrant + EGF-responsive genes, t-test, P ≥ 1e-2). Estrogen-responsive genes were presented previously (Carroll et al. 2006).
Transfection of MCF7 cells
MCF7 cells were maintained in phenol red-free medium (Invitrogen) supplemented with 10% CDT-FBS as described previously (Lupien et al. 2008) prior to transfection. MCF7 cells were transfected with the mock (pcDNA3.1) or AP-1 dominant-negative (TAM67) vectors (1 μg per well) using LipoD293 DNA transfection reagent according to the manufacturer's instructions (SignaGen). Forty-eight hours after transfection, cells were stimulated with control (ddH2O) or the EGF (100 ng/mL). For cell proliferation assays, cell number was determined every 24 h after EGF addition. For expression assays, RNA was extracted 3 h following EGF stimulation.
RT-qPCR
Collected RNA was processed for RT-qPCR as described previously (Krum et al. 2008). Primer sequences used for in RT-qPCR are listed in Supplemental Table 1.
Sequence analysis and cluster analysis
Genome-wide distribution as well as sequence conservation analysis of the different clusters derived from the ERα estrogen versus EGF cistromes was determined using the Cis-Elements Annotation Systems (CEAS) (Ji et al. 2006). Enriched motifs within clusters as well as the association of trends in gene expression with cluster binding sites were identified as described in Lupien et al. (2008).
Oncomine Concepts Map
We compared our various gene lists (list of genes in each gene list can be found in Supplemental Table 3A,B) with expression profiles from breast tumors compiled on Oncomine (Compendia Bioscience; http://www.oncomine.org). Using the Oncomine Concepts Map tools, we established significant association between our gene lists and Oncomine overexpressed or underexpressed Gene Expression Signature derived from independent breast cancer studies. Node connection figures can be generated with Cytoscape (http://www.cytoscape.org). Gene Expression Signatures used in Figures 1D and 4A and Supplemental Figure S2 are derived from van de Vijver et al. (2002), Zhao et al. (2004), Miller et al. (2005), Minn et al. (2005), Wang et al. (2005), Chin et al. (2006), Ginestier et al. (2006), Hess et al. (2006), Ivshina et al. (2006), Richardson et al. (2006), Sotiriou et al. (2006), Yu et al. (2006), Desmedt et al. (2007), Saal et al. (2007), Boersma et al. (2008), and Finak et al. (2008).
Acknowledgments
We thank Dr. Nancy Colburn (NCI-Frederick) for providing the dominant-negative AP-1 construct (TAM67). This work was supported by grants from the NIDDK (R01DK074967 to M.B.), the NCI (P01 CA8011105, and the DF/HCC Breast Cancer SPORE Grant P50C89393 to M.B.), the DFCI Women's Cancers Program, and the US Department of Defense Breast Cancer Research Program Awards (W81XWH-08-1-0214 to M.L.).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1944810.
Supplemental material is available at http://www.genesdev.org.
References
- Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK 1992. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat 24: 85–95 [DOI] [PubMed] [Google Scholar]
- Boersma BJ, Reimers M, Yi M, Ludwig JA, Luke BT, Stephens RM, Yfantis HG, Lee DH, Weinstein JN, Ambs S 2008. A stromal gene signature associated with inflammatory breast cancer. Int J Cancer 122: 1324–1332 [DOI] [PubMed] [Google Scholar]
- Bunone G, Briand PA, Miksicek RJ, Picard D 1996. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J 15: 2174–2183 [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, et al. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122: 33–43 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, et al. 2006. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38: 1289–1297 [DOI] [PubMed] [Google Scholar]
- Chen D, Washbrook E, Sarwar N, Bates GJ, Pace PE, Thirunuvakkarasu V, Taylor J, Epstein RJ, Fuller-Pace FV, Egly J-M, et al. 2002. Phosphorylation of human estrogen receptor α at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene 21: 4921–4931 [DOI] [PubMed] [Google Scholar]
- Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, Lapuk A, Neve RM, Qian Z, Ryder T, et al. 2006. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell 10: 529–541 [DOI] [PubMed] [Google Scholar]
- Cunliffe HE, Ringner M, Bilke S, Walker RL, Cheung JM, Chen Y, Meltzer PS 2003. The gene expression response of breast cancer to growth regulators: Patterns and correlation with tumor expression profiles. Cancer Res 63: 7158–7166 [PubMed] [Google Scholar]
- Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, Korach KS 1996. Physiological coupling of growth factor and steroid receptor signaling pathways: Estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci 93: 12626–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt C, Piette F, Loi S, Wang Y, Lallemand F, Haibe-Kains B, Viale G, Delorenzi M, Zhang Y, d'Assignies MS, et al. 2007. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res 13: 3207–3214 [DOI] [PubMed] [Google Scholar]
- Dhar A, Hu J, Reeves R, Resar LM, Colburn NH 2004. Dominant-negative c-Jun (TAM67) target genes: HMGA1 is required for tumor promoter-induced transformation. Oncogene 27: 4466–4476 [DOI] [PubMed] [Google Scholar]
- Dowsett M 2001. Overexpression of HER-2 as a resistance mechanism to hormonal therapy for breast cancer. Endocr Relat Cancer 8: 191–195 [DOI] [PubMed] [Google Scholar]
- Dudek P, Picard D, 2008. Genomics of signaling crosstalk of estrogen receptor α in breast cancer cells. PLoS ONE 3: e1859 doi: 10.1371/journal.pone.0001859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhoute J, Lupien M, Meyer CA, Verzi MP, Shivdasani RA, Liu XS, Brown M 2009. Cell-type selective chromatin remodeling defines the active subset of FOXA1-bound enhancers. Genome Res 19: 372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, et al. 2008. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14: 518–527 [DOI] [PubMed] [Google Scholar]
- Font De Mora J, Brown M 2000. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol 20: 5041–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, et al. 2009. An oestrogen-recepto-α-bound human chromatin interactome. Nature 462: 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Harper ME, Hutcheson IR, Madden TA, Barrow D, Knowlden JM, McClelland RA, Jordan N, Wakeling AE, Nicholson RI 2003. The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology 144: 5105–5117 [DOI] [PubMed] [Google Scholar]
- Ginestier C, Cervera N, Finetti P, Esteyries S, Esterni B, Adelaide J, Xerri L, Viens P, Jacquemier J, Charafe-Jauffret E, et al. 2006. Prognosis and gene expression profiling of 20q13-amplified breast cancers. Clin Cancer Res 12: 4533–4544 [DOI] [PubMed] [Google Scholar]
- Hanstein B, Eckner R, DiRenzo J, Halachmi S, Liu H, Searcy B, Kurokawa R, Brown M 1996. p300 is a component of an estrogen receptor coactivator complex. Proc Natl Acad Sci 93: 11540–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess KR, Anderson K, Symmans WF, Valero V, Ibrahim N, Mejia JA, Booser D, Theriault RL, Buzdar AU, Dempsey PJ, et al. 2006. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol 24: 4236–4244 [DOI] [PubMed] [Google Scholar]
- Howell A, DeFriend D, Robertson J, Blamey R, Walton P 1995. Response to a specific antioestrogen (ICI 182780) in tamoxifen-resistant breast cancer. Lancet 345: 29–30 [DOI] [PubMed] [Google Scholar]
- Hua S, Kallen CB, Dhar R, Baquero MT, Mason CE, Russell BA, Shah PK, Liu J, Khramtsov A, Tretiakova MS, et al. 2008. Genomic analysis of estrogen cascade reveals histone variant H2A.Z associated with breast cancer progression. Mol Syst Biol 4: 188 doi: 10.1038/msb.2008.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS 2008. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature 456: 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignar-Trowbridge DM, Nelson KG, Bidwell MC, Curtis SW, Washburn TF, McLachlan JA, Korach KS 1992. Coupling of dual signaling pathways: Epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci 89: 4658–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, Lindahl T, Pawitan Y, Hall P, Nordgren H, et al. 2006. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res 66: 10292–10301 [DOI] [PubMed] [Google Scholar]
- Ji X, Li W, Song J, Wei L, Liu XS 2006. CEAS: Cis-regulatory element annotation system. Nucleic Acids Res 34: W551–W554 doi: 10.1093/nar/gkl322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel PB, Smith J, Sturgill TW, Fisher TL, Blenis J, Lannigan DA 1998. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol Cell Biol 18: 1978–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li W, Meyer CA, Gottardo R, Carroll JS, Brown M, Liu XS 2006. Model-based analysis of tiling-arrays for ChIP-chip. Proc Natl Acad Sci 103: 12457–12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S 2001. Estrogen receptor-mediated cross-talk with growth factor signaling pathways. Breast Cancer 8: 3–9 [DOI] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. 1995. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270: 1491–1494 [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI 2003. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144: 1032–1044 [DOI] [PubMed] [Google Scholar]
- Krum SA, Miranda-Carboni GA, Lupien M, Eeckhoute J, Carroll JS, Brown M 2008. Unique ERα cistromes control cell type-specific gene regulation. Mol Endocrinol 22: 2393–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisue H, Kurebayashi J, Otsuki T, Tang CK, Kurosumi M, Yamamoto S, Tanaka K, Doihara H, Shimizu N, Sonoo H 2000. Anti-HER2 antibody enhances the growth inhibitory effect of anti-oestrogen on breast cancer cells expressing both oestrogen receptors and HER2. Br J Cancer 82: 46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa H, Arteaga CL 2003. ErbB (HER) receptors can abrogate antiestrogen action in human breast cancer by multiple signaling mechanisms. Clin Cancer Res 9: 511S–515S [PubMed] [Google Scholar]
- Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL 2000. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res 60: 5887–5894 [PubMed] [Google Scholar]
- Lee AV, Yee D 1995. Insulin-like growth factors and breast cancer. Biomed Pharmacother 49: 415–421 [DOI] [PubMed] [Google Scholar]
- Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, et al. 2007. Whole-genome cartography of estrogen receptor α binding sites. PLoS Genet 3: e87 doi: 10.1371/journal.pgen.0030087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gao H, Marstrand TT, Strom A, Valen E, Sandelin A, Gustafsson JA, Dahlman-Wright K 2008. The genome landscape of ERα- and ERβ-binding DNA regions. Proc Natl Acad Sci 105: 2604–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GN, Turck CW, Schaufele F, Stallcup MR, Kushner PJ 2001. Growth factors signal to steroid receptors through mitogen-activated protein kinase regulation of p160 coactivator activity. J Biol Chem 276: 22177–22182 [DOI] [PubMed] [Google Scholar]
- Lupien M, Jeyakumar M, Hebert E, Hilmi K, Cotnoir-White D, Loch C, Auger A, Dayan G, Pinard GA, Wurtz JM, et al. 2007. Raloxifene and ICI182,780 increase estrogen receptor-α association with a nuclear compartment via overlapping sets of hydrophobic amino acids in activation function 2 helix 12. Mol Endocrinol 21: 797–816 [DOI] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M 2008. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132: 958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Krum SA, Rhodes DR, Liu XS, Brown M 2009. Coactivator function defines the active estrogen receptor-α cistrome. Mol Cell Biol 29: 3413–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu R, Cardillo M, Cho C, Harris L, Hijazi M, Perez C, Rosenberg K, Yang D, Tang C 1996. The significance of heregulin in breast cancer tumor progression and drug resistance. Breast Cancer Res Treat 38: 57–66 [DOI] [PubMed] [Google Scholar]
- Macedo LF, Sabnis GJ, Goloubeva OG, Brodie A 2008. Combination of anastrozole with fulvestrant in the intratumoral aromatase xenograft model. Cancer Res 68: 3516–3522 [DOI] [PubMed] [Google Scholar]
- Martin LA, Pancholi S, Chan CM, Farmer I, Kimberley C, Dowsett M, Johnston SR 2005. The anti-oestrogen ICI 182,780, but not tamoxifen, inhibits the growth of MCF-7 breast cancer cells refractory to long-term oestrogen deprivation through down-regulation of oestrogen receptor and IGF signalling. Endocr Relat Cancer 12: 1017–1036 [DOI] [PubMed] [Google Scholar]
- Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, Pawitan Y, Hall P, Klaar S, Liu ET, et al. 2005. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci 102: 13550–13555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J 2005. Genes that mediate breast cancer metastasis to lung. Nature 436: 518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moasser MM 2007. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 26: 6469–6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC 2003. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERα or ERβ. J Cell Biochem 90: 315–326 [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Hutcheson IR, Harper ME, Knowlden JM, Barrow D, McClelland RA, Jones HE, Wakeling AE, Gee JM 2001. Modulation of epidermal growth factor receptor in endocrine-resistant, oestrogen receptor-positive breast cancer. Endocr Relat Cancer 8: 175–182 [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, Gorman CM, Parker MG, Sliwkowski MX, Slamon DJ 1995. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene 10: 2435–2446 [PubMed] [Google Scholar]
- Pietras RJ, Marquez DC, Chen HW, Ayala R, Ramos LB, Slamon DJ 2003. Improved antitumor therapy with Herceptin and Faslodex for dual targeting of HER-2 and estrogen receptor signalling pathways in human breast cancers with overexpression of HER-2/neu gene. Breast Cancer Res Treat 82: S12, Abstract 22 [Google Scholar]
- Rhodes DR, Kalyana-Sundaram S, Tomlins SA, Mahavisno V, Kasper N, Varambally R, Barrette TR, Ghosh D, Varambally S, Chinnaiyan AM 2007. Molecular concepts analysis links tumors, pathways, mechanisms, and drugs. Neoplasia 9: 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S 2006. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell 9: 121–132 [DOI] [PubMed] [Google Scholar]
- Robertson JFR, Steger GG, Neven P, Barni S, Gieseking F, Nole F, Pritchard KI, O'Malley FP, Simon SD, Kaufman B, et al. 2010. Activity of fulvestrant in HER2-overexpressing advanced breast cancer. Ann Oncol 21: 1246–1253 [DOI] [PubMed] [Google Scholar]
- Ross-Innes CS, Stark R, Holmes KA, Schmidt D, Spyrou C, Russel R, Massie CE, Vowler SL, Eldridge M, Carroll JS 2010. Cooperative interaction between retinoic acid receptor-α and estrogen receptor in breast cancer. Genes Dev 24: 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, Koujak S, Ferrando AA, Malmstrom P, Memeo L, et al. 2007. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci 104: 7564–7569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R, Massarweh SA, Shou J, Bharwani L, Arpino G, Rimawi M, Osborne CK 2005. Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: Implicated role of growth factor signaling and estrogen receptor coregulators. Cancer Chemother Pharmacol 56: 10–20 [DOI] [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R 2004. Mechanisms of tamoxifen resistance: Increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96: 926–935 [DOI] [PubMed] [Google Scholar]
- Smith CL 1998. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod 58: 627–632 [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. 2001. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci 98: 10869–10874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. 2003. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci 100: 8418–8423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, et al. 2006. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98: 262–272 [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, et al. 2002. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347: 1999–2009 [DOI] [PubMed] [Google Scholar]
- Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F, et al. 2009. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 457: 854–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, et al. 2005. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365: 671–679 [DOI] [PubMed] [Google Scholar]
- Yu K, Ganesan K, Miller LD, Tan P 2006. A modular analysis of breast cancer reveals a novel low-grade molecular signature in estrogen receptor-positive tumors. Clin Cancer Res 12: 3288–3296 [DOI] [PubMed] [Google Scholar]
- Zhao H, Langerod A, Ji Y, Nowels KW, Nesland JM, Tibshirani R, Bukholm IK, Karesen R, Botstein D, Borresen-Dale AL, et al. 2004. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell 15: 2523–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]