Abstract
Human skin and its immune cells provide essential protection of the human body from injury and infection. Recent studies reinforce the importance of keratinocytes as sensors of danger through alert systems such as the inflammasome. In addition, newly identified CD103+ dendritic cells are strategically positioned for cross-presentation of skin-tropic pathogens and accumulating data highlight a key role of tissue-resident rather than circulating T cells in skin homeostasis and pathology. This Review focuses on recent progress in dissecting the functional role of skin immune cells in skin disease.
The skin, as the primary interface between the body and the environment, provides a first line of defence against microbial pathogens and physical and chemical insults. Immunosurveillance of such a large and exposed organ presents unique challenges for immune sentinels and effector cells. If an immune response is inadequate then overwhelming infections or tumours may ensue, but if an immune response is excessive then chronic inflammation and autoimmunity may develop. Controlling the extent of an immune response is thus a major challenge for maintaining skin integrity, which is of paramount importance for host survival. Therefore, both active defence mechanisms and tolerogenic pathways are used by the host to achieve immune homeostasis, ensuring that immune responses in the skin are properly adjusted to various challenges.
Owing to its accessibility, the skin is an ideal organ system in which to study both tissue and whole-organism responses to local and systemic insults. A growing body of data supports the notion that the skin has essential immunological functions, both during tissue homeostasis and in various pathological conditions.
Although early studies highlighted individual cell types in the skin, it was the visionary concept of the skin-associated lymphoid tissue (SALT), first described by Streilein in 1983 (ref. 1), and later the ‘skin immune system’ (ref. 2) that provided a modern interpretation and overall paradigm for investigators interested in cutaneous immunology. The initial SALT concept introduced the idea of distinct circuiting immune cells that continually traffic in a directed manner between the skin, the draining lymph nodes and the circulation, thereby providing optimal immunosurveillance.
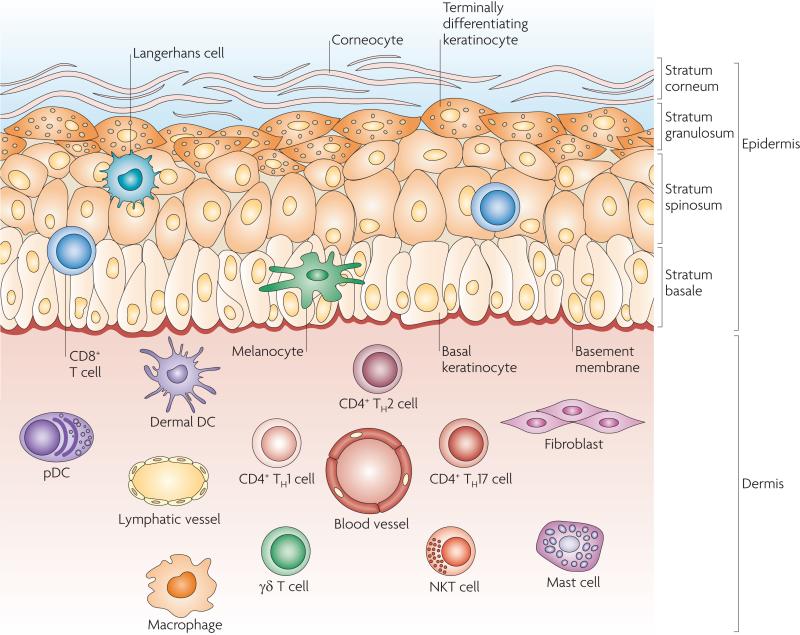
Although considerable attention was directed at the function of epidermal Langerhans cells3, it became apparent that other types of dendritic cells (DCs) and innate immune cells present in the dermis also have a relevant role, resulting in the emergence of the concept of a ‘dermal immune system’ (ref. 4). Human skin has two main compartments: the epidermis and the dermis (FIG. 1). The epidermis is the outer compartment and contains four strata. The stratum basale is the bottom layer of the epidermis and is responsible for constantly renewing the cells of the epidermis. This layer contains just one row of undifferentiated epidermal cells, known as basal keratinocytes, that divide frequently. Basal keratinocytes differentiate and move to the next layer (the stratum spinosum; also known as the prickle cell layer) to begin a maturation process, but also divide to replenish the basal layer. Cells that move into the stratum spinosum change from being columnar to being polygonal in shape and start to synthesize keratins that are distinct from the basal-layer keratins. Keratinocytes in the stratum granulosum are characterized by dark clumps of cytoplasmic material and these cells actively produce keratin proteins and lipids. The stratum corneum, as the ultimate product of maturing keratinocytes, is the outermost of the four strata of the epidermis and is largely responsible for the barrier function of the skin. Cells in this layer, known as corneocytes, are dead keratinocyte-derived cells that are devoid of organelles. They provide the barrier that excludes many toxic agents and prevents dehydration5. Specialized cells of the epidermis include melanocytes, which produce the pigment melanin, and Langerhans cells, which are the main skin-resident immune cell. In addition, T cells, mainly CD8+ T cells, can be found in the stratum basale and stratum spinosum6.
Langerhans cell
A type of dendritic cell that is resident in the epidermal layer of the skin.
Keratinocytes
The major cell type of the epidermis, constituting more than 90% of epidermal cells. Keratinocytes form an effective barrier against the entry of foreign matter and infectious agents into the body and minimize moisture loss.
Figure 1. Skin anatomy and cellular effectors.
The structure of the skin reflects the complexity of its functions as a protective barrier, in maintaining the body temperature, in gathering sensory information from the environment and in having an active role in the immune system. The epidermis contains the stratum basale, the stratum spinosum, the stratum granulosum and the outermost layer, the stratum corneum, which is responsible for the vital barrier function of the skin. Specialized cells in the epidermis include melanocytes, which produce pigment (melanin), and Langerhans cells. Rare T cells, mainly CD8+ cytotoxic T cells, can be found in the stratum basale and stratum spinosum. The dermis is composed of collagen, elastic tissue and reticular fibres. It contains many specialized cells, such as dendritic cell (DC) subsets, including dermal DCs and plasmacytoid DCs (pDCs), and T cell subsets, including CD4+ T helper 1 (TH1), TH2 and TH17 cells, γδ T cells and natural killer T (NKT) cells. In addition, macrophages, mast cells and fibroblasts are present. Blood and lymphatic vessels and nerves (not shown) are also present throughout the dermis.
The epidermis has a simple histology, but the underlying dermis is anatomically more complicated, with greater cell diversity. It contains many specialized immune cells, including DCs, CD4+ T helper (TH) cells, γδ T cells and natural killer T (NKT) cells. Moreover, macrophages, mast cells, fibroblasts and nerve-related cell types are also present (FIG. 1). The dermis is drained by lymphatic and vascular conduits, through which migrating cells can traffic4.
In this review, we focus on new concepts relating to the main skin cell types that have immunological functions and act as immune sentinels. We discuss previously unappreciated roles of tissue-resident epithelial and immune cells in tissue homeostasis and pathology.
γδ T cells
T cells that express heterodimers consisting of the γ- and δ-chains of the T cell receptor (TCR). They enter tissues such as the gut and skin without priming in lymphoid tissues, express limited or invariant TCRs and display a ‘pseudo-memory’ T cell phenotype allowing them to respond rapidly to antigen challenge.
Keratinocytes as immune sentinels
Similar to gut epithelial cells, keratinocytes can sense pathogens and mediate immune responses to discriminate between harmless commensal organisms and harmful pathogens. Eukaryotic cells sense microbial products using receptors that recognize various evolutionarily conserved microbial components termed pathogen-associated molecular patterns (pAMps), which include lipopolysaccharide (LpS), peptidoglycan, flagellin and nucleic acids7. The Toll-like receptors (TLRs) are the best studied of these receptors and ligation of TLRs by pAMps leads to the activation of host cell signalling pathways and subsequent innate and adaptive immune responses. Epidermal keratinocytes express several TLRs, located either on the cell surface (TLR1, TLR2, TLR4, TLR5 and TLR6) or in endosomes (TLR3 and TLR9)8 (FIG. 2). In addition, TLR7 expression is induced through triggering of TLR3 by double-stranded RNA, which makes keratinocytes responsive to TLR7 agonists of the imidazoquinoline antiviral immune response modifier family9. TLR expression by keratinocytes might be crucial for promoting skin immune responses, as activation of these receptors on human keratinocytes leads to a predominant TH1-type immune response and to the production of type I interferons (IFNs)10.
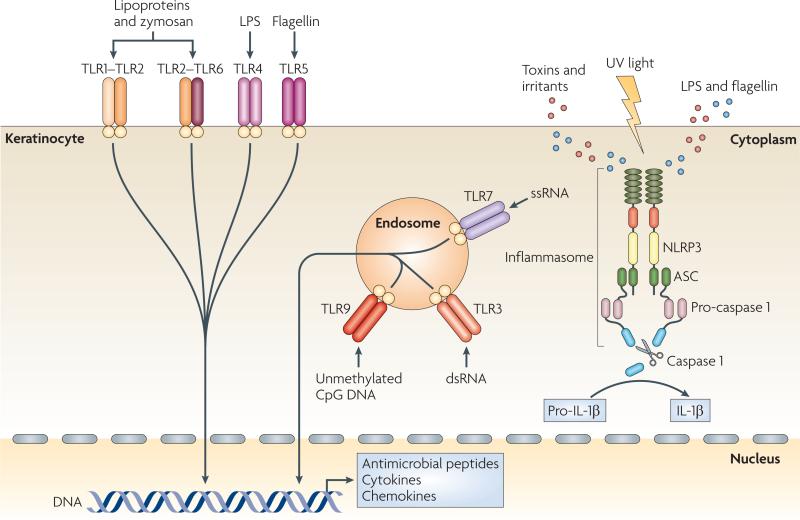
Figure 2. Keratinocytes as sensors of danger.
Keratinocytes are central skin sentinels and can recognize foreign and dangerous agents, for example pathogen-associated molecular patterns (PAMPs) of microbial origin and danger-associated molecular pattern (DAMPs), such as irritants and toxins, through Toll-like receptors (TLRs) and the inflammasome machinery. TLRs are transmembrane receptors that are present on the cell surface or on the surface of endosomal compartments. Lipopolysaccharide (LPS) stimulates TLR4; bacterial lipoproteins and fungal zymosan stimulate TLR1–TLR2 and TLR2–TLR6 heterodimers; bacterial flagellin activates TLR5; unmethylated CpG motifs present in DNA function as stimulators of endosomal TLR9; double-stranded RNA (dsRNA) activates endosomal TLR3; and single-stranded RNA (ssRNA) activates TLR7, the expression of which is induced by TLR3 triggering (not shown). PAMP recognition by TLRs leads to activation of host cell signalling pathways and subsequent innate and adaptive immune responses with antimicrobial peptide, cytokine and chemokine production. Keratinocytes also express NLR family, pyrin domain containing 3 (NLRP3), which belongs to the newly identified class of proteins encoded by the nucleotide-binding domain, leucine-rich repeat-containing (NLR) gene family. These proteins can recognize PAMPs that are in the cytoplasm (such as LPS and flagellin), DAMPs and ultraviolet (UV) light, and activate the inflammasome complex. This multimeric complex is formed by an NLR, an adaptor protein termed ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and pro-caspase 1, and its assembly leads to the activation of caspase 1, which processes pro-interleukin-1β (pro-IL-1β) into biologically active IL-1β.
A recently discovered class of proteins that are encoded by the nucleotide-binding domain, leucine-rich repeat-containing (NLR) gene family can also recognize PAMPs and endogenous danger-associated molecular patterns (DAMPs), such as irritants and toxins. Activation of these receptors results in the activation of pro-inflammatory signalling pathways through the inflammasome — a large multiprotein complex formed by an NLR, the adaptor protein ASC (apoptosis- associated speck-like protein containing a caspase recruitment domain) and pro-caspase 1 (ref. 11). The assembly of the inflammasome leads to the activation of caspase 1, which cleaves pro-interleukin-1β (pro-IL-1β) and pro-IL-18 to generate the active pro-inflammatory cytokines11. It has recently been established that ultraviolet (UV) irradiation activates the inflammasome in human keratinocytes12,13. In addition, contact sensitizers, such as haptens, applied to the skin induce inflammasome-dependent IL-1β and IL-18 processing and secretion and thus allergic contact dermatitis reactions14.
Thus, following skin exposure to haptens or high doses of uv irradiation, intracellular sensors contained in the inflammasome complex in keratinocytes are activated, leading to the activation of caspase 1 and to the processing and secretion of key pro-inflammatory cytokines. This in turn results in the activation of tissue-resident immune cells that induce and perpetuate an inflammatory response.
Hapten
A molecule that can bind antibody but is thought not to elicit an immune response itself. Antibodies that are specific for a hapten can be generated when the hapten is chemically linked to a protein carrier that can elicit a T cell response.
Allergic contact dermatitis
A cutaneous inflammatory condition caused by a T cell-mediated hypersensitivity to defined allergens.
β-defensins and cathelicidins
Members of a family of small antimicrobial polypeptides that are abundant in neutrophils and epithelial cells. They contribute to host defence by disrupting the cytoplasmic membrane of microorganisms such as Escherichia coli or Candida albicans.
Keratinocytes produce innate immune mediators
The production of antimicrobial peptides (AMPs) is an evolutionarily conserved defence mechanism of eukaryotic cells against pathogens. They are produced at damaged epithelium surfaces, where they prevent microbial invasion of the host by direct killing of the pathogen, recruitment of host immune cells and modulation of cytokine production15,16. In the skin, keratinocytes are an important source of cationic AMPs, namely β-defensins and cathelicidins. During skin infections, the local production of AMps by keratinocytes can be increased by T cell-derived cytokines. In particular, IL-17A and IL-22, which are produced by TH17 cells17, increase AMP production by keratinocytes18 and are therefore important regulators of skin and mucosal immunity19, providing a link between keratinocytes and adaptive immune cells. AMPs are also expressed at high levels in the skin of patients with psoriasis, and are thought to be responsible for the lack of skin infection observed in these individuals20. Furthermore, it has been suggested that keratinocytes can contribute to the breaking of self tolerance in patients with psoriasis by producing the cathelicidin antimicrobial peptide LL37 (ref. 21) (see later).
In addition, a link between AMP production, TLR expression and vitamin D has been described: keratinocytes surrounding a wound have increased expression of TLR2, LL37 and 25-hydroxyvitamin D3 1α hydroxylase (also known as CYP27B1), which converts inactive 25-hydroxyvitamin D3 (25(OH)VD3) to active 1,25-dihydroxyvitamin D3 (1,25(OH)2VD3)22. 1,25(OH)2VD3 also acts in synergy with T cell-derived IL-17A to enhance LL37 expression by keratinocytes23. Thus, the skin epithelium is an effective cathelicidin-regulating environment that might be dysregulated in diseases with increased IL-17A production, such as psoriasis (see later).
In addition to AMPs, keratinocytes constitutively secrete, or are induced to release, numerous cytokines, including IL-1, IL-6, IL-10, IL-18 and tumour necrosis factor (TNF)24. Of particular interest with regard to the skin in health and disease is the production of IL-1 by keratinocytes. IL-1 is a pleiotropic cytokine with a broad range of biological effects, including the activation of TH cells and DCs and the promotion of B cell maturation and clonal expansion25. In healthy skin, keratinocytes constitutively synthesize both pro-IL-1α and pro-IL-1β but cannot process them or secrete them in their active forms. Following exposure to stimuli such as UV irradiation, keratinocytes process and release IL-1β through activation of the inflammasome12. Regulation of IL-1α secretion in keratinocytes is less clear. A recent study has shown that loss of caspase 8 leads to the secretion of active IL-1α from a large reservoir of pro-IL-1α in mouse epidermal keratinocytes, suggesting a negative regulatory role for caspase 8 (ref. 26). A role for IL-1α in skin inflammation is suggested in a transgenic mouse model of IL-1α overexpression by keratinocytes, and these mice have an inflammatory skin phenotype27. There are also other IL-1 family members, such as IL-1F6, that have a role in skin immunopathology. Transgenic overexpression of IL-1F6 by keratinocytes led to an inflammatory skin phenotype28. In addition, the expression of IL-1F6 was shown to be increased in psoriatic epithelium, suggesting a possible role in the immunopathogenesis of psoriasis. Keratinocytes might also condition DCs to promote a dysregulated immune response, for example through the secretion of thymic stromal lymphopoietin in allergic inflammation29.
Keratinocytes are also an important source of chemokines and express chemokine receptors, and therefore can modulate an immune response by attracting different cell types into the skin. By expressing CC-chemokine ligand 20 (CCL20), CXC-chemokine ligand 9 (CXCL9), CXCL10 and CXCL11 activated keratinocytes selectively attract effector T cells to the skin during diseases that are characterized by T cell infiltration, such as psoriasis and cutaneous T cell lymphoma24. Activated keratinocytes can also recruit neutrophils to the inflamed epidermis of patients with psoriasis by producing CXCL1 and CXCL8 (also known as IL-8)24. Furthermore, keratin ocytes regulate the trafficking of Langerhans cell precursors to the epithelium through the expression of CCL20 (ref. 30).
So, keratinocytes are pro-inflammatory effector cells that are strategically positioned at the outermost layer of the body to react in a timely fashion to harmful insults by the coordinated production of AMPs, pro-inflammatory cytokines and chemokines.
Keratinocytes as non-professional antigen-presenting cells
Keratinocytes were first shown to express MHC class II molecules in studies of graft-versus-host disease31, and subsequent studies showed that keratinocytes express MHC class II molecules in several skin disorders characterized by significant T cell infiltrates31, thus suggesting that they might act as non-professional antigen-presenting cells (APCs). We now know that IFNγ can upregulate MHC class II expression on primary human keratinocytes and keratinocyte cell lines in vitro31.
Keratinocytes generally induce T cell anergy or tolerance rather than T cell activation in certain in vitro and in vivo models32. However, keratinocytes can support superantigen-driven proliferation in resting T cells, indicating that they can provide requisite signals for T cell proliferation33. A recent study showed that keratinocytes could induce functional responses in epitope-specific CD4+ and CD8+ memory T cells: they could process peptide antigen and present it to CD4+ T cells, resulting in the production of both TH1- and TH2-type cytokines, and could process virally encoded or exogenous peptide and present it to CD8+ T cells, resulting in cytokine production and target cell lysis34. Therefore, it seems that although keratinocytes cannot prime naive T cells, they can potentially induce a recall immune response in antigen-experienced T cells.
The emerging view is that keratinocytes display features of APCs with the potential for both antigen-specific tolerization and activation.
Tolerance
Denotes lymphocyte non-responsiveness to antigen, but implies an active process, not simply a passive lack of response.
LL37
A member of the cathelicidin family of antimicrobial peptides. LL37 has been proposed to have a specific role in psoriasis pathogenesis, contributing to breaking the tolerance to self DNA.
Graft-versus-host disease (GVHD)
A disease that results from donor allogeneic T cells that are transferred along with an allograft (such as a bone marrow, liver or gut allograft) attacking target recipient organs or tissues (such as the skin or gut). GVHD occurs in graft recipients that cannot eliminate the host-reactive donor T cells owing to immunosuppression, immunological immaturity or tolerance.
T cell anergy
A state of T cell unresponsiveness to stimulation with antigen. It can be induced by stimulation with a large amount of specific antigen in the absence of the engagement of co-stimulatory molecules.
Keratinocytes as instigators of inflammation
A key insight into the immunological role of keratinocytes came from an analysis of human skin reactions following topical application of contact sensitizers, such as poison ivy, which showed that keratinocytes are activated before T cells enter the skin35. This finding, together with those of studies of keratinocyte-derived cytokines36–38, led to the conclusion that epidermal keratinocytes could function as instigators of cutaneous inflammation.
Studies carried out in mice showed that dysregulation of keratinocyte function can trigger systemic autoimmune responses by lymphocytes. Overexpression of CD40 ligand in keratinocytes led to a greater than 90% reduction in epidermal Langerhans cells and increased dermal DC numbers, suggesting enhanced migration of CD40-activated Langerhans cells39. This was associated with massive lymphadenopathy and autoantibody formation, suggesting that lymphocyte tolerance to skin antigens was disrupted. Moreover, mice with targeted deletion of JUN and JUNB (both components of the transcription factor AP1) in keratinocytes spontaneously developed chronic inflammation in the skin and joints, with some features resembling psoriasis and psoriatic arthritis40. Targeted inhibition of the nuclear factor-κB (NF-κB) pathway by deleting its crucial regulatory kinase, IκB kinase-β (IKKβ), in keratinocytes also resulted in enhanced cutaneous inflammation41, which supports the role for NF-κB as a double-edged sword in inflammation42. Furthermore, mice with constitutive activation of the transcription factor signal transducer and activator of transcription 3 (STAT3), which is downstream of cytokine receptors such as the IL-23 receptor, in keratinocytes developed a psoriasis-like condition43. Thus, genetic alteration of key signalling pathways involved in inflammatory immune responses in keratinocytes can alter skin homeostasis and induce immunopathology.
Alterations in the expression of keratinocyte-derived molecules that are known to activate immune cells may also modulate the threshold for the development of skin tumours. For example, constitutive expression of the natural-killer group 2, member D (NKG2D) ligand retinoic acid early transcript 1 (RAE1) by keratinocytes results in downregulation of the expression of the activating receptor NKG2D on NK cells, γδ T cells and CD8+ αβ T cells. This in turn resulted in defects in NK cell-mediated cytotoxicity and enhanced tumour susceptibility44. Moreover, an acute rather than sustained upregulation of RAE1 expression by keratinocytes leads to an inflammatory phenotype that involves the redistribution of γδ T cells and Langerhans cells in the epidermal compartment, followed by an influx of unconventional αβ T cells, suggesting that acute changes in NKG2D ligand expression may initiate a rapid, multifaceted immune response45.
Remarkably, all of these model systems that target molecular events in keratinocytes result in a phenotype involving both local and distant organs and systemic alterations of immune responses, thus supporting a pivotal role for keratinocytes in modulating systemic immune responses.
Plasmacytoid DC
A dendritic cell (DC) that lacks myeloid markers such as CD11c and CD33 but expresses high levels of HLA-DR and CD123. These cells produce high levels of type I interferon after activation (for example, when stimulated through Toll-like receptors).
Cross-presentation
The initiation of a CD8+ T cell response to an antigen that is not present within antigen-presenting cells (APCs). This exogenous antigen must be taken up by APCs and then re-routed to the MHC class I pathway of antigen presentation.
Birbeck granules
Membrane-bound rod- or tennis racket-shaped structures with a central linear density, found in the cytoplasm of Langerhans cells. Their formation is induced by langerin.
Skin-resident DCs as immune sentinels
Skin DCs can be classified according to their localization in distinct anatomical compartments of the skin: Langerhans cells are the main DC subset in the epidermis, where they constitutively reside in the suprabasal layers and are regularly spaced among keratinocytes, whereas dermal DCs reside in the dermis immediately below the dermal–epidermal junction and are dispersed throughout the whole dermal compartment (FIGS 1,3 and TABLE 1). In addition to their different anatomical locations, different DC types in the skin might have specific functional properties, such as secretion of pro-inflammatory mediators (inflammatory DCs), production of type I IFNs (plasmacytoid DCs (pDCs)) or cross-presentation (CD103 (also known as integrin αe)+ skin DCs). Progress in the field of skin DC research gained momentum when a subset of langerin (also known as CLEC4K and CD207)+ DCs were described in mouse dermis that are clearly distinct from langerin+ Langerhans cells that reside in the epidermis46–49. A distinguishing feature of Langerhans cells is the expression of epithelial cell adhesion molecule (EPCAM), which is not expressed by langerin+ dermal DCs49. A comprehensive review on Langerhans cells has been recently published50, and therefore we only briefly discuss the role of Langerhans cells as immune sentinels.
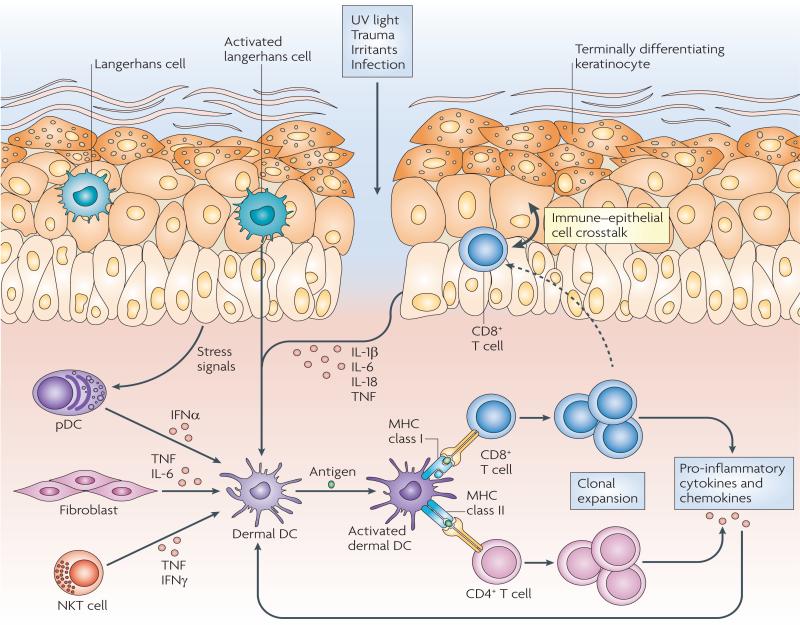
Figure 3. Skin-resident immune sentinels.
Ultraviolet (UV) light, trauma, irritants or infection (essentially any type of barrier disruption) triggers a coordinated immune response to maintain skin homeostasis. Skin-resident immune cells are key sentinels for restoring homeostasis but can also be effector cells during tissue pathology. Epidermal Langerhans cells are key immunological sentinels. Keratinocytes sense and react to noxious stimuli by producing pro-inflammatory cytokines (such as interleukin-1β (IL-1β), IL-6, IL-18 and tumour necrosis factor (TNF)), which in turn activate dermal dendritic cells (DCs) in the presence or absence of antigen encounter. Innate immune cells, such as plasmacytoid DCs (pDCs), activated by stress signals derived from keratinocytes, can also contribute to dermal DC activation by releasing interferon-α (IFNα). Fibroblasts can produce TNF and IL-6 and natural killer T (NKT) cells can produce TNF and IFNγ, thereby contributing to the local inflammatory response. Dermal DCs activate and promote the clonal expansion of skin-resident memory CD4+ or CD8+ T cells. T cell-derived pro-inflammatory cytokines and chemokines in turn can further stimulate epithelial and mesenchymal cells, including keratinocytes and fibroblasts, thus amplifying the inflammatory reaction. Moreover, skin-resident T cells can migrate into the epidermis, engaging in an immune–epithelial cell crosstalk.
Table 1.
Skin dendritic cells and macrophages
| Cell types | Location | Main surface markers* | Sentinel role |
|---|---|---|---|
| Langerhans cells | Epidermis | CD1a, CD207 (langerin) and MHC class II | Antigen-presenting role during certain infections and possibly tolerance induction |
| Inflammatory dendritic epidermal cells | Inflamed epidermis | CD11b, CD23 (FcεRII), CD206 (MMR), FcεRI, IgE and MHC class II | Responds to antibody–allergen complexes in atopic dermatitis |
| Dermal DCs | Dermis | CD1alow, CD1c (BDCA1), CD206 (MMR), CD209 (DC-SIGN) and MHC class II | Antigen presentation and cytokine and chemokine secretion |
| Inflammatory DCs (also known as TIP DCs) | Inflamed dermis | CD11c | TNF and nitric oxide production |
| Macrophages | Dermis | CD163, factor XIIIa, CD16, CD32 and CD64 | Antimicrobial activity and production of pro-and anti-inflammatory mediators |
| Plasmacytoid DCs | Dermis | CD45RA, CD123, CD303 (BDCA2) and MHC class II | IFNα production, functional role in psoriasis and recognition of self-DNA–LL37 complexes |
Alternative protein names are given in brackets. DC, dendritic cell; FcεR, Fc receptor for IgE; IFNα, interferon-α; TIP, TNF- and iNOS-producing; TNF, tumour necrosis factor.
DCs in the epidermis
Langerhans cells are characterized by a special type of intracytoplasmic organelle known as the Birbeck granule. Traditionally Langerhans cells have been distinguished from other cells by the expression of langerin in mice and CD1a in humans51. Although they have long been recognized as the prototypic APC in the skin, surprisingly little is known about their function in vivo.
Langerhans cells are among the first DCs to come into contact with microbial antigens. In vitro studies have shown that Langerhans cells take up and process lipid antigens and microbial fragments for presentation to effector T cells52. In addition, human Langerhans cells have been shown to preferentially induce the differentiation of TH2 cells and can prime and cross-prime naive CD8+ T cells53. Although initial evidence pointed to a role for Langerhans cells in cross-presentation in vivo54, recent data suggest that important cross-presenting APCs in the skin are langerin+CD103+ DCs, most likely of dermal origin55,56.
Given the role of Langerhans cells as the outermost sentinel of the skin immune system, immunoderma tologists have long focused on a potential role for Langerhans cells in the induction of contact hypersensitivity reactions57. However, the observation that the removal of Langerhans cells by topical application of steroids resulted in an enhancement rather than a reduction of contact hypersensitivity raised the possibility that Langerhans cells were potential inhibitors of contact hyper sensitivity58. More recent data using Langerhans cell-deficient mice indicate that Langerhans cells are indeed dispensable for the induction of certain types of cell-mediated immune responses and may actually dampen inflammation and generate tolerogenic responses59. These observations depend on the Langerhans cell ablation model that was used (a detailed discussion can be found elsewhere50,59). In the context of infection, in vivo studies showed that for cytolytic viruses, such as herpes viruses, langerin+CD103+ DCs that are not Langerhans cells and that are probably of dermal origin56 may be the key APC population involved in mounting an immune response60,61.
An additional epidermal DC subset known as inflammatory dendritic epidermal cells (IDECs), which can be distinguished from Langerhans cells by the expression of the macrophage mannose receptor CD206, is found in the inflamed epidermis of patients with atopic dermatitis62. IDECs overexpress high-affinity Fc receptor for Ige (FcεRI), which facilitates their reactivity to IgE-bound allergens, resulting in a pro-inflammatory allergen-specific response63. Although IDECs are found in the epidermis, it has been suggested that they are a population of inflammatory DCs that can populate both the epidermis and the dermis64.
Taken together, these recent insights into the role of epidermal DCs have revealed an unexpected role for Langerhans cells in the induction of tolerance. These findings will require confirmation in further studies, especially for human Langerhans cells, and raise the question of the true role of Langerhans cells in tissue homeostasis and disease.
Contact hypersensitivity
The inflammatory reaction that occurs after the first exposure to a ‘sensitizer’ hapten or antigen. This step requires dendritic cell migration to lymph nodes to prime contact-antigen-specific T cells.
Langerhans cell-deficient mice
Two main Langerhans cell-deficient mouse models have been developed using diphtheria toxin ablation. One model, in which diphtheria toxin receptor is constitutively expressed under the control of the human langerin promoter, shows selective depletion of langerin+ epidermal Langerhans cells. The other model, which uses the mouse langerin promoter, displays conditional depletion of all langerin+ DCs, including Langerhans cells in the epidermis, langerin+ DCs in the dermis and langerin+ DCs in lymph nodes.
DCs and macrophages in the dermis
Dermal DCs migrate rapidly to lymph nodes and colonize micro-anatomical areas in the paracortex of lymph nodes65. Recent data from a model of allergic contact dermatitis showed that dermal DCs and not Langerhans cells isolated from draining lymph nodes induced T cell proliferation66. On the basis of the discovery of dermal langerin+ DCs some data on the role of Langerhans cells during tissue homeostasis and pathology needed to be revisited50. It now seems that in mice the main sub-type of migratory DCs that present antigens from lytic viruses, such as herpes simplex virus (HSV), to CD8+ T cells is dermal langerin+CD103+ DCs56. This cell subset has similarities with the mouse CD8+ cross-presenting DCs found in lymphoid organs but is different from langerin+CD103– Langerhans cells and langerin– dermal DCs. An additional source of dermal DCs is a population of monocytes that patrol the skin mainly during inflammatory conditions67,68. An important role for newly formed monocyte-derived DCs in mouse dermis during infection has been recently described68.
In humans, several research groups have recently attempted to identify cell surface molecules that can be used to distinguish various DC subsets that reside in the dermis under normal conditions69–71. CD1c (also known as BDCA1) is a useful cell surface marker for dermis-based myeloid DCs69,72,73. Future studies will show whether the strongly stimulatory CD1a+ subset of dermal CD1c+ DCs in humans corresponds to the CD103+ cross-presenting DC subset that has been described in mouse skin56,72,74. Dermal DCs can exist in an immature state with cytoplasmic ruffles and express pathogen recognition receptors such as TLR2, TLR4, CD206 and DC-SIGN (also known as CD209)75. More mature dermal DCs have cytoplasmic veils and express higher levels of co-stimulatory molecules, such as CD83, and lower levels of pattern recognition receptors. Dermal DCs also express low-density lipoprotein-related protein 1 (LRP1; also known as CD91), which functions as a possible heat shock protein recognition receptor76.
Activated dermal DCs participate in the inflammatory response64 by secreting cytokines and chemokines to generate a cytokine network. In some instances the cytokine network is beneficial and contributes to the eradication of infectious agents but in other settings it underlies a pathological tissue response with persistent inflammation. Dermal DCs that produce both TNF and inducible nitric oxide synthase (iNOS) are known as IDCs or TIP (TNF- and iNOS-producing) DCs77,78. This type of DC has been proposed to have a major role in psoriasis78.
pDCs are rare in healthy skin but have been implicated in the pathogenesis of systemic lupus erythematosus and psoriasis79,80. It has been suggested that in psoriasis early activation of pDCs triggers an innate immune response, followed by the activation of myeloid DCs and adaptive immune responses81. Indeed, the cathelicidin LL37 bound to self-DNA fragments released by stressed or dying cells in the skin was shown to trigger TLR9 activation in pDCs, resulting in IFNα production and activation of an adaptive immune response21. This study raises the possibility that high levels of cathelicidins in psoriatic skin can break tolerance to self DNA, leading to a sustained activation of pDCs and type I IFN production.
Skin macrophages are predominantly sessile dermal cells but they can migrate to lymph nodes under inflammatory conditions82. A recent study of the phenotypic profile of dermal DCs and macrophages in normal human skin identified a population of macrophage-like cells that expressed CD163, a scavenger receptor that is expressed by most tissue macrophages, and factor XIIIa69, a component of the coagulation cascade with a potential function in wound healing. Insights into potential precursors of tissue-resident macrophages and DCs have been provided by mouse studies that traced the development of these cell types from circulating monocytes during infection. A subset of mouse CD115+LY6C+LY6G+CCR2+ inflammatory monocytes differentiates into inflammatory DCs, whereas patrolling LY6C–LY6G–CX3CR1+ monocytes differentiate into alternatively activated macrophages67. It has been proposed that the human counterparts of these inflammatory monocytes are CD14+CD16– circulating monocytes67.
There is a diverse range of DCs and macrophages in the dermis that enable highly differential immunological responses. The most recently discovered are dermal langerin+CD103+ cross-presenting DCs in mouse skin, which raises important questions about their wider functional role and their human counterpart.
The functional role of skin T cells
Normal healthy skin contains more than 2 × 1010 skin-resident T cells, which is more than twice the total number of T cells in the blood83. Although the presence of lymphocytes in normal healthy epidermis has been suspected since 1949 (ref. 84), these cells received little attention for almost 40 years. In the late 1980s it was shown that normal epidermis harbours a phenotypically heterogeneous population of T cells, most of which are memory CD8+ αβ T cells85,86. Epidermal T cells are mainly distributed in the basal and suprabasal keratinocyte layer, often in close proximity with Langerhans cells86. In the dermis, T cells are preferentially clustered around postcapillary venules and are often situated just beneath the dermal–epidermal junction or adjacent to cutaneous appendages. CD4+ and CD8+ T cells are present in equal numbers and most are memory T cells that express cutaneous lymphocyte-associated antigen (CLA)87. Skin-specific memory T cells gain skin-homing properties after a process known as imprinting, which involves contact with tissue-derived DCs and possibly mesenchymal cells88,89. Vitamin D has been suggested to have a key role in the guidance of memory T cells to the skin through the upregulation of CCR10 expression90.
Conventional T cells in the skin
The three main types of CD4+ TH cells — that is, TH1, TH2 and TH17 cells — have been found in the skin during various inflammatory diseases. For example, during infection of the skin with intracellular organisms TH1 cells producing IFNγ and lymphotoxin are present and activate macrophages to kill intracellular organisms. Traditionally, TH1 cell responses were associated with autoimmunity and immune-mediated pathologies, such as psoriasis, whereas TH2 cell responses were linked to allergic diseases, such as asthma and atopic dermatitis. More recently, however, TH17 cells have been shown to have a potential role in both psoriasis91 and atopic dermatitis92, and recent reports have shown that TH17 cells are essential for the first-line defence against various fungal and bacterial infections17. In fact, a severely impaired IL-17-mediated immune response due to genetic defects underlies autosomal-dominant hyper-IgE syndrome and autosomal-recessive susceptibility to mycobacterial disease93,94 and could also be responsible for chronic mucocutaneous candidiasis95. Interestingly, these diseases are characterized by recurrent and persistent infections of the skin and mucosal membranes. As already mentioned, a putative mechanism for host defence against microorganisms involving IL-17 and IL-22 in the skin is the upregulation of AMP production by keratinocytes18. Therefore, TH17 cell-derived cytokines may be seen as a link between immune and epithelial cells that optimizes the host immune response to skin-tropic pathogens. A subset of circulating T cells with skin-homing potential that produce IL-22 but not IL-17 or IFNγ (termed TH22 cells) has been recently identified96,97. pDCs were efficient in generating TH22 cells in a TNF- and IL-6-dependent manner96. TH22 cells were also identified in skin cell cultures from patients with atopic dermatitis98. It will be interesting to further explore the functional role of these cells in conditions of skin pathology and homeostasis.
Alternatively activated macrophage
A macrophage stimulated by interleukin-4 (IL-4) or IL-13 that expresses arginase 1, mannose receptor CD206 and IL-4 receptor-α. There may be pathogen-associated molecular patterns expressed by helminths that can also drive alternative activation of macrophages.
Skin-resident T cells as immune sentinels
Initial views of skin immunosurveillance emphasized the importance of immune cells that circulate between skin-draining lymph nodes and peripheral tissue and that can react quickly to antigen challenges1,99,100. Recently, however, it has been proposed that skin-resident T cells rather than recruited T cells have a major role in skin immune homeostasis and pathology81 (FIG. 3). Skin-resident memory T cells are strategically positioned as the first-line defence against secondary antigen challenge and are therefore thought to be important effector cells of tissue pathology.
To assign a role to skin-resident versus circulating T cells in skin pathology, one should consider not only that normal skin contains more than twice as many T cells as the blood but also that 98% of CLA+ skin-homing lymphocytes in the body reside in the skin under physiological conditions83. Studies of skin inflammation have provided evidence supporting a key role for skin-resident memory T cells101,102. For example, typical psoriasis lesions developed spontaneously in healthy samples of skin from patients with psoriasis that were grafted onto immunodeficient mice known as AGR mice, which are deficient for type I and type II IFN receptors and recombination activating gene 2 (Rag2)101. The development of psoriasis lesions in these immuno-deficient host mice suggests that the skin-resident memory T cells that are present in the graft are sufficient for disease development. In a subsequent study, investigation of the kinetics of T cell expansion showed that an increase in the number of dermal T cells preceded the development of typical psoriatic changes in the epidermis102. Progression towards psoriasiform changes took place only when T cells entered the epidermal compartment, implying that T cell activation and expansion preceded epithelium pathology and that crosstalk between intraepithelial T cells and keratinocytes was required for the development of typical skin pathology. Entrance of T cells into the epidermis required the interaction between very late antigen 1 (VLA1; also known as α1β1 integrin) expressed by T cells and collagen IV on the basement membrane, thus providing a key checkpoint for in situ migration of tissue-resident T cells.
In addition to the above mentioned human xenotrans-plantation model, convincing evidence supporting and extending the importance of skin-resident memory T cells in mouse skin comes from recent studies focusing on models of HSV infection. Resident memory T cells together with CD4+ T cells were shown to be activated by skin DCs, resulting in the local proliferation of antigen-specific memory CD8+ T cells103. In a further study tissue-resident memory T cells were shown to express CD103 and VLA1, to undergo homeostatic proliferation and to provide protection from pathogen challenge104.
Comparable observations in lung infection models led to the proposal that pathogen-specific recall immune responses involve three phases: a first phase dominated by tissue-resident memory T cells, a second phase that involves nonspecific recruitment of circulating memory T cells and a third phase that follows the migration of antigen-specific DCs to draining lymph nodes 24–72 hours after infection and involves the homing to the skin of pathogen-specific effector T cells previously expanded in draining lymph nodes105.
Taken together these studies raise important questions about the functional role for tissue-resident memory T cells in controlling localized infection and the role of these cells in inflammatory tissue pathology. A focus on tissue-resident rather than circulating T cells would have major implications for the development of new therapeutics targeted at epithelial inflammatory disorders.
Unconventional T cells in the skin
γδ T cells106 and NKT cells107 constitute the majority of unconventional or innate-like T cells (FIG. 4). In the skin, human γδ T cells make up a small proportion of the total T cells in the dermis (2–9%) and the epidermis (1–10%); however, in mice, the Vγ5+ dendritic epidermal T cells (DETCs) constitute more than 90% of epidermal T cells108. DETCs are thought to recognize a specific antigen that is restricted to the epidermis (and thymus) and to downregulate αβ T cell-mediated inflammation, thus contributing to local immune surveillance and immunoregulation109,110. In particular, skin-resident γδ T cells that express NKG2D have been linked to the regulation of skin cancer. Engagement of NKG2D by one of its several identified ligands, including MHC class I polypeptide-related sequence A (MICA) and MICB in humans and RAE1α in mice, provides a co-stimulatory function and results in target cell destruction111. These ligands are upregulated during cell stress and are expressed by various tumour cells, including melanoma cells112. Accordingly, mouse γδ T cells have been shown to negatively regulate inflammation as well as carcinogenesis112,113, whereas αβ T cells promoted skin tumour responses114.
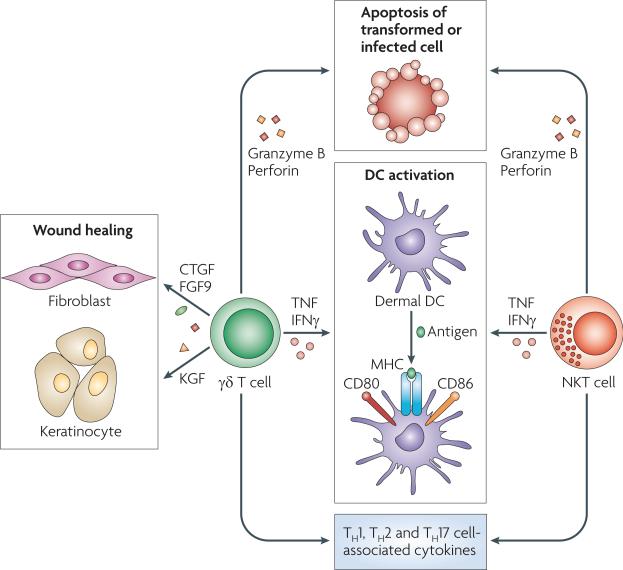
Figure 4. Unconventional T cells in the skin.
Unconventional T cells, such as γδ T cells and natural killer T (NKT) cells, are involved in skin immunosurveillance. Both γδ T cells and NKT cells are cytolytic and release granzyme B and perforin and cause apoptosis of transformed or infected cells. They activate dermal dendritic cells (DCs) by producing tumour necrosis factor (TNF) and interferon-γ (IFNγ). Moreover, γδ T cells produce growth factors that are essential for wound healing, such as connective tissue growth factor (CTGF), fibroblast growth factor 9 (FGF9; also known as GAF) and keratinocyte growth factor (KGF). Finally, both γδ T cells and NKT cells produce cytokines that are usually associated with T helper 1 (TH1), TH2 and TH17 cells.
Less is known about the role of γδ T cells in human skin. They have been shown to be increased in numbers in the skin of patients affected by melanoma, Langerhans cell histiocytosis, skin leishmaniasis, leprosy, psoriasis and chronic cutaneous lupus erythematosus, suggesting their involvement in a wide range of human skin pathologies. It has been proposed that highly restricted intraepithelial Vδ1 T cells act as immune sentinels by responding to stressed epithelial cells, thus controlling epithelial cell integrity115. Moreover, human γδ T cells have been shown to produce growth factors that are important in wound healing, such as connective tissue growth factor (CTGF), fibroblast growth factor 9 (FGF9; also known as GAF), keratinocyte growth factor (KGF) and insulin-like growth factor 1 (IGF1)116. Finally, they could contribute to antimicrobial defence by producing certain AMPs, such as cathelicidins117. Further studies are required to better elucidate the function of γδ T cells in human skin and their involvement in the immunopathogenesis of skin diseases.
A protective role for invariant NKT cells (iNKT cells) in the skin has been suggested, owing to their ability to recognize bacterial glycolipids and hence serve as antimicrobial immune sentinels107,118. iNKT cells, which were identified in psoriatic plaques, might also have a role in tissue pathology as a result of dysregulated immune responses119. Although keratinocytes in normal human skin express low levels of CD1d, there is significant induction of expression in psoriatic plaques in which CD1d+ keratinocytes are juxtaposed to the glycolipid-rich stratum corneum120. This suggests that self-derived glycolipids presented by CD1d-restricted NKT cells might contribute to keratinocyte activation. Similarly, CD1d expression by iNKT cells is higher in the elicitation sites of allergic contact dermatitis than in normal skin, and iNKT cells have been shown to be, at least in part, the source of the IFNγ and IL-4 that are present in allergic contact dermatitis121, indicating their contribution to disease onset. Finally, it has been shown that α-galactosylceramide-activated iNKT cells can modulate DC trafficking from the skin to draining lymph nodes by inducing high systemic levels of TNF in a contact hypersensitivity mouse model122.
Progress in our understanding of unconventional T cells such as γδ T cells and iNKT cells has revealed a key role for such cells in the regulation of both skin inflammation and skin carcinogenesis, raising the question of whether skin-specific ligands and stimuli regulate these cell populations.
Invariant NKT cell
A cell type thought to be particularly important in bridging innate and adaptive immunity. iNKT cells are typified by a capacity for self-recognition and rapid release of cytokines such as interferon-γ.
Dysregulated skin immune responses
Numerous skin disorders, such as psoriasis, atopic dermatitis and contact dermatitis are associated with dysregulation of immune responses in the skin. Key insights into the roles of immune sentinels during a skin immune response have been provided by contact dermatitis models. These include the sentinel role of DCs, the effector role of antigen-specific T cells, the pro-inflammatory role of keratinocytes and the important role of the innate immune system123–126.
Here we focus on the immunopathogenesis of psoriasis as a model for inflammatory pathology of the skin. Some of the most convincing human skin immunology data are based on psoriasis studies127. These studies are of relevance not only to other inflammatory skin disorders but also to many common immune-mediated inflammatory disorders, such as rheumatoid arthritis or Crohn's disease owing to shared genetic variants, immunological pathways and therapeutic targets with psoriasis.
Psoriasis is a lifelong inflammatory skin disease characterized by sharply demarcated, scaly, erythematous plaques. Effector cells of the innate immune system, such as keratinocytes, pDCs and iNKT cells, and innate inflammatory mediators, such as LL37 and type I IFNs, have been shown to have a role in the pathogenesis of psoriasis21,80. In addition, a large body of clinical128 and experimental101,129 evidence supports the idea that T cells have an essential role in psoriasis. Consistent with other autoimmune-type diseases, psoriasis has been traditionally considered a TH1-type disease130. Some reports have also suggested the presence of activated autoreactive T cells in psoriasis131.
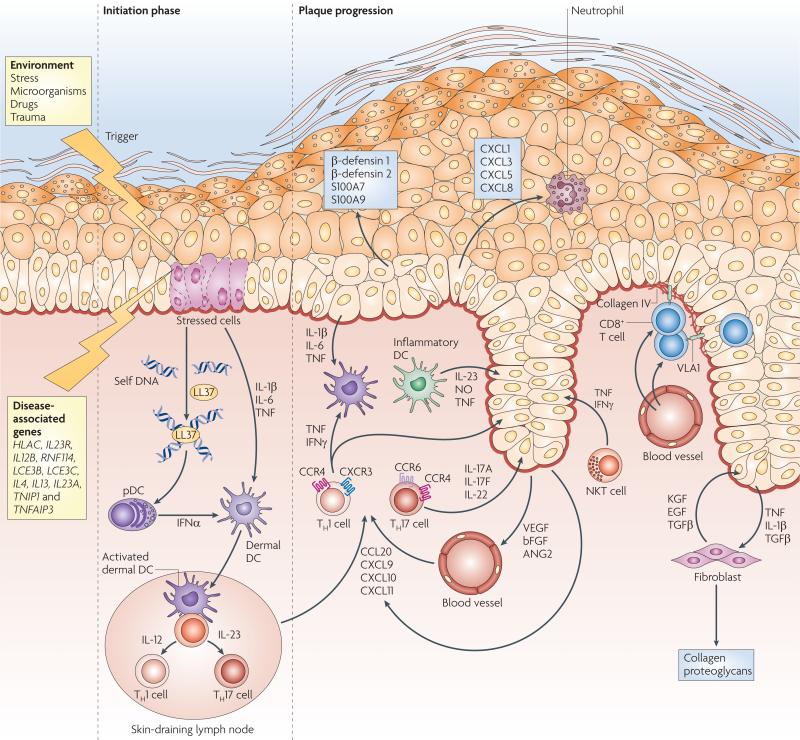
The current view of psoriasis pathogenesis proposes that a combination of environmental and genetic factors confers susceptibility to the disease and that a dysregulated immune response leads to a series of linked cellular changes in the skin127,132. An important genetic component of psoriasis susceptibility is the PSORS1 locus, which is located in the MHC region on chromosome 6p21 (ref. 133). In addition, genome-wide association studies have implicated a further ten psoriasis-associated gene variants127. These psoriasis-associated genes function in various biological pathways, including epidermal cell differentiation134, NF-κB signalling135 and TH2-type responses135. Interestingly, variants in the TH17 cell-associated genes IL23A, IL12B and IL23R135–137 have been associated with psoriasis susceptibility, supporting experimental and clinical data linking TH17 cells to psoriasis pathogenesis91,138. Current models of immuno-pathogenesis (FIG. 5) focus on a scenario in which pDCs are activated by LL37–self DNA complexes and TH1 and TH17 cells interact with skin-resident dermal DCs, contributing to a psoriatic phenotype. In the dermis, IL-12 and IL-23 secreted by dermal DCs can induce the activation of T cells that release pro-inflammatory TH1- and TH17-type cytokines. In the epidermis, VLA1+CD8+ T cells also contribute to disease pathogenesis, for example by producing IFNγ102. The resulting cytokine milieu of IFNγ, TNF, IL-17A and IL-22 affects keratinocytes, increasing their proliferation and stimulating the production of pro-inflammatory mediators and AMPs, which in turn sustains and amplifies the chronic inflammatory disease process.
Figure 5. Psoriasis immunopathogenesis.
Environmental factors trigger psoriasis in genetically predisposed individuals carrying susceptibility alleles of disease-associated genes. In the initiation phase, stressed keratinocytes release self DNA that forms complexes with the cathelicidin antimicrobial peptide LL37, which in turn activates plasmacytoid dendritic cells (pDCs) to produce interferon-α (IFNα). Keratinocyte-derived interleukin-1β (IL-1β), IL-6 and tumour necrosis factor (TNF) and pDC-derived IFNα activate dermal DCs. Activated dermal DCs then migrate to the skin-draining lymph nodes to present an as yet unknown antigen (either of self or of microbial origin) to naive T cells and promote their differentiation into T helper 1 (TH1) and/or TH17 cells. TH1 cells (expressing cutaneous leukocyte antigen (CLA; not shown), CXC-chemokine receptor 3 (CXCR3) and CC-chemokine receptor 4 (CCR4)) and TH17 cells (expressing CLA and chemokine receptors CCR4 and CCR6) migrate via lymphatic and blood vessels into psoriatic dermis, attracted by the keratinocyte-derived chemokines CCL20, CXCL9, CXCL10 and CXCL11, which ultimately leads to the formation of a psoriatic plaque. TH17 cells secrete IL-17A, IL-17F and IL-22, which stimulate keratinocyte proliferation and the release of β-defensin 1, β-defensin 2, S100A7 and S100A9 and the neutrophil-recruiting chemokines CXCL1, CXCL3, CXCL5 and CXCL8. Moreover, inflammatory DCs produce IL-23, nitric oxide (NO) radicals and TNF. At the dermo–epidermal junction, memory CD8+ cells expressing very-late antigen-1 (VLA1) bind to collagen IV, allowing entry into the epidermis and contributing to disease pathogenesis. Cross-talk between keratinocytes, producing TNF, IL-1β and transforming growth factor-β (TGFβ), and fibroblasts, which in turn release keratinocyte growth factor (KGF), epidermal growth factor (EGF) and TGFβ, contribute to tissue reorganization and deposition of extracellular matrix (for example, collagen and proteoglycans). Figure is modified, with permission, from REF. 127 © (2009) Massachusetts Medical Society.
Conclusions and future directions
Studies of skin immune sentinels have made considerable progress since the early days when the skin was considered purely a passive protective shield. Studies of epidermal cells have unveiled unexpected immuno logical sentinel functions of keratinocytes. Essential roles for skin DCs in cross-presentation of pathogen-derived antigens and a new role for tissue-resident T cells in maintaining skin homeostasis are gaining support. Progress in understanding the role of immune sentinels will depend on our ability to answer key questions such as how to transfer insights from mouse model systems into translational research studies focusing on human pathology and ultimately how to develop new therapeutics targeting human skin immune sentinels.
Acknowledgements
We apologize to all the authors whose work could not be discussed and cited owing to space limitations. We thank R. Trembath, A. Hayday, J. Barker and F. Geissmann for discussions. We acknowledge support by the following grant funding bodies: Wellcome Trust Programme GR078173MA, National Institute of Health RO1AR040065, National Insitute for Health Research Comprehensive Biomedical Research Centre at Guy's and St. Thomas’ Hospital and King's College London, Medical Research Council UK Programme G0601387, and Dunhill Medical Trust.
Footnotes
DATABASES
UniProtKB: http://www.uniprot.org
ASC | caspase 1 | caspase 8 | CCL20 | CCR2 | CCR10 | CD1a | CD1c | CD1d | CD14 | CD16 | CD83 | CD103 | CD115 | CD163 | CD206 | CTGF | CX3CR1 | CXCL1 | CXCL8 | CXCL9 | CXCL10 | CXCL11 | CYP27B1 | DC-SIGN | EPCAM | factor XIIIa | FGF9 | IFNγ | IGF1 | IL-1α | IL-1β | IL-1F6 | IL-4 | IL-6 | IL-10 | IL-17A | IL-18 | IL-22 | iNOS | JUN | JUNB | KGF | langerin | LL37 | LRP1 | LY6C | LY6G | MICA | MICB | NKG2D | NLRP3 | RAE1α | TLR1 | TLR2 | TLR3 | TLR4 | TLR5 | TLR6 | TLR7 | TLR9 | TNF
FURTHER INFORMATION
Frank O. Nestle's homepage: http://www.kcl.ac.uk/schools/medicine/depts/dermatology/research/nestle
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Streilein JW. Skin-associated lymphoid tissues (SALT): origins and functions. J. Invest. Dermatol. 1983;80:12S–16S. doi: 10.1111/1523-1747.ep12536743. [This is a milestone paper introducing the concept of SALT for the first time and describing the skin in conjunction with draining lymph nodes as an immune-competent organ.] [DOI] [PubMed] [Google Scholar]
- 2.Bos JD, Kapsenberg ML. The skin immune system (SIS): its cellular constituents and their interactions. Immunol. Today. 1986;7:235–240. doi: 10.1016/0167-5699(86)90111-8. [DOI] [PubMed] [Google Scholar]
- 3.Stingl G, Bergstresser PR. Dendritic cells: a major story unfolds. Immunol. Today. 1995;16:330–333. doi: 10.1016/0167-5699(95)80148-0. [DOI] [PubMed] [Google Scholar]
- 4.Nickoloff BJ, editor. Dermal Immune System. CRC; Boca Raton: 1993. [Google Scholar]
- 5.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp. Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 6.Krueger GG, Stingl G. Immunology/inflammation of the skin — a 50-year perspective. J. Invest. Dermatol. 1989;92:32S–51S. doi: 10.1111/1523-1747.ep13074960. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Lebre MC, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Invest. Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 9.Kalali BN, et al. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J. Immunol. 2008;181:2694–2704. doi: 10.4049/jimmunol.181.4.2694. [DOI] [PubMed] [Google Scholar]
- 10.Miller LS, Modlin RL. Human keratinocyte Toll-like receptors promote distinct immune responses. J. Invest. Dermatol. 2007;127:262–263. doi: 10.1038/sj.jid.5700559. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 12.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Curr. Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 13.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [References 12 and 13 are key papers showing that the inflammasome machinery is responsible for UV-induced secretion of IL-1β by human keratinocytes.] [DOI] [PubMed] [Google Scholar]
- 14.Watanabe H, et al. Activation of the IL-1β-processing inflammasome is involved in contact hypersensitivity. J. Invest. Dermatol. 2007;127:1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 15.Gilliet M, Lande R. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr. Opin. Immunol. 2008;20:401–407. doi: 10.1016/j.coi.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 18.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolls JK, McCray PB, Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nature Rev. Immunol. 2008;8:829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 21.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [A landmark paper showing that LL37 can convert inert self DNA into a potent pro-inflammatory trigger for pDC IFNα production through TLR9-dependent mechanisms, suggesting that this pathway might drive autoimmunity in the context of skin inflammation but also other autoimmune-type disorders.] [DOI] [PubMed] [Google Scholar]
- 22.Schauber J, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peric M, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J. Immunol. 2008;181:8504–8512. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albanesi C, Scarponi C, Giustizieri ML, Girolomoni G. Keratinocytes in inflammatory skin diseases. Curr. Drug Targets Inflamm. Allergy. 2005;4:329–334. doi: 10.2174/1568010054022033. [DOI] [PubMed] [Google Scholar]
- 25.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee P, et al. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–523. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groves RW, Mizutani H, Kieffer JD, Kupper TS. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1α in basal epidermis. Proc. Natl Acad. Sci. USA. 1995;92:11874–11878. doi: 10.1073/pnas.92.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumberg H, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 30.Dieu-Nosjean MC, et al. Macrophage inflammatory protein 3α is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 2000;192:705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickoloff BJ, Turka LA. Immunological functions of non-professional antigen-presenting cells: new insights from studies of T-cell interactions with keratinocytes. Immunol. Today. 1994;15:464–469. doi: 10.1016/0167-5699(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 32.Gaspari AA, Katz SI. Induction of in vivo hyporesponsiveness to contact allergens by hapten-modified Ia+ keratinocytes. J. Immunol. 1991;147:4155–4161. [PubMed] [Google Scholar]
- 33.Nickoloff BJ, et al. Accessory cell function of keratinocytes for superantigens. Dependence on lymphocyte function-associated antigen-1/intercellular adhesion molecule-1 interaction. J. Immunol. 1993;150:2148–2159. [PubMed] [Google Scholar]
- 34.Black AP, et al. Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells. Eur. J. Immunol. 2007;37:1485–1493. doi: 10.1002/eji.200636915. [DOI] [PubMed] [Google Scholar]
- 35.Griffiths CE, Nickoloff BJ. Keratinocyte intercellular adhesion molecule-1 (ICAM-1) expression precedes dermal T lymphocytic infiltration in allergic contact dermatitis (Rhus dermatitis). Am. J. Pathol. 1989;135:1045–1053. [PMC free article] [PubMed] [Google Scholar]
- 36.Kupper TS. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J. Invest. Dermatol. 1990;94:146S–150S. doi: 10.1111/1523-1747.ep12876130. [DOI] [PubMed] [Google Scholar]
- 37.Luger TA, Schwarz T. Evidence for an epidermal cytokine network. J. Invest. Dermatol. 1990;95:100S–104S. doi: 10.1111/1523-1747.ep12874944. [DOI] [PubMed] [Google Scholar]
- 38.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 39.Mehling A, et al. Overexpression of CD40 ligand in murine epidermis results in chronic skin inflammation and systemic autoimmunity. J. Exp. Med. 2001;194:615–628. doi: 10.1084/jem.194.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zenz R, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 41.Pasparakis M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 42.Eckmann L, et al. Opposing functions of IKKβ during acute and chronic intestinal inflammation. Proc. Natl Acad. Sci. USA. 2008;105:15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sano S, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nature Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- 44.Oppenheim DE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nature Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 45.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nature Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [This paper provides insights into the early phases of tissue immunosurveillance, establishing that upregulation of the NKG2D ligand RAE1α can promote considerable reorganization of the skin immune compartment.] [DOI] [PubMed] [Google Scholar]
- 46.Bursch LS, et al. Identification of a novel population of Langerin dendritic cells. J. Exp. Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ginhoux F, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poulin LF, et al. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [References 46–48 describe a newly discovered population of skin DCs: langerin+ non-Langerhans cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagao K, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl Acad. Sci. USA. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nature Rev. Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 51.Romani N, et al. Epidermal Langerhans cells — changing views on their function in vivo. Immunol. Lett. 2006;106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Hunger RE, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J. Clin. Invest. 2004;113:701–708. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoitzner P, et al. Langerhans cells cross-present antigen derived from skin. Proc. Natl Acad. Sci. USA. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waithman J, et al. Skin-derived dendritic cells can mediate deletional tolerance of class I-restricted self-reactive T cells. J. Immunol. 2007;179:4535–4541. doi: 10.4049/jimmunol.179.7.4535. [DOI] [PubMed] [Google Scholar]
- 56.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nature Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [This paper establishes that CD103+ DCs are the key cross-presenting APCs in the skin.] [DOI] [PubMed] [Google Scholar]
- 57.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [This key paper describes the concept of immature DCs (specialized for antigen processing) and mature DCs (specialized for antigen presentation) for the first time, using the example of Langerhans cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grabbe S, Steinbrink K, Steinert M, Luger TA, Schwarz T. Removal of the majority of epidermal Langerhans cells by topical or systemic steroid application enhances the effector phase of murine contact hypersensitivity. J. Immunol. 1995;155:4207–4217. [PubMed] [Google Scholar]
- 59.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur. J. Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 60.Allan RS, et al. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 61.Zhao X, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wollenberg A, et al. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J. Invest. Dermatol. 2002;118:327–334. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 63.Bieber T. The pro- and anti-inflammatory properties of human antigen-presenting cells expressing the high affinity receptor for IgE (FcεRI). Immunobiology. 2007;212:499–503. doi: 10.1016/j.imbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Guttman-Yassky E, et al. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J. Allergy Clin. Immunol. 2007;119:1210–1217. doi: 10.1016/j.jaci.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Fukunaga A, Khaskhely NM, Sreevidya CS, Byrne SN, Ullrich SE. Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J. Immunol. 2008;180:3057–3064. doi: 10.4049/jimmunol.180.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 68.Lopez-Bravo M, Ardavin C. In vivo induction of immune responses to pathogens by conventional dendritic cells. Immunity. 2008;29:343–351. doi: 10.1016/j.immuni.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+ BDCA-1+ dendritic cells and CD163+ FXIIIA+ macrophages. J. Clin. Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ochoa MT, Loncaric A, Krutzik SR, Becker TC, Modlin RL. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209 macrophages. J. Invest. Dermatol. 2008;128:2225–2231. doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nestle FO, Nickoloff BJ. Deepening our understanding of immune sentinels in the skin. J. Clin. Invest. 2007;117:2382–2385. doi: 10.1172/JCI33349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J. Immunol. 1993;151:6535–6545. [PubMed] [Google Scholar]
- 73.Lenz A, Heine M, Schuler G, Romani N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J. Clin. Invest. 1993;92:2587–2596. doi: 10.1172/JCI116873. [References 72 and 73 provided the first functional characterization of human dermal DCs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larregina AT, et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nature Immunol. 2001;2:1151–1158. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 75.Angel CE, et al. CD14+ antigen-presenting cells in human dermis are less mature than their CD1a+ counterparts. Int. Immunol. 2007;19:1271–1279. doi: 10.1093/intimm/dxm096. [DOI] [PubMed] [Google Scholar]
- 76.Boyman O, et al. Activation of dendritic antigen-presenting cells expressing common heat shock protein receptor CD91 during induction of psoriasis. Br. J. Dermatol. 2005;152:1211–1218. doi: 10.1111/j.1365-2133.2005.06701.x. [DOI] [PubMed] [Google Scholar]
- 77.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 78.Lowes MA, et al. Increase in TNF-α and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl Acad. Sci. USA. 2005;102:19057–19062. doi: 10.1073/pnas.0509736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 80.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [References 79 and 80 establish the functional role of pDCs and their production of IFNα in the pathogenesis of immune-mediated diseases such as systemic lupus erythematosus and psoriasis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyman O, Conrad C, Tonel G, Gilliet M, Nestle FO. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol. 2007;28:51–57. doi: 10.1016/j.it.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 82.van Furth R, Nibbering PH, van Dissel JT, Diesselhoff-den Dulk MM. The characterization, origin, and kinetics of skin macrophages during inflammation. J. Invest. Dermatol. 1985;85:398–402. doi: 10.1111/1523-1747.ep12277056. [DOI] [PubMed] [Google Scholar]
- 83.Clark RA, et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [This is an important paper showing that normal skin harbours a large number of skin-homing memory T cells, which are 2.8-fold more abundant than T cells circulating in the blood.] [DOI] [PubMed] [Google Scholar]
- 84.Andrew W, Andrew NV. Lymphocytes in the normal epidermis of the rat and of man. Anat. Rec. 1949;104:217–241. doi: 10.1002/ar.1091040207. [DOI] [PubMed] [Google Scholar]
- 85.Bos JD, et al. The skin immune system (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J. Invest. Dermatol. 1987;88:569–573. doi: 10.1111/1523-1747.ep12470172. [DOI] [PubMed] [Google Scholar]
- 86.Foster CA, et al. Human epidermal T cells predominantly belong to the lineage expressing alpha/beta T cell receptor. J. Exp. Med. 1990;171:997–1013. doi: 10.1084/jem.171.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol. Today. 1993;14:75–78. doi: 10.1016/0167-5699(93)90062-P. [DOI] [PubMed] [Google Scholar]
- 88.Mora JR, et al. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edele F, et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J. Immunol. 2008;181:3745–3749. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 90.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nature Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in immunopathogenesis of psoriasis. J. Invest. Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 92.Di Cesare A, Di Meglio P, Nestle FO. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J. Invest. Dermatol. 2008;128:2569–2571. doi: 10.1038/jid.2008.283. [DOI] [PubMed] [Google Scholar]
- 93.de Beaucoudrey L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [Studying patients with immune deficiencies, these authors describe IL-12Rβ1- and STAT3-dependent signals as key components of TH17 cell differentiation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Milner JD, et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eyerich K, et al. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J. Invest. Dermatol. 2008;128:2640–2645. doi: 10.1038/jid.2008.139. [DOI] [PubMed] [Google Scholar]
- 96.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nature Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 97.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nature Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 98.Nograles KE, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 2009;123:1244–1252. doi: 10.1016/j.jaci.2009.03.041. [References 96–98 describe TH22 cells as a skin-homing TH cell population that secretes IL-22 but not IL-17 or IFNγ.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nature Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 100.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nature Rev. Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boyman O, et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 2004;199:731–736. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Conrad C, et al. α1β1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nature Med. 2007;13:836–842. doi: 10.1038/nm1605. [References 101 and 102 both use a new psoriasis xenotransplantation model to show evidence for a functional role of tissue-resident T cells in the pathogenesis of psoriasis. They also identify the binding of VLA1 on T cells to collagen IV as a key checkpoint for T cell entry into the epidermis during inflammation.] [DOI] [PubMed] [Google Scholar]
- 103.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 104.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [Using an HSV infection model, this paper describes a unique and protective skin-resident memory T cell subset, supporting a role of tissue-resident T cells in the memory response to infection.] [DOI] [PubMed] [Google Scholar]
- 105.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nature Rev. Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 106.Hayday A, Tigelaar R. Immunoregulation in the tissues by γδ T cells. Nature Rev. Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 107.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 108.Bergstresser PR, Sullivan S, Streilein JW, Tigelaar RE. Origin and function of Thy-1+ dendritic epidermal cells in mice. J. Invest. Dermatol. 1985;85:85S–90S. doi: 10.1111/1523-1747.ep12275516. [DOI] [PubMed] [Google Scholar]
- 109.Girardi M. Immunosurveillance and immunoregulation by γδ T cells. J. Invest. Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 110.Boyden LM, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal γδ T cells. Nature Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strid J, Tigelaar RE, Hayday AC. Skin immune surveillance by T cells — a new order? Semin. Immunol. 2009;21:110–120. doi: 10.1016/j.smim.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 112.Girardi M, et al. Regulation of cutaneous malignancy by γδ T cells. Science. 2001;294:605–609. [PubMed] [Google Scholar]
- 113.Girardi M, et al. Resident skin-specific γδ T cells provide local, nonredundant regulation of cutaneous inflammation. J. Exp. Med. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberts SJ, et al. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc. Natl Acad. Sci. USA. 2007;104:6770–6775. doi: 10.1073/pnas.0604982104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Holtmeier W, Kabelitz D. γδ T cells link innate and adaptive immune responses. Chem. Immunol. Allergy. 2005;86:151–183. doi: 10.1159/000086659. [DOI] [PubMed] [Google Scholar]
- 116.Toulon A, et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 2009;206:743–750. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agerberth B, et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 118.Nickoloff BJ. Skin innate immune system in psoriasis: friend or foe? J. Clin. Invest. 1999;104:1161–1164. doi: 10.1172/JCI8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nickoloff BJ, Bonish B, Huang BB, Porcelli SA. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J. Dermatol. Sci. 2000;24:212–225. doi: 10.1016/s0923-1811(00)00120-1. [DOI] [PubMed] [Google Scholar]
- 120.Bonish B, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-γ production by NK-T cells. J. Immunol. 2000;165:4076–4085. doi: 10.4049/jimmunol.165.7.4076. [DOI] [PubMed] [Google Scholar]
- 121.Gober MD, Fishelevich R, Zhao Y, Unutmaz D, Gaspari AA. Human natural killer T cells infiltrate into the skin at elicitation sites of allergic contact dermatitis. J. Invest. Dermatol. 2008;128:1460–1469. doi: 10.1038/sj.jid.5701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gorbachev AV, Fairchild RL. Activated NKT cells increase dendritic cell migration and enhance CD8+ T cell responses in the skin. Eur. J. Immunol. 2006;36:2494–2503. doi: 10.1002/eji.200636075. [DOI] [PubMed] [Google Scholar]
- 123.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol. Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 124.Gober MD, Gaspari AA. Allergic contact dermatitis. Curr. Dir. Autoimmun. 2008;10:1–26. doi: 10.1159/000131410. [DOI] [PubMed] [Google Scholar]
- 125.Lonsdorf AS, Enk AH. Immunologie des allergischen kontaktekzems. Hautarzt. 2009;60:32–41. doi: 10.1007/s00105-008-1642-8. in German. [DOI] [PubMed] [Google Scholar]
- 126.Martin SF, Jakob T. From innate to adaptive immune responses in contact hypersensitivity. Curr. Opin. Allergy Clin. Immunol. 2008;8:289–293. doi: 10.1097/ACI.0b013e3283088cf9. [DOI] [PubMed] [Google Scholar]
- 127.Nestle FO, Kaplan DH, Barker J. Psoriasis. N. Engl. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 128.Ellis CN, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110–3116. [PubMed] [Google Scholar]
- 129.Prinz JC, et al. Selection of conserved TCR VDJ rearrangements in chronic psoriatic plaques indicates a common antigen in psoriasis vulgaris. Eur. J. Immunol. 1999;29:3360–3368. doi: 10.1002/(SICI)1521-4141(199910)29:10<3360::AID-IMMU3360>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 130.Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-γ, interleukin-2, and tumor necrosis factor-α, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Invest. Dermatol. 1999;113:752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 131.Valdimarsson H, Baker BS, Jonsdottir I, Powles A, Fry L. Psoriasis: a T-cell-mediated autoimmune disease induced by streptococcal superantigens? Immunol. Today. 1995;16:145–149. doi: 10.1016/0167-5699(95)80132-4. [DOI] [PubMed] [Google Scholar]
- 132.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 133.Capon F, Munro M, Barker J, Trembath R. Searching for the major histocompatibility complex psoriasis susceptibility gene. J. Invest. Dermatol. 2002;118:745–751. doi: 10.1046/j.1523-1747.2002.01749.x. [DOI] [PubMed] [Google Scholar]
- 134.de Cid R, et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nature Genet. 2009;41:211–215. doi: 10.1038/ng.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nair RP, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nature Genet. 2009;41:199–204. doi: 10.1038/ng.311. [A comprehensive whole-genome scan of psoriasis confirming association with gene variants in the IL-23 pathway and pointing towards new gene variants involved in the NF-κB pathway.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cargill M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Capon F, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum. Genet. 2007;122:201–206. doi: 10.1007/s00439-007-0397-0. [References 136 and 137 are the first description of an association of psoriasis with IL23R gene variants in whole-genome association studies.] [DOI] [PubMed] [Google Scholar]
- 138.van der Fits L, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]