Abstract
Purpose of review
This review summarizes recent advances in the understanding of the molecular physiology and regulation of the thiazide-sensitive cotransporter (NCC).
Recent findings
Mutations of With-No-Lysine (WNK) Kinases 1 and 4 result in hyperactivity of NCC and FHHt (Familial Hyperkalemic Hypertension), a genetic syndrome of hypertension. Recent studies have shown that WNK1 and WNK4 activate the STE20 family protein kinases SPAK and OSR1, resulting in phosphorylation and activation of NCC. Additionally, a mouse knock-in model for a WNK4 FHHt mutant demonstrated increased SPAK/OSR1 and NCC phosphorylation. It is unclear how these studies fit with the data indicating that WNK4 inhibits NCC and the FHHt mutations of WNK4 are loss-of-function mutations. Another WNK kinase, WNK3, regulates NCC, activating NCC and antagonizing WNK4's effect. Recent studies examining the hormonal regulation of NCC have implicated angiotensin II and aldosterone in regulation of the WNK4-SPAK-NCC pathway. Angiotensin II may also play a role in pressure natriuresis via actions on NCC.
Summary
NCC is subject to a complex regulatory network of kinases which appear sensitive to alterations of the hormonal and physiologic milieu.
Keywords: NCC, Gordon's syndrome, FHHt, WNK
Introduction
The thiazide-sensitive sodium-chloride cotransporter (NCC) localizes to the apical membrane of the distal convoluted tubule (DCT) that is responsible for reabsorbing 5–10% of the filtered load of sodium in the kidney [1]. Encoded by the SLC12A3 gene, NCC is a member of the family of cation-chloride cotransporters which includes the sodium-potassium-chloride cotransporters (NKCC1 and NKCC2) as well as the potassium-chloride cotransporters. Medications that inhibit NCC (thiazide diuretics) are a first line treatment for essential hypertension [2]. While important in human disease, the mechanisms underlying regulation of this cotransporter in the kidney have remained largely unknown until recently. NCC was first cloned in 1994, ushering in an era of study of the molecular regulation of the cotransporter [1]. Since that time, the physiology of NCC in genetic hypertension, hormonal regulation, and other systems have been investigated. This article serves to review the recent discoveries in these fields.
Regulation of NCC by WNK Kinases
The form of genetic hypertension known as Familial Hyperkalemic Hypertension (FHHt, also known as Gordon's Syndrome and Pseudohypoaldosteronism type II) has long been thought to involve NCC [3]. FHHt is manifested by arterial hypertension, hyperkalemia, and metabolic acidosis, the mirror image of the well-known syndrome of decreased NCC activity, Gitelman's Syndrome [3, 4]. In 2001, mutations in the WNK (With-No-Lysine (K)) kinases WNK1 and WNK4 were linked to FHHt [5]. This family of kinases was given the designation WNK as they lack a strictly conserved lysine residue with the catalytic domain [6]. They range in size from 1200 to 1700 amino acids with highly conserved sequences [7, 8]. WNK1, WNK3, and WNK4 are expressed in the DCT, and in addition, a shorter transcript of WNK1 lacking its kinase domain is expressed exclusively in the kidney (ks-WNK1) [7, 8].
The discovery of a link between the WNK kinases and FHHt gave way to a flurry of studies using the Xenopus laevis oocyte expression system. This work described a cascade of inhibitory effects involving WNK kinases and NCC [9, 10]. NCC activity is inhibited by WNK4, WNK1 inhibits WNK4's effect, and ks-WNK1 inhibits full length WNK1 [11–14]. The role of the kinase and protein-protein interaction domains in these activities is unclear, with various studies producing conflicting results [11, 12]. The changes in activity are mediated by changes in NCC surface expression, with reduced NCC cell surface expression in the presence of WNK4 [12, 15]. Further studies using both oocytes and transfected mammalian cells demonstrated that WNK4's effect on NCC is dynamin-independent and therefore not mediated by clathrin-mediated endocytosis [15, 16]. Subsequent studies demonstrated that WNK4 sharply decreases movement of NCC from the trans-Golgi network to the plasma membrane, with NCC instead accumulating and degrading in lysosomes [15, *17]. Furthermore, NCC associates with the adaptor protein AP-3, which is involved in lysosomal transport [*17]. The addition of WNK4 enhances this association, suggesting that WNK4 stimulates NCC–AP3 interaction, thereby stimulating NCC's transport to the lysosome [*17]. Notably, this paper represents the first reported use of an externally tagged NCC to measure surface expression.
Recent work has described a role for WNK3 in the regulation of NCC. WNK3 stimulates NCC activity, with kinase-dead WNK3 having an inhibitory effect on the cotransporter [18]. WNK3 antagonizes the action of WNK4, and vice versa, with FHHt disease-causing mutants exerting a dominant negative effect on WNK4's ability to oppose WNK3, creating a state of effective WNK3 excess [19]. In addition, WNK3 associates with both WNK4 and WNK1, and ks-WNK1 inhibited WNK3's kinase activity [19]. This study demonstrated that the carboxy-terminal domain of WNK3 is essential for its effect, but work using chimeras has provided evidence that these effects of WNK3 and WNK4 are dependent on their amino-terminal domains [19, *20]. However, a recent study of WNK3 carboxy-terminal splice variants demonstrated that these variants had differential effects on NCC activity [*21]. Given these conflicting data, further work is needed to clarify the roles of the amino and carboxy-termini of WNK3 and WNK4. These interesting studies should form the basis for future investigations utilizing transgenic animal models and/or mammalian cells to further characterize the role of WNK3 in NCC regulation.
The cell models demonstrating inhibition of NCC by WNK4 and loss-of-function of WNK4 FHHt mutants have now been supported by a transgenic whole animal model. Mice transgenic for these WNK4 mutations develop hypertension remediable with thiazide diuretics, thus recapitulating the clinical syndrome of FHHt [22]. In addition, mice transgenic for an additional normal WNK4 gene exhibit hypotension when compared with wild-type mice [22]. This data reinforces the cell-based data. Echoing the results of the prior animal study, another group created a different FHHt WNK4 mutant knock-in mouse that developes hypertension treatable with thiazide diuretics [23]. This study demonstrated increased NCC phosphorylation as well as phosphorylation of two recently described stimulators of NCC activity, STE20/SPS-1 related proline/alanine rich kinase and oxidative-stress-responsive kinase-1 (SPAK and OSR1). This data led the authors to hypothesize that wild type WNK4 acts through SPAK/OSR1 to increase NCC activity and that the WNK4 mutation is a gain-of-function mutation [23].
Regulation of NCC by OSR1/SPAK
OSR1 and SPAK are closely related kinases belonging to the STE20 kinase subfamily, possessing a conserved serine motif and C-terminal domain [24–26]. Immunoprecipitation and yeast two-hybrid studies revealed that WNK1 and WNK4 interact with SPAK and OSR1 [25, 27–29]. The SPAK/OSR1 conserved C-terminal domain interact with specific RFx[V/I] motifs present on the WNK kinases [26, 28, 29]. Both WNK1 and WNK4 phosphorylate OSR1 and SPAK at a Thr residue (Thr233 in SPAK/Ser323 in OSR1) and a Ser residue (Ser 373 in SPAK/Ser323 in OSR1) [25]. While both phosphorylate and activate SPAK/OSR1, WNK1 may be more efficient at phosphorylating OSR1/SPAK than WNK4 [25].
Activation of sodium-chloride cotransporters by low chloride concentrations is a long-recognized phenomena [30, 31]. In fact, all three of the sodium-dependent cation-chloride cotransporters are phosphorylated in their amino-terminus and activated in response to low chloride concentrations [26, 32–34]. It is now evident that SPAK and OSR1 are responsible for this process. Recent studies have demonstrated that SPAK/OSR1 phosphorylate NCC at three specific residues: Thr46, Thr55, and Thr60 in human NCC [**35]. Exposure of HEK293 and mpkDCT cells to hypotonic, low chloride incubation enhances NCC phosphorylation at those sites [**35]. Interaction at an N-terminal RFxI motif appears to facilitate phosphorylation [**35]. Mutation of Thr60 to a non-phosphorylatable Ala, results in decreased phosphorylation at each of the three residues, implying a central role for Thr60 in this process [**35]. Furthermore, mutation of Thr60 sharply decreased NCC activity in response to low-chloride incubation in both mammalian cells and oocytes [**35, 36]. Interestingly, mutations of Thr60 have been associated with Gitelman's syndrome, the genetic disorder of hypotension secondary to a loss-of-function mutation of NCC [37].
Although this WNK-SPAK-NCC pathway is consistent with data indicating that WNK1 stimulates NCC at baseline and that FHHt-causing WNK1 mutations are gain of function mutations, it implies a different mechanism to this effect. It remains to be seen whether this pathway or WNK4 inhibition by WNK1 is the primary physiologic pathway by which WNK1 stimulates NCC. It is possible that these two pathways operate in parallel, providing dual mechanisms of action (Figure 1). Importantly, unlike WNK4, there is no available transgenic animal model to study the effects of mutant WNK1. Mice heterozygous for a gene-trapping WNK1 mutation (WNK1 deficient) did display decreased blood pressure compared to controls, but a homozygous WNK1 gene-trap mutation was embryonic lethal [38].
Figure 1.
Contrasting theories into the mechanism of FHHt mutations. A. Mutant WNK4 as a Loss of Function. WNK4 acts to inhibit NCC activity. Mutant WNK4 is unable to inhibit NCC, resulting in a net increase in NCC activity. B. Mutant WNK4 as a Gain of Function. In the normal state, WNK4 activates OSR1/SPAK, which in turn activates NCC, resulting in enhanced NCC activity. The mutant WNK4 is a gain of function mutation, resulting in increased OSR1/SPAK activation and therefore increased NCC activity. C. Mutant WNK1 acting via WNK4. WNK1 normally acts to inhibit WNK4. Since WNK4 inhibits NCC, this has the net result of increasing NCC activity. Mutant WNK1 is a gain of function, enhancing its ability to inhibit WNK4 and therefore increasing NCC activity. D. Mutant WNK1 Acting Via OSR1/SPAK. WNK1 normally acts to increase OSR1/SPAK phosphorylation, which increases NCC activity. Mutant WNK1 is a gain of function mutation, resulting in increased OSR1/SPAK activity, which in turn increases NCC activity.
The WNK4-SPAK-NCC cell-based data indicating that WNK4 stimulates NCC has been considerably more controversial since there is body of data indicating that WNK4 inhibits NCC. This was further examined in an FHHt-causing WNK4 mutant knock-in mouse that was found to have a hypertensive and hyperkalemic phenotype compared to wild type animals [23]. Of note, this study utilized a different FHHt mutation than the prior animal model (WNK4D561A vs. WNK4Q562E). These mice displayed increased phosphorylation of both NCC and SPAK/OSR1 [23]. Therefore, the authors hypothesized that wild-type WNK4 acts through SPAK/OSR1 to phosphorylate and increase the surface expression of NCC and that mutant WNK4 is a gain-of-function mutation [23]. This hypothesis is in opposition to the wealth of data indicating that wild-type WNK4 decreases NCC surface expression and mutant WNK4 is a loss-of-function mutation (Figure 1) [11, 12, 22]. It has not been reported whether the mouse generated by Lalioti, et al also has altered phosphorylation of NCC. However, recent work in a Xenopus oocyte model has demonstrated that mutations of the key SPAK/OSR phosphorylation site (rat Thr58, human Thr60) on NCC do not prevent the effects of WNK3 or WNK4 on NCC [*21]. This suggests that WNK kinases may have effects on NCC independent of SPAK/OSR1. Two recent studies exploring hormonal mechanisms of NCC regulation have provided hints that may eventually reconcile these seemingly disparate hypotheses [**39, **40]. These studies suggest that WNK4 may stimulate NCC function through SPAK only under certain physiologic or hormonal conditions.
Regulation of NCC By Hormones and Other Mechanisms
Although aldosterone has traditionally been thought to mediate physiologic effects on sodium reabsorption and blood pressure through regulation of ENaC, a number of studies have shown that it also regulates NCC [41–43]. This would be consistent with the expression of the mineralocorticoid receptors in the DCT of humans, rats and rabbits [44–46]. Recent work has suggested a role for OSR1/SPAK in an aldosterone effect on NCC (Figure 2) [39]. In wild-type mice, increased phosphorylation of NCC and OSR1/SPAK were seen with low salt diets, an effect blocked by spironolactone [39]. Administration of exogenous aldosterone recapitulated this effect [39]. However, the previously described WNK4 FHHt knockin mouse demonstrated increased phosphorylation that was not effected by a low salt diet or aldosterone. Function of NCC was not measured in this study. These findings implied that under conditions of renin-angiotensin-aldosterone stimulation that aldosterone mediates increased phosphorylation of SPAK and NCC. FHHt mutations seem to render this phosphorylation constitutive.
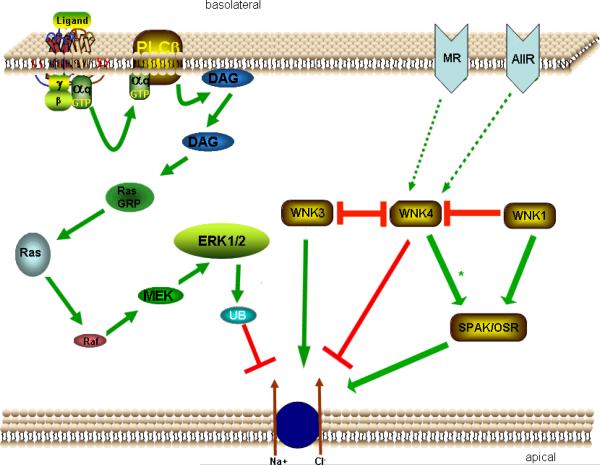
Figure 2.
Proposed Schematic of Molecular Pathways of NCC Regulation. Binding of ligand to G-protein coupled (αq) receptor stimulates phospholipase C (PLCβ). PLCβ activation triggers diacylglerol (DAG) release, in turn stimulating RasGRP1, Ras, Raf, MEK1/2, and ERK1/2. ERK1/2 causes enhanced ubiquitination of NCC, ultimately reducing NCC activity. WNK4 appears to directly inhibit NCC activity. WNK1 and WNK3 appear to inhibit WNK4. WNK3 may directly activate NCC as well. WNK1 and WNK4 stimulate OSR1/SPAK. OSR1/SPAK activates NCC. Stimulation of the mineralcorticoid receptor (MR) and/or angiotensin II receptor (AIIR) may act through WNK4 to activate OSR1/SPAK (* = Under certain hormonal/physiologic stimuli, WNK4 acts to stimulate OSR1/SPAK), stimulating NCC.
Angiotensin II (AII) has also been implicated as a potential regulator of the WNK-SPAK-NCC stimulatory cascade. Studies have demonstrated that AII acutely increases the surface expression of NCC in the rat DCT and that Angiotensin Converting Enzyme inhibitors blocked the increase in surface expression [47, 48]. A recent study indicated that co-injection of AII receptors, NCC and WNK4 into oocytes followed by exposure to AII increases thiazide-sensitive 22Na+ uptake as compared to NCC and WNK4 alone [**40]. Exposure to AII prevents the inhibition of WNK4. In this system, AII has no effect on NCC function in the absence of WNK4. A SPAK dominant-negative prevents the effect of AII in the presence of WNK4 (Figure 2). Additionally, this study showed a qualitative increase in SPAK and NCC phosphorylation in mammalian cells exposed to AII. These studies suggest that under certain physiologic conditions that activate the renin-angiotensin-aldosterone system, WNK4 may be stimulatory for NCC and perhaps under other physiologic conditions WNK4 inhibits NCC. Future studies should further clarify the effects of angiotensin and aldosterone on WNK4, SPAK, and NCC in whole animal and mammalian cell models.
AII has also been studied as a mediator for NCC's role in pressure natriuresis. DCT salt reabsorption (and presumably NCC activity) decreases in response to acute increases in blood pressure [*49]. In this study, AII blunts the diuretic effect [*49]. However, a second study, using a rat arterial constriction model of acute hypertension, showed that NCC expression at the apical surface (a presumed surrogate for NCC activity) decreases in response to acute increases in blood pressure [*50]. Clamping AII at a constant level prevented this effect, suggesting that NCC retraction from the apical membrane contributes to pressure natriuresis and that this effect is mediated (at least in part) by a decrease in AII [*50]. These seemingly disparate results may be in fact due to a number of possible factors. As the first study did not separate NCC and ENaC activity, differential effects on NCC and ENaC in pressure diuresis could account for the differences. Similarly, potential biphasic dose effects of AII on NCC (the studies used vastly different doses of AII), and differences in experimental models (AII infusion vs. vasoconstrictive) may play a role in the contrasting results.
Lastly, work in our laboratory has uncovered an additional kinase pathway that regulates NCC activity [51]. Using a mammalian cell line with native NCC activity, phorbol esters (PE), an analog of diacylglycerol, markedly diminished NCC activity. Although diacylglycerol has been historically linked to activation of protein kinase C, utilizing specific inhibitors and RNA interference, a more complex interaction was demonstrated. PEs stimulate Ras Guanyl nucleotide Releasing Protein 1 (RasGRP1), which activates the small G-protein Ras, triggering a cascade ultimately activating ERK1/2 MAPK (Extra-cellular signal-Regulated Kinases 1 and 2 Mitogen Activated Protein Kinase (Figure 2)). ERK1/2 activation then triggers reduced NCC activity [51]. Our recent data (unpublished observations) suggests that ERK1/2 activation enhances ubiquitination of NCC, in turn triggering endocytosis of NCC. As DAG activation is often associated with G-protein-coupled receptor activation, this pathway may mediate the effects of various hormones on NCC.
Conclusions
The last two years have seen a remarkable increase in our understanding of the molecular regulation of NCC (Figure 2). Another WNK kinase (WNK3) has been found to interact with and regulate NCC and WNK4, adding another layer of complexity to an intricate pathway. Additionally two other kinases (SPAK and OSR1) have been implicated as key stimulators of NCC activity and potential transducers of WNK signaling to NCC. Two mice transgenic for different WNK4 FHHt mutations have demonstrated thiazide-sensitive hypertension. However, one of those transgenic mice has demonstrated increased phosphorylation of NCC at SPAK phosphorylation sites. This suggested that WNK4 may activate NCC and that certain WNK4 mutations could result in increased activation of SPAK and thus NCC. However a wealth of data indicates that WNK4 inhibits NCC and that the FHHt mutation is a gain-of-function. This data may be reconciled by very recent studies indicating WNK4 may be stimulatory for NCC only under certain hormonal or physiologic conditions. Further studies examining whether WNK4 can both activate NCC via SPAK and inhibit NCC directly depending on the conditions will be essential to advancing the field. On the other hand all WNK1 studies seem to indicate that WNK1 stimulates NCC. Further studies are necessary to determine if WNK1 stimulates NCC through WNK4 inhibition and SPAK activation in a parallel fashion or if one of these pathways is the more physiologically relevant. Phorbol ester stimulation of RasGRP1 revealed that ERK1/2 is yet another kinase that inhibits NCC activity, and this may be an important pathway for G-protein-coupled hormone receptor regulation of NCC. Importantly, the investigation of the molecular physiology of NCC has now begun to focus on the hormonal regulation of NCC. This key step in our understanding of the physiology of NCC is still in its infancy, but the process of understanding the role and mechanism of angiotensin II and aldosterone effects on NCC has begun. Future work will focus on clarifying these controversies and further exploring these pathways.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gamba G, Miyanoshita A, Lombardi M, et al. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J. Biol. Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- [2].Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- [3].Gordon RD, Hodsman GP. The syndrome of hypertension and hyperkalaemia without renal failure: long term correction by thiazide diuretic. Scott Med J. 1986;31:43–4. doi: 10.1177/003693308603100114. [DOI] [PubMed] [Google Scholar]
- [4].Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians. 1966;79:221–35. [PubMed] [Google Scholar]
- [5].Wilson FH, Disse-Nicodeme S, Choate KA, et al. Human Hypertension Caused by Mutations in WNK Kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- [6].Xu B, English JM, Wilsbacher JL, et al. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275:16795–801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- [7].Xu BE, Min X, Stippec S, et al. Regulation of WNK1 by an autoinhibitory domain and autophosphorylation. J Biol Chem. 2002;277:48456–62. doi: 10.1074/jbc.M207917200. [DOI] [PubMed] [Google Scholar]
- [8].Lenertz LY, Lee BH, Min X, et al. Properties of WNK1 and implications for other family members. J Biol Chem. 2005;280:26653–8. doi: 10.1074/jbc.M502598200. [DOI] [PubMed] [Google Scholar]
- [9].Huang CL, Yang SS, Lin SH. Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens. 2008;17:519–25. doi: 10.1097/MNH.0b013e32830dd580. [DOI] [PubMed] [Google Scholar]
- [10].Flatman PW. Cotransporters, WNKs and hypertension: an update. Curr Opin Nephrol Hypertens. 2008;17:186–92. doi: 10.1097/MNH.0b013e3282f5244e. [DOI] [PubMed] [Google Scholar]
- [11].Wilson FH, Kahle KT, Sabath E, et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci U S A. 2003;100:680–4. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest. 2003;111:1039–45. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang CL, Zhu X, Wang Z, et al. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J Clin Invest. 2005;115:1379–87. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol. 2006;290:F619–24. doi: 10.1152/ajprenal.00280.2005. [DOI] [PubMed] [Google Scholar]
- [15].Cai H, Cebotaru V, Wang YH, et al. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int. 2006;69:2162–70. doi: 10.1038/sj.ki.5000333. [DOI] [PubMed] [Google Scholar]
- [16].Golbang AP, Cope G, Hamad A, et al. Regulation of the expression of the Na/Cl cotransporter by WNK4 and WNK1: evidence that accelerated dynamin-dependent endocytosis is not involved. Am J Physiol Renal Physiol. 2006;291:F1369–76. doi: 10.1152/ajprenal.00468.2005. [DOI] [PubMed] [Google Scholar]
- *[17].Subramanya AR, Liu J, Ellison DH, et al. WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J. Biol. Chem. 2009 doi: 10.1074/jbc.M109.008185. M109.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using an externally tagged NCC, this paper demonstrates that WNK4 diverts NCC to the lysosome. Furthermore, the paper describes an association between NCC and AP-3 that is enhanced by WNK4 which may play a role in lysosomal shuttling.
- [18].Rinehart J, Kahle KT, de Los Heros P, et al. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci U S A. 2005;102:16777–82. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang CL, Zhu X, Ellison DH. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest. 2007;117:3403–11. doi: 10.1172/JCI32033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[20].San-Cristobal P, Ponce-Coria J, Vazquez N, et al. WNK3 and WNK4 amino-terminal domain defines their effect on the renal Na+-Cl− cotransporter. Am J Physiol Renal Physiol. 2008;295:F1199–206. doi: 10.1152/ajprenal.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates that the amino-terminal domains of WNK3 and WNK4 are essential for their actions upon the cotransporter.
- *[21].Glover M, Zuber AM, O'Shaughnessy KM. Renal and Brain Isoforms of WNK3 Have Opposite Effects on NCCT Expression. J Am Soc Nephrol. 2009;20:1314–1322. doi: 10.1681/ASN.2008050542. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrates that C-terminal splice variants of WNK3 have differential effects on NCC. Additionally, mutations of NCC phosphorylation sites are shown to have no effect on WNK3 or WNK4's effect on NCC.
- [22].Lalioti MD, Zhang J, Volkman HM, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–32. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- [23].Yang SS, Morimoto T, Rai T, et al. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5:331–44. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- [24].Tamari M, Daigo Y, Nakamura Y. Isolation and characterization of a novel serine threonine kinase gene on chromosome 3p22-21.3. J Hum Genet. 1999;44:116–20. doi: 10.1007/s100380050121. [DOI] [PubMed] [Google Scholar]
- [25].Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vitari AC, Thastrup J, Rafiqi FH, et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397:223–31. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Anselmo AN, Earnest S, Chen W, et al. WNK1 and OSR1 regulate the Na+, K+, 2Clâ^' cotransporter in HeLa cells. Proceedings of the National Academy of Sciences. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gagnon KBE, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol. 2006;290:C134–142. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- [29].Moriguchi T, Urushiyama S, Hisamoto N, et al. WNK1 Regulates Phosphorylation of Cation-Chloride-coupled Cotransporters via the STE20-related Kinases, SPAK and OSR1. J. Biol. Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- [30].Lytle C, Forbush B., 3rd Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: modulation by cytoplasmic Cl. Am J Physiol. 1996;270:C437–48. doi: 10.1152/ajpcell.1996.270.2.C437. [DOI] [PubMed] [Google Scholar]
- [31].Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277:50812–9. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- [32].Darman RB, Forbush B. A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J Biol Chem. 2002;277:37542–50. doi: 10.1074/jbc.M206293200. [DOI] [PubMed] [Google Scholar]
- [33].Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1) J Biol Chem. 2003;278:27347–53. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- [34].Gimenez I, Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2) Am J Physiol Renal Physiol. 2005;289:F1341–5. doi: 10.1152/ajprenal.00214.2005. [DOI] [PubMed] [Google Scholar]
- **[35].Richardson C, Rafiqi FH, Karlsson HKR, et al. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]; Key paper that demonstrates that the kinases SPAK and OSR1 phosphorylate NCC, implying a role for OSR1/SPAK in the WNK kinases' effect on NCC.
- [36].Pacheco-Alvarez D, Cristobal PS, Meade P, et al. The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem. 2006;281:28755–63. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- [37].Maki N, Komatsuda A, Wakui H, et al. Four novel mutations in the thiazide-sensitive Na-Cl cotransporter gene in Japanese patients with Gitelman's syndrome. Nephrol Dial Transplant. 2004;19:1761–6. doi: 10.1093/ndt/gfh239. [DOI] [PubMed] [Google Scholar]
- [38].Zambrowicz BP, Abuin A, Ramirez-Solis R, et al. Wnk1 kinase deficiency lowers blood pressure in mice: A gene-trap screen to identify potential targets for therapeutic intervention. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14109–14114. doi: 10.1073/pnas.2336103100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[39].Chiga M, Rai T, Yang SS, et al. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int. 2008;74:1403–9. doi: 10.1038/ki.2008.451. [DOI] [PubMed] [Google Scholar]; This study describes increased NCC and SPAK phosphorylation with low salt diets in control mice and no changes in FHHt-causing mutant knock-in mice. These findings implicate the WNK-SPAK-NCC pathway in aldosterone action.
- **[40].San-Cristobal P, Pacheco-Alvarez D, Richardson C, et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proceedings of the National Academy of Sciences. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that AII increases NCC activity in oocytes also expressing the AII receptor in the presence of WNK4 and SPAK. This provides the first evidence that WNK4 and SPAK may lie in the signaling pathway between AII and NCC.
- [41].Masilamani S, Wang X, Kim GH, et al. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol. 2002;283:F648–57. doi: 10.1152/ajprenal.00016.2002. [DOI] [PubMed] [Google Scholar]
- [42].Nielsen J, Kwon T-H, Masilamani S, et al. Sodium transporter abundance profiling in kidney: effect of spironolactone. Am J Physiol Renal Physiol. 2002;283:F923–933. doi: 10.1152/ajprenal.00015.2002. [DOI] [PubMed] [Google Scholar]
- [43].Velazquez H, Bartiss A, Bernstein P, Ellison DH. Adrenal steroids stimulate thiazide-sensitive NaCl transport by rat renal distal tubules. Am J Physiol. 1996;270:F211–9. doi: 10.1152/ajprenal.1996.270.1.F211. [DOI] [PubMed] [Google Scholar]
- [44].Rundle SE, Smith AI, Stockman D, Funder JW. Immunocytochemical demonstration of mineralocorticoid receptors in rat and human kidney. J Steroid Biochem. 1989;33:1235–42. doi: 10.1016/0022-4731(89)90435-4. [DOI] [PubMed] [Google Scholar]
- [45].Krozowski ZS, Rundle SE, Wallace C, et al. Immunolocalization of renal mineralocorticoid receptors with an antiserum against a peptide deduced from the complementary deoxyribonucleic acid sequence. Endocrinology. 1989;125:192–8. doi: 10.1210/endo-125-1-192. [DOI] [PubMed] [Google Scholar]
- [46].Farman N, Oblin ME, Lombes M, et al. Immunolocalization of gluco- and mineralocorticoid receptors in rabbit kidney. Am J Physiol. 1991;260:C226–33. doi: 10.1152/ajpcell.1991.260.2.C226. [DOI] [PubMed] [Google Scholar]
- [47].Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, et al. ANG II provokes acute trafficking of distal tubule Na+-Cl(−) cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–9. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- [48].Sandberg MB, Maunsbach AB, McDonough AA. Redistribution of distal tubule Na+-Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol. 2006;291:F503–8. doi: 10.1152/ajprenal.00482.2005. [DOI] [PubMed] [Google Scholar]
- *[49].Zhao D, Navar LG. Acute angiotensin II infusions elicit pressure natriuresis in mice and reduce distal fractional sodium reabsorption. Hypertension. 2008;52:137–42. doi: 10.1161/HYPERTENSIONAHA.108.111435. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article shows that distal tubule activity decreases in response in acute changes in blood pressure.
- *[50].Lee DH, Riquier AD, Yang LE, et al. Acute hypertension provokes acute trafficking of distal tubule Na-Cl cotransporter (NCC) to subapical cytoplasmic vesicles. Am J Physiol Renal Physiol. 2009;296:F810–8. doi: 10.1152/ajprenal.90606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that NCC participates in pressure natriuresis with acute trafficking of NCC to subapical vesicles. Clamping AII levels prevented this effect, implying a role for AII in this process.
- [51].Ko B, Joshi LM, Cooke LL, et al. Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci U S A. 2007;104:20120–5. doi: 10.1073/pnas.0709506104. [DOI] [PMC free article] [PubMed] [Google Scholar]