Abstract
Summary
Hypoparathyroidism, a disorder characterized by low parathyroid hormone (PTH), is generally treated with oral calcium and vitamin D supplementation. We investigated the effects of PTH(1–84) treatment in 30 hypoparathyroid subjects for 24 months. PTH(1–84) treatment in hypoparathyroidism significantly reduced supplemental calcium and 1,25-dihydroxyvitamin D requirements without generally altering serum and urinary calcium levels.
Introduction
Hypoparathyroidism, a disorder characterized by low PTH, is associated with hypocalcemia, hypercalciuria, and increased bone mineral density (BMD). Conventional therapy with calcium and 1,25-dihydroxyvitamin D can maintain the serum calcium concentration, but doses are high, and control is variable. We investigated the effects of human PTH(1–84) treatment in hypoparathyroidism.
Methods
Thirty subjects with hypoparathyroidism were treated in an open-label study of PTH(1–84) 100 μg every other day by subcutaneous injection for 24 months, with monitoring of calcium and vitamin D supplementation requirements, serum and 24 h urinary calcium excretion, and BMD by dual energy X-ray absorptiometry.
Results
Requirements for supplemental calcium decreased significantly (3,030±2,325 to 1,661±1,267 mg/day (mean± SD); p<0.05), as did requirements for supplemental 1,25-dihydroxyvitamin D (0.68±0.5 to 0.40±0.5 μg/day; p< 0.05). Serum calcium levels and 24 h urinary calcium excretion were mostly unchanged at 24 months. BMD increased at the lumbar spine by 2.9±4% from baseline (p<0.05), while femoral neck BMD remained unchanged and distal one third radial BMD decreased by 2.4±4% (p<0.05).
Conclusion
PTH(1–84) treatment in hypoparathyroidism significantly reduces supplemental calcium and 1,25-dihydroxyvitamin D requirements without generally altering serum and urinary calcium levels.
Keywords: Calcium, Hypoparathyroidism, PTH(1–84)
Introduction
Hypoparathyroidism is due to deficient or absent parathyroid hormone (PTH). The disorder can be congenital or acquired [1–3]. Acquired hypoparathyroidism is most commonly the result of inadvertent removal of or irreversible damage to all parathyroid glands. Hypoparathyroidism can also be due to immune-mediated destruction of the parathyroid glands, activating mutations in the extracellular calcium-sensing receptor or part of DiGeorge syndrome, a genetic disorder. The typical biochemical constellation in untreated hypoparathyroidism includes low or absent PTH levels in the circulation, hypocalcemia, relatively high urinary calcium excretion, hyperphosphatemia, and reduced levels of 1,25-dihydroxyvitamin D [4, 5].
Conventional management of hypoparathyroidism consists of oral calcium and 1,25-dihydroxyvitmain D. While normal serum calcium can be generally maintained with this approach, it may also exacerbate the attendant hyper-calciuria. Thus, patients with hypoparathyroidism who are treated with calcium and 1,25-dihydroxyvitamin D can develop worsening hypercalciuria, leading, in some cases, to nephrocalcinosis, nephrolithiasis, or renal insufficiency [6–8]. In addition, it is a challenge to maintain the serum calcium concentration within normal limits with calcium and vitamin D therapy. Often, there are major swings in either direction leading to symptomatic hypercalcemic or hypocalcemic emergencies. Reducing calcium and calcitriol requirements in hypoparathyroidism by the use of PTH has important advantages with regard to safety and efficacy. The reduced calcium and vitamin D requirements could also potentially lessen the risk of hypercalcemia and hypercalciuria. An additional possible advantage is that use of PTH would reduce the risk of soft tissue deposition of calcium (nephrocalcinosis, nephrolithiasis, and possibly, in other soft tissues).
Administration of teriparatide, a PTH molecule fore-shortened to an amino terminus region [PTH(1–34)], has been shown to maintain serum calcium in the normal range [9–11] and to reduce urinary calcium excretion in hypoparathyroid subjects. Use of the native, full length molecule, PTH(1–84), has not been investigated. This study was designed to investigate the effects of PTH(1–84) in hypoparathyroidism.
Methods
Subjects
Thirty subjects with documented hypoparathyroidism participated in the study. The diagnosis of hypoparathyroidism was established by the simultaneous presence of serum calcium and PTH concentrations below the lower limits of normal on at least two prior occasions separated by an interval of at least 30 days. Hypoparathyroidism had to have been present for at least 3 years so as to establish a chronic state of PTH deprivation. Stability of supplemental calcium and vitamin D intake had to be present for 6 months prior to enrollment. Patients were excluded if they had been on a bisphosphonate within 5years priortostudy entryorfor greaterthan6months duration at any time or if they were women within 5 years of menopause. Patients were also excluded if they used any of the following medications: estrogens, progestins, raloxifene, calcitonin, systemic corticosteroids, fluoride, lithium, statins, loop diuretics, or methotrexate. Potentially confounding disorders were also exclusionary criteria, if present: Paget's disease of bone, diabetes mellitus, chronic liver or renal disease, acromegaly, Cushing's syndrome, rheumatoid arthritis, or multiple myeloma.
Patients were recruited from the Metabolic Bone Diseases Unit of Columbia University Medical Center and from the Hypoparathyroidism Association, a patient advocacy group. The study was approved by the Institutional Review Board of Columbia University Medical Center. All subjects gave written informed consent.
Protocol
Dose finding study
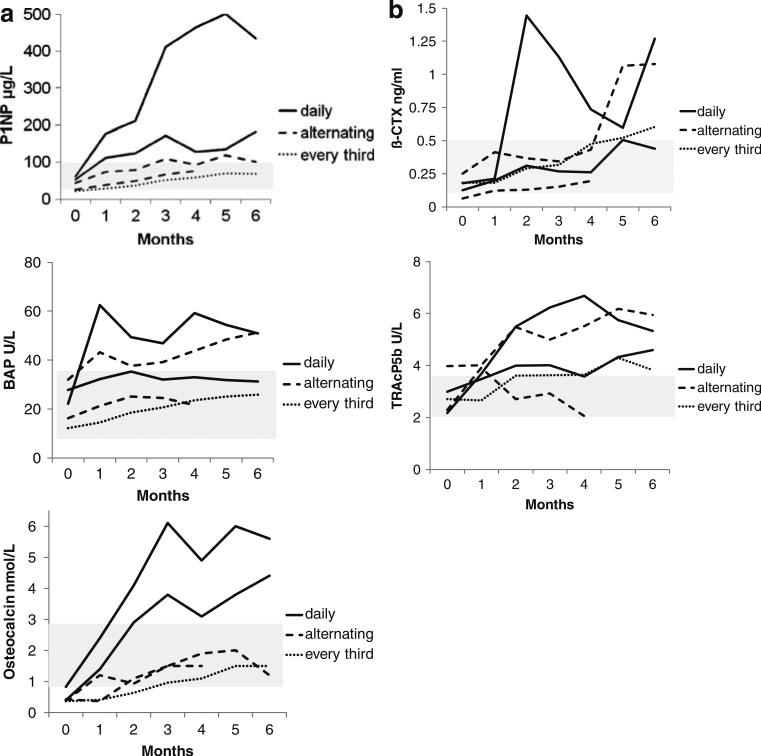
A dosing study was first performed to determine which dose of PTH(1–84) would lead to decreased requirements for calcium and vitamin D supplementation. Since bone turnover is known to be suppressed in hypoparathyroidism, we anticipated that the dose of PTH(1–84) that increased bone turnover would also lead to less requirements for supplemental calcium and vitamin D. PTH(1–84) was provided by NPS Pharmaceuticals (Bedminster, NJ, USA). Five hypoparathyroid subjects underwent a preliminary dosing study for 6 months in which three regimens were compared: PTH(1–84) 100 μg SC daily, every other day or every third day. Changes in biochemical markers of bone turnover (propeptide of type 1 procollagen, bone specific alkaline phosphate, serum β-C-telopeptide, and tartrate-resistant acid phosphatase) were assessed monthly. The changes in bone turnover markers in each patient are shown in Fig. 1. The dosage of 100 μg every other day SC was selected as the treatment dose for the larger trial.
Fig. 1.
Changes in turnover markers in dosing study in five patients. The five patients were assigned to either PTH(1–84) 100 μg every day (n=2), every other day (n=2), or every third day (n=1) for 6 months. a Change in bone formation markers. b Change in bone resorption markers
Treatment study
For the overall study, subjects were treated with PTH(1–84) at the selected dosage of 100 μg every other day subcutaneously for 24 months. Blood was collected three times prior to treatment and at months 1, 2, 3, 4, 5, 6, 9, 12, 18, and 24. Blood sampling was performed 48 h after the last PTH injection. A 24-h urinary calcium excretion was measured at baseline and at 3, 6, 9, and 12 months. The 24-h urinary calcium collection was begun 24 h after the last PTH injection. Bone mineral density (BMD) by dual energy X-ray absorptiometry (DXA) was performed twice at baseline and at 6 and 12 months.
Biochemistries were measured by automated techniques. The average value of the three pretreatment serum calcium determinations was used for the baseline calcium value. Serum PTH was measured by immunoradiometric assay [12] and urinary calcium by atomic absorption spectrophotometry.
In addition to the time points listed above, serum calcium was measured 1 and 2 weeks after institution of PTH(1–84). If the serum calcium remained at or above the pretreatment level, supplemental calcium was reduced by 500 mg decrements until a goal of 1,500 mg calcium supplementation was reached. After the serum calcium had been reduced to 1,500 mg daily, the 1,25-dihydroxyvitamin D was reduced by 0.25 μg decrements. This was repeated until the goal of 0.25 μg 1,25-dihydroxyvitamin D daily was achieved. If symptoms of hypocalcemia, such as numbness or paresthesias were present, variations from the protocol were permitted to allow reductions in calcium and calcitriol dosing. Serum calcium was measured 1 week after each reduction in calcium or 1,25-dihydroxvitamin D supplementation to ensure stability of the serum calcium concentration.
BMD of the lumbar spine, femoral neck, and distal one third radius was measured by DXA. Short-term in vivo precision error (root-mean-square standard deviation) was 0.026 g/cm2 for L1–L4 (1.1%), 0.041 g/cm2 for the femoral neck (2.4%), and 0.033 g/cm2 (1.8%) for the forearm. BMD was measured twice at baseline; the average value was used for the baseline measurement.
Statistical analysis
Data are expressed as mean±SD. Estimates of change in indices from baseline were assessed with paired t tests. A value of p<0.05 was considered significant. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) for Windows (version 11.0; SPSS, Chicago, IL, USA).
Results
Baseline characteristics
The demographics of the hypoparathyroid population reflected a predominance of female subjects (Tables 1 and 2). The two major etiologies of hypoparathyroidism were postsurgical and autoimmune. In addition, one patient had been previously diagnosed with autosomal dominant hypoparathyroidism and one with autoimmune polyglandular syndrome type 1. Duration of hypoparathyroidism ranged from 3 to 45 years. Baseline biochemical and BMD measurements are shown in Table 3. Most subjects had normal serum calcium levels on replacement therapy with calcium and vitamin D; a few had levels below the lower limits of normal. All patients had previously been documented to have hypoparathyroidism by concomitant hypocalcemia and low PTH levels. Serum creatinine was in the normal range. Among the postsurgical patients, six were hyperthyroid at study enrollment, while one was hypothyroid. BMD T-score was normal or above the normal range.
Table 1.
Baseline characteristics
| Mean±SD (N=30) | Cohort range | |
|---|---|---|
| Age (years) | 49±12 | 25–68 |
| Sex | ||
| Male | 8 | |
| Female | 22 | |
| Premenopausal | 14 | |
| Postmenopausal | 8 | |
| Etiology | ||
| Postoperative | 15 | |
| Idiopathic | 11 | |
| Autoimmune | 1 | |
| Autosomal dominant hypoparathyoridism | 1 | |
| DiGeorge | 2 | |
| Duration of hypoparathyroidism (years) | 19±15 | 3–46 |
| Fractures in adulthood: number of patients (%) | 7 (23%) | |
| Kidney stones: number of patients (%) | 5 (16%) |
Table 2.
Doses of hypoparathyroid treatments
| Mean±SD (N=30) | Median (N=30) | Cohort range | |
|---|---|---|---|
| Calcium supplement dose (mg/day) | 3,030±2,325 | 2,000 | 0–9,000 |
| 1,25-dihydroxvitamin D supplement dose (μg/day) | 0.68±0.5 | 0.5 | 0–3 |
| Daily chole- or ergocalciferol (vitamin D) dose (IU/day; n = 13a) | 5,909±10,748 | 4,000 | 400–28,571 |
| Thiazide dose (mg/day; n = 10) | 25±11 | 25 | 6.25–50 |
Twelve patients were taking both daily chole- or ergocalciferol (vitamin D) and 1,25-dihydroxvitamin D supplement
Table 3.
Baseline biochemistries and bone mineral density measurements
| Mean±SD (N=30) | Cohort range | Normal range | |
|---|---|---|---|
| Serum calcium (mmol/L)a | 2.1±0.2 | 1.6 to 2.5 | 2.1 to 2.6 |
| PTH (ng/L) | 4.4±4 | 1 to 14 | 10 to 65 |
| Phosphate (mmol/L) | 1.4±0.2 | 0.8 to 1.9 | 0.8 to 1.3 |
| Magnesium (mmol/L) | 0.74±0.1 | 0.6 to 0.8 | 0.6 to 0.9 |
| Total alkaline phosphatase activity (U/L) | 66±18 | 37 to 116 | 33 to 96 |
| Urinary calcium excretion (mmol/day) | 6.5±1 | 1.3 to 12.5 | <7.5 male |
| <6.25 female | |||
| Serum creatinine (μmol/L) | 88.4±796 | 45.8 to 137.3 | 38 to 69 |
| Glomerular filtration rate estimation by modification of diet in renal disease (mL/min/1.73 m2) | 74.9±21 | 38 to 123 | >60 |
| 25-hydroxyvitamin D (nmol/L) | 150.0±165 | 22.0 to 806.2 | 22 to 130 |
| 1,25-dihydroxyvitamin D (pmol/L) | 97.2±52 | 36.4 to 260 | 39 to 156 |
| Lumbar spine BMD (g/cm2)b | 1.24±0.3 | 0.89 to 1.91 | |
| T-score | 1.7±2 | –1.4 to +7.9 | |
| Femoral neck BMD (g/cm2)b | 0.95±0.2 | 0.64 to 1.30 | |
| T-score | 0.7±2 | –1.9 to +3.6 | |
| Radius one third BMD (g/cm2)b | 0.73±0.1 | 0.58 to 0.90 | |
| T-score | –0.03±1.0 | –1.9 to +169 |
Mean value of three measurements per patient
Mean value of two measurements per patient
Calcium and vitamin D supplementation
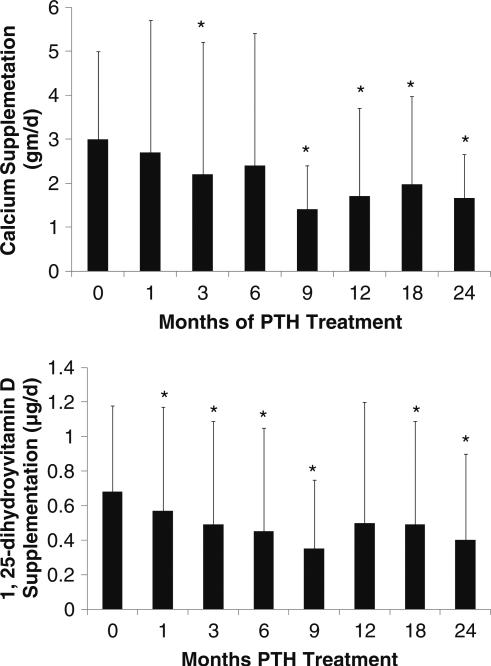
Throughout the larger 2-year study, requirements for supplemental calcium fell significantly from 3,030±2,325 to 1,661±1,267 mg/day (p<0.05; Fig. 2). The number of subjects on calcium supplementation that was greater than 1,500 mg/day decreased from 22 (73%) at study entry to 12 (40%) at the study conclusion. Two subjects at study entry were not on any calcium supplementation (but were taking 0.5 or 1.0 μg of 1,25-dihydroxyvitamin D daily); at the study conclusion, two subjects (one the same, the other different) were not on any calcium supplementation.
Fig. 2.
Changes in calcium and 1,25-dihydroxyvitamin D supplementation. Calcium requirements decreased at 3, 9, 12, 18, and 24 months from baseline while 1,25-dihydroxyvitamin D requirements decreased by 1 month. Data are mean±SD; *p<0.05 as compared to baseline
1,25-dihydroxyvitamin D supplementation declined from the baseline mean of 0.68±0.5 to 0.40±0.5 μg/day (p<0.05; Fig. 2). The number of subjects on a dose of 1,25-dihydroxyvitamin D that was greater than 0.25 μg/day fell from 25 (83%) at study entry to 15 (50%) at study conclusion. The number of subjects who did not require any 1,25-dihydroxyvitamin D increased from one to eight. The reductions in the amounts of calcium and vitamin D supplementation were similar, regardless of the etiology of the hypoparathyroidism. The number of subjects on hydrochlorothiazide for hypercalciuria decreased from ten at baseline to three at the study conclusion. Those who were on thiazides did not differ from the group in terms of baseline calcium and calcitriol supplementation. Adjustments in thiazides were not part of the algorithm and were undertaken at the discretion of the patient's primary care physician. There was no change in the number of subjects who were taking ergo- or cholecalciferol. The six hyper-thyroid subjects and one hypothyroid subject did not respond differently to PTH treatment than the remainder of the subjects.
Serum and urinary calcium levels
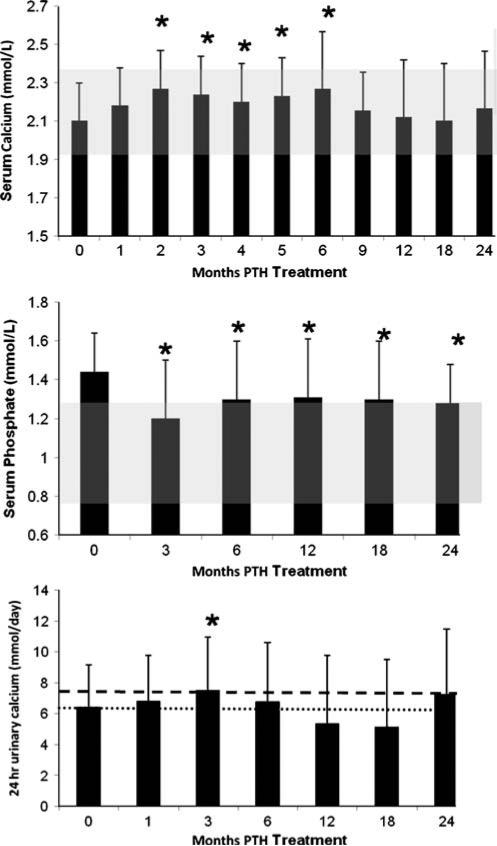
The serum calcium was maintained in the lower half of the normal range and during months 9 to 24, was not different from baseline values (Fig. 3). During the first 6 months of the study, there were small but significant increases baseline (e.g., at 2 weeks, 2.1±0.2 to 2.2±0.3 mmol/L; p=0.03). Thereafter, serum calcium values were not different from baseline. Transient, mild hypercalcemia, defined as a serum calcium greater than 2.6 mmol/L, occurred sporadically and in isolated fashion. It was not time or dose dependent. These isolated hypercalcemic values represented only 4% of all measurements. Nine subjects (30%) developed a mild elevation at some point during the trial. Among those whose serum calcium rose above normal, the average value was 2.8 mmol/L. The highest serum calcium was 3.2 mmol/ L, occurring in one subject at 6 months of PTH treatment. The 24-h urinary calcium excretion changed significantly, but minimally, at only one time point (3 months; Fig. 3).
Fig. 3.
Changes in serum calcium, serum phosphate, and urinary calcium. Serum calcium increased at months 2 to 6 but was no different from baseline at 1, 9, 12, 18, and 24 months. Serum phosphate decreased into the normal range at month 3 and remained in the normal range through 24 months. The shaded area shows the normal ranges of serum calcium and phosphate. Urinary calcium increased at 3 months but, otherwise, did not change. The heavier and lighter dashed lines shows the upper limit of normal urinary calcium levels in men and women, respectively. Data are mean±SD; *p<0.05 as compared to baseline
Other indices of mineral metabolism
Serum phosphate levels fell from 1.44±0.2 to 1.29± 0.2 mmol/L p<0.05 (Fig. 3). 25-hydroxyvitamin D levels decreased significantly from baseline (150.0±165 to 81.1± 45 nmol/L; p<0.05). 1,25-dihydroxyvitamin D levels did not change (97.2±52 to 111.2±60 pmol/L). Total alkaline phosphatase activity increased significantly from 66±18 at baseline to 85±22 U/L by month 3 and remained significantly above baseline at month 24 (73±21 U/L; p<0.05). The new steady-state level of the alkaline phosphatase activity was well within normal limits. Magnesium fell minimally (0.74±0.1 to 0.72±0.1 mmol/L; p<0.05). Serum creatinine did not change significantly (88.4±796 to 79.6± 796 μmol/L; p=NS) nor did glomerular filtration rate as calculated by modification of diet in renal disease (74.9± 21 to 76.4±20 mL/min/1.73 m2; p=NS).
Bone mineral density
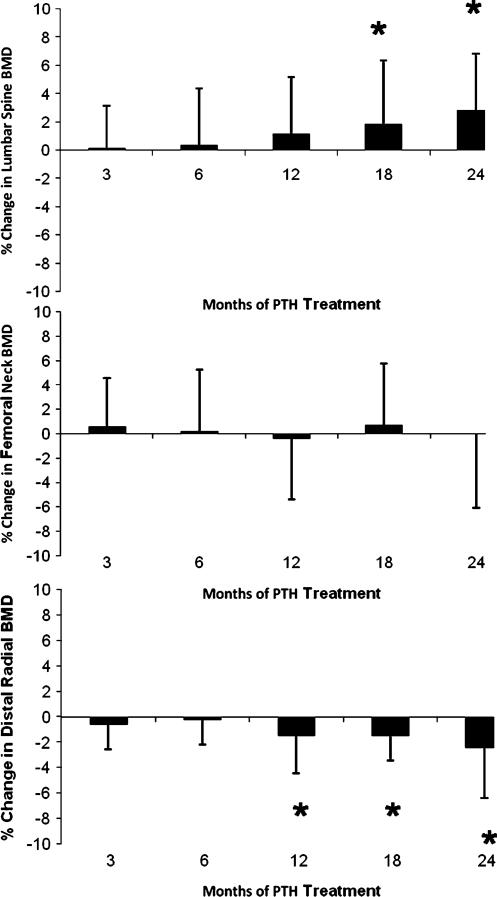
Lumbar spine BMD increased from baseline by 2.9±4% (p <0.05) from 1.24±0.3 to 1.27±0.3 g/cm2 (T-score, +1.7±2 to +1.9±2; Fig. 4). Femoral neck BMD did not change significantly from baseline (+0.95±0.2 g/cm2; T-score 0.7± 2; Fig. 4), while the distal one third radius BMD decreased by 2.4±4% (p<0.05; +0.73±0.1 to 0.70±0.1 g/cm2; T-score, −0.03±2 to −0.26±1; Fig. 4).
Fig. 4.
Changes in bone mineral density. Lumbar spine bone mineral density (BMD) increased, while the femoral neck did not change and the distal one third radius BMD decreased. Data are mean±SD; *p< 0.05 as compared to baseline
Discussion
The results of this study show that in patients with hypoparathyroidism of various etiologies, treatment with PTH(1–84) at 100 μg every other day for 2 years significantly reduced calcium and 1,25-dihydroxyvitamin D supplementation while maintaining stable serum calcium concentrations. Hypercalcemia was uncommon. BMD increased at the lumbar spine, a site enriched in cancellous bone and decreased at the distal one third radius, a site enriched in cortical bone. Femoral neck BMD did not change. These data help to elucidate the effect of PTH replacement on calcium metabolism and skeletal mass in hypoparathyroidism.
The current mainstay of treatment for hypoparathyroidism is calcium and vitamin D supplementation. The amounts of supplemental calcium required to maintain the serum calcium in the normal range are typically much higher than usual recommendations. As a result, this approach carries with it a number of concerns. Fluctuations in serum calcium can occur, from levels above or below normal, as doses of calcium and/or vitamin D are adjusted to the ambient calcium concentration and to symptoms. Such variability in the circulating concentration of a critically important cation for organ and cellular function can have long-term deleterious effects. Vitamin D toxicity can also develop, as manifested by hypercalcemia and hypercalciuria, with its adverse effects on the renal, central nervous, cardiovascular, and other systems.
Treatment of hypoparathyroidism with PTH is appealing because it provides the hormone that is missing. Replacement therapy with PTH should lead to a restoration of or improvement in calcium homeostasis. Illustrating this point, we were able to maintain the serum calcium within the normal range, despite significantly reducing the intake of supplemental calcium and vitamin D. With PTH administration, urinary calcium did not increase, with the exception of only one time point in which the change was modest and transient. In fact, PTH treatment achieved higher serum calcium levels for a significant period of time (months 2–6), with only transiently higher urinary calcium levels (month 3). Higher serum levels of calcium were, thus, attained without incurring additional renal risks. The observation that PTH is not associated with hypercalciuria has been seen also when PTH(1–34) was used in hypoparathyroidism [10]. With another dosing regimen, perhaps, urinary calcium excretion might decrease, a finding that would take advantage of the physiologic actions of PTH to conserve urinary calcium excretion. It is difficult to compare our results with the data of Winer et al. with PTH(1–34) [10] because of the different dosages and frequencies of administration.
In the study of Winer et al., there were no differences in BMD when the PTH group was compared to those who were treated with 1,25-dihydroxyvitamin D alone [9]. Our results differ in that the lumbar spine BMD increased significantly. Since PTH is known to be anabolic for cancellous bone, these findings could indicate that new, younger bone is being formed as a result of PTH treatment. A more detailed examination of skeletal features using high-resolution imaging or bone biopsy would be necessary to elucidate which changes in microarchitectural parameters contribute to the increase in trabecular BMD. Such results would also be of great interest in terms of a comparison between effects of PTH as a therapy for osteoporosis or as replacement therapy for hypoparathyroidism. Along with the increase in lumbar spine BMD, we observed a decrease in the distal one third radius, a site of cortical bone. Again, these results speak to the effects of PTH to cause endosteal resorption. These data do not imply that the bone is weakened because salutary effects on microarchitecture and bone size could well provide biomechanical advantages despite the reduction in BMD. Again, a more detailed skeletal assessment would be required to answer this question. Overall, these changes in trabecular and cortical skeletal compartments recall the pattern seen with PTH treatment of osteoporosis in individuals who do not have hypoparathyroidism [13].
The study has a number of limitations. There was no control arm of subjects with hypoparathyroidism who were not treated with PTH. However, it is unlikely that the magnitude of decreases in oral calcium and 1,25-dihydroxyvitamin D supplementation that were observed would have occurred in the absence of PTH. The relative stability of supplemental calcium and vitamin D dosages prior to study entry also argue against this. It is also possible that further decreases in calcium and calcitriol requirements or even complete cessation of calcitriol administration might have been observed with a more frequent dosing regimen of PTH(1–84). However, dosing that is frequent enough to obviate the need for any calcitirol could be associated with excessive bone turnover. Our study design may have led us to miss detection of peak serum and urinary calcium levels if these occurred within 24 h of PTH administration. A more detailed pharmacokinetic assessment would be necessary to address this point. Finally, we do not have data on dietary calcium intake. However, relative to the large amounts of supplemental calcium that these patients were taking, it is unlikely that dietary calcium would have significantly influenced our findings.
Despite these limitations, this report demonstrates that PTH(1–84) can be used as a therapeutic agent in hypoparathyroidism effectively and safely. Future studies are needed to determine further how PTH(1–84) may influence bone quality and how it may be used to even greater advantage in this disorder. Finally, the study looks ahead to a definitive clinical trial with larger numbers of subjects and with a randomized, placebo-controlled design.
Acknowledgments
Funding DK067619, DK 069350, FD-R-02525, and NPS Pharmaceuticals.
Footnotes
Conflicts of interest None.
References
- 1.Marx S. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med. 2000;343:1863–1875. doi: 10.1056/NEJM200012213432508. [DOI] [PubMed] [Google Scholar]
- 2.Thakker R. Genetics of endocrine and metabolic disorders: parathyroid. Rev Endocr Metab Disord. 2004;5:37–51. doi: 10.1023/B:REMD.0000016123.21743.fe. [DOI] [PubMed] [Google Scholar]
- 3.Rubin M, Levine M. Primer of metabolic bone diseases. American Society of Bone and Mineral Research; Washington: 2008. Hypoparathyroidism. [Google Scholar]
- 4.Kao PC, van Heerden JA, Grant CS, Klee GG, Khosla S. Clinical performance of parathyroid hormone immunometric assays. Mayo Clin Proc. 1992;67:637–645. doi: 10.1016/s0025-6196(12)60717-4. [DOI] [PubMed] [Google Scholar]
- 5.Michelangeli VP, Heyma P, Colman PG, Ebeling PR. Evaluation of a new, rapid and automated immunochemilumino-metric assay for the measurement of serum intact parathyroid hormone. Ann Clin Biochem. 1997;34(Pt 1):97–103. doi: 10.1177/000456329703400115. [DOI] [PubMed] [Google Scholar]
- 6.Christiansen C, Rodbro P, Christensen MS, Hartnack B, Transbol I. Deterioration of renal function during treatment of chronic renal failure with 1, 25-dihydroxycholecalciferol. Lancet. 1978;2:700–703. doi: 10.1016/s0140-6736(78)92702-2. [DOI] [PubMed] [Google Scholar]
- 7.Kurokawa K. Calcium-regulating hormones and the kidney. Kidney Int. 1987;32:760–771. doi: 10.1038/ki.1987.272. [DOI] [PubMed] [Google Scholar]
- 8.Litvak J, Moldawer MP, Forbes AP, Henneman PH. Hypocalcemic hypercalciuria during vitamin D and dihydrotachysterol therapy of hypoparathyroidism. J Clin Endocrinol Metab. 1958;18:246–252. doi: 10.1210/jcem-18-3-246. [DOI] [PubMed] [Google Scholar]
- 9.Winer KK, Ko CW, Reynolds JC, et al. Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1–34) versus calcitriol and calcium. J Clin Endocrinol Metab. 2003;88:4214–4220. doi: 10.1210/jc.2002-021736. [DOI] [PubMed] [Google Scholar]
- 10.Winer KK, Yanovski JA, Cutler GB., Jr Synthetic human parathyroid hormone 1–34 vs calcitriol and calcium in the treatment of hypoparathyroidism. JAMA. 1996;276:631–636. [PubMed] [Google Scholar]
- 11.Winer KK, Yanovski JA, Sarani B, Cutler GB., Jr A randomized, cross-over trial of once-daily versus twice-daily parathyroid hormone 1–34 in treatment of hypoparathyroidism. J Clin Endocrinol Metab. 1998;83:3480–3486. doi: 10.1210/jcem.83.10.5185. [DOI] [PubMed] [Google Scholar]
- 12.Nussbaum SR, Zahradnik RJ, Lavigne JR, et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem. 1987;33:1364–1367. [PubMed] [Google Scholar]
- 13.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]