Abstract
Background
The atrioventricular (AV) node is essential for the sequential excitation and optimized contraction of the adult multichambered heart; however, relatively little is known about its formation from the embryonic AV canal. A recent study demonstrated that signaling by Alk3, the type 1a receptor for bone morphogenetic proteins, in the myocardium of the AV canal was required for the development of both the AV valves and annulus fibrosus. To test the hypothesis that bone morphogenetic protein signaling also plays a role in AV node formation, we investigated conduction system function and AV node morphology in adult mice with conditional deletion of Alk3 in the AV canal.
Methods and Results
High-resolution optical mapping with correlative histological analysis of 28 mutant hearts revealed 4 basic phenotypic classes based on electrical activation patterns and volume-conducted ECGs. The frequency of AV node conduction and morphological abnormalities increased from no detectable anomalies (class I) to severe defects (class IV), which included the presence of bypass tracts, abnormal ventricular activation patterns, fibrosis of the AV node, and twin AV nodes.
Conclusion
The present findings demonstrate that bone morphogenetic protein signaling is required in the myocardium of the AV canal for proper AV junction development, including the AV node.
Keywords: atrioventricular node, genes, electrophysiology
In the normal heart, the atrioventricular (AV) node electrically connects the atrial and ventricular chambers, which are otherwise insulated from one another by the annulus fibrosus. Slow impulse propagation within the AV node is critical to allow complete filling of the ventricles and thus efficient cardiac function. The AV node, annulus fibrosus, and mitral and tricuspid valves form the AV junction, and these structures are derived at least in part from the embryonic AV canal.1 In the mouse, detailed microscopy studies defined the AV node primordium that arises from the AV canal at approximately embryonic day (E) 11.2,3 This observation was confirmed with both the CCS-lacZ and minK-lacZ conduction system markers.4,5 Between E13 and E16, the node develops its characteristic compact, ovoid shape.6 Although substantial information is available on mechanisms of AV valve development, considerably less is known about the formation and maturation of the AV node itself.7,8
Bone morphogenetic proteins (BMPs) are multifunctional signaling molecules expressed throughout development in a multitude of tissues, including the AV canal.9 They are involved in such processes as cell proliferation, migration, differentiation, and apoptosis (reviewed in Chen et al10). In particular, BMP2 is expressed in the myocardium of the AV canal in E11 to E12 mouse embryos.11 Germline knockout of the type 1a BMP receptor, Alk3, leads to embryonic death before gastrulation.12 Cardiomyocyte-specific deletion of Alk3 was lethal to the embryo at mid gestation.13 Mutant hearts exhibited abnormalities in trabeculation, formation of the compact layer and interventricular septum, and endocardial cushion formation. To study the role of BMP signaling specifically in the AV canal, Gaussin et al14 conditionally inactivated Alk3 in the AV canal myocardium by expressing Cre recombinase under the transcriptional control of a chicken GATA-6 (cGATA6) enhancer. These studies demonstrated a requirement for BMP signaling in AV valve development and in formation of the annulus fibrosus. In cGATA6-Cre/Alk3 mice, the posterior tricuspid valve was displaced downward, and disruption of the annulus fibrosus led to the formation of accessory conduction pathways in a portion of mutants, a constellation of findings reminiscent of Ebstein's anomaly.14 To test the hypothesis that BMP signaling in AV myocardium is also critical for the development of the AV node, we analyzed the structure and function of the AV node in cGATA6-Cre/Alk3 mice using a combination of high-resolution optical mapping and correlative histology.
Methods
Animals
To target the deletion of Alk3 in cardiac myocytes of the AV canal, mice hemizygous for both cGATA6-Cre15 and the conventional null Alk3 allele12 were bred to mice homozygous for the floxed Alk3 allele.13 This breeding strategy generated 25% of mice in which Cre was present together with a conventional null allele and a floxed allele for Alk3, according to a previously established breeding strategy.14 In that genotype (Alk3null/flox, Cre+), Cre action resulted in AV canal–targeted deletion of Alk3 (cGATA6-Cre/Alk3). The 2 control groups were Cre− (Alk3wt/flox, Cre−) and Cre+ (Alk3wt/flox, Cre+) mice. Both control and mutant mice used in the present study ranged in age from 12 to 23 months.
ECGs and Optical Mapping
Surface ECGs were performed under anesthesia as described previously.16 The methods used to optically map the mouse heart have been described previously.17 Briefly, hearts were isolated and perfused by the Langendorff method with warm (37°C), oxygenated (95% O2, 5% CO2) Tyrode's solution. The Tyrode's solution contained (in mmol/L) NaCl 114, NaHCO3 25, dextrose 10, KCl 4.6, CaCl2 1.5, Na2PO4 1.2, and MgCl2 0.7. Once the heart rate stabilized, it was stained with a bolus of the voltage-sensitive dye Di-4-ANEPPs (8 μL of a 2-mmol/L stock solution dissolved in DMSO). The heart was stimulated with a platinum electrode, and volume-conducted ECGs (vECGs) were recorded with Ag-AgCl electrodes placed near the heart. We defined breakthroughs as points on the cardiac surface at which the impulse could be seen radiating out in all directions.
Drug Treatments
Drugs were added to warm Tyrode's solution and perfused through the heart continuously. Initial adenosine treatments ranged from 20 to 80 μmol/L and continued at increasing doses until AV block or asystole was observed. Procainamide treatments began at 45 μg/mL and continued at increasing dosages until AV conduction was restored or asystole was induced.
Histology
After optical mapping, hearts were perfused with KCl 50 mmol/L followed by 10% neutral-buffered formalin. They were further fixed by immersion in 10% neutral-buffered formalin, embedded in paraffin, and sectioned at 6-μm intervals. Staining with Masson's trichrome (Poly Scientific, Bay Shore, NY) was performed according to the manufacturer's protocol.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Activation Pattern of Control Hearts
To assay conduction system function, we used high-resolution optical mapping.17 No difference was detected between the 2 control groups in terms of activation patterns (data not shown), average PR intervals (mean±SEM except where indicated: Cre− 39±3 ms, n=3; Cre+ 40±3 ms, n=3), and AV nodal morphology. Anterior and posterior maps are presented. For orientation purposes, the anterior maps were obtained from the front of the heart with right and left ventricles in view, whereas the posterior maps were recorded from the back of the heart. In the normal heart, after the AV delay, impulses traveled down the His bundle, split to the right and left bundle branches, and were rapidly delivered to the ventricles via the Purkinje fiber network. On the anterior epicardial surface, 2 breakthroughs that began near the apex of the right and left ventricles were typically observed (Figure 1A). vECGs recorded simultaneously showed normal AV conduction in control hearts (Figure 1B). On the posterior surface, 2 different patterns were observed. In the first, the impulse came around from the anterior side (Figure 1C), and in the second, the impulse emerged from the apex and propagated along the septal surface (Figure 1D).
Figure 1.
Representative activation maps showing normal impulse conduction across the ventricular epicardium of control hearts. The change in color represents time, with red indicating the earliest and purple the latest activation. A, Anterior view, right (RV) and left (LV) ventricles in view, apex (A) at the bottom. Note 2 breakthroughs (⋆) on the ventricular surface toward the apex of the heart. B, Representative vECG collected from a control heart. Atrial (P) and ventricular (QRS) depolarizations were clearly defined. C and D, Views from the posterior surface of the ventricle showing 2 types of normal activation patterns. Arrows show direction of impulse propagation. In C, the impulse wrapped around from the anterior surface. In D, breakthroughs occurred along the septal surface.
Class I: Normal Activation Patterns and Nodal Morphology
We studied a total of 28 mutant hearts. Of these, 9 hearts displayed activation patterns that were indistinguishable from controls (Figure 2). vECGs in these class I mutants showed similar PR intervals compared with control mice (not shown). The hearts of 3 class I mutants were processed for histological analysis of the AV node. Sections were stained with Masson's trichrome to visualize fibrous tissue in blue. In control hearts, the AV node was a clearly discernible compact structure isolated from the chamber myocardium by fibrous tissue (Figure 3A through 3D). No morphological anomalies were detected in the AV nodes of any of the 3 class I mutant hearts analyzed (Figure 3E and 3F).
Figure 2.

Control and class I mutant ventricular activation patterns. A and B, Anterior views of control and mutant hearts showed similar activation patterns. C and D, Posterior views of the same hearts were also similar. A indicates apex; LV, left ventricle; and RV, right ventricle.
Figure 3.
Histological analysis of control and class I and class II mutant hearts. A and B, Cre+ control. C and D, Cre− control. E and F, Class I mutant. G and H, Class II mutant. B, D, F, and H are high-magnification pictures of the boxed areas in A, C, E, and G, respectively. Hearts were sectioned and stained with Masson's trichrome, and fibrous tissue appears in blue. In all control as well as class I and class II mutants, the AV node appeared compact and ovoid. Ao indicates aorta; AVN, AV node; His, His bundle; LV, left ventricle; and RV, right ventricle. Scale bar 500 μm (A, C, E, and G) and 200 μm (B, D, F, and H).
Class II: Intermittent Bypass Conduction
The second group of mutant mice was identified during the optical mapping studies. These cGATA6-Cre/Alk3 mice had normal surface ECGs, but the isolated hearts showed evidence of a bypass tract (Figure 4A and 4B). This behavior was observed in 6 of 28 mice studied. As observed clinically in individuals with ventricular preexcitation,18 impulse conduction in this class of mutants switched between AV nodal conduction and bypass pathways (Figure 4C through 4E). Shorts runs of preferential conduction over accessory pathways could be detected by vECG, but impulse propagation in the mutant hearts was primarily through the AV node. In the majority of class II cGATA6-Cre/Alk3 mice (5 of 6), AV impulses from the apex and accessory impulses from the base were seen to collide on the posterior epicardial surface (Figure 4D), although the vECG was normal at the time (Figure 4D). These findings suggest that AV conduction was preserved, but accessory conduction pathways were also present. Histology data supported this notion, because sections through 2 class II cGATA6-Cre/Alk3 hearts revealed no detectable difference in AV node morphology compared with controls (Figure 3G and 3H).
Figure 4.
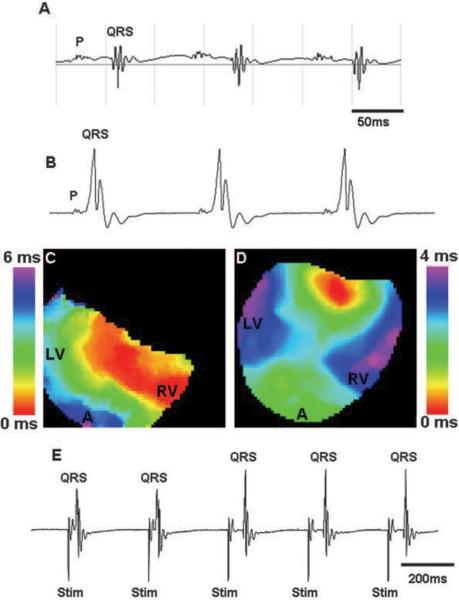
Class II mutants exhibited intermittent use of bypass tracts. A, Surface ECG of a class II mutant showed normal AV conduction. B, vECG of the same heart after isolation and Langendorff perfusion clearly demonstrated conduction through a bypass tract. C and D, Activation maps showed bypass conduction (C) followed by a collision beat (D) where signals were coming both from the apex and the base of the ventricle in a single heart. E, vECG data from the same heart demonstrated intermittent use of the bypass during pacing. First 2 beats had a short PR interval, which indicates accessory pathway conduction, whereas the next 3 beats were conducted through the AV node. P indicates atrial depolarization; QRS, ventricular depolarization; LV, left ventricle; RV, right ventricle; A, apex; and Stim, stimulus.
Class III: Primary Bypass Conduction
AV conduction in class III animals occurred exclusively or predominantly through accessory pathways associated with short PR intervals (mean interval 10±2 ms, n=7). Mice with this behavior were recognized previously14 and accounted for 7 of 28 animals in the present study. The PR interval in this class was greatly abbreviated or not present, and a delta wave was observed (Figure 5A). The impulse in class III mutant hearts was observed by optical mapping to travel from the base to the apex of the ventricle (Figure 5C). Such a pattern was never seen in control hearts. Six class III hearts were treated with the type 1A antiarrhythmic agent procainamide. In 1 heart, treatment was able to block conduction over the bypass track and restored AV nodal conduction (Figure 5B and 5D).19 Gross morphological examination of this heart revealed a clear external connection between the coronary sinus and the ventricle (Figure 5E and 5F). The remaining 5 treated hearts exhibited either AV block or bypass, and normal AV conduction was never restored (n=5; Figure 5G and 5H). These data suggest that AV nodal conduction in these hearts was severely compromised. In agreement with this interpretation, histological examination of 4 class III hearts demonstrated obvious AV nodal defects (n=3). Two hearts exhibited fibrosis within the AV node. The fibrosis pushed apart the nodal cells, slightly altering the morphology and likely impairing the ability of impulses to propagate through the node (Figure 6A and 6B). Fibrosis was never detected in control AV nodes (Figure 3A through 3D). The morphology of the third node was extremely disrupted. Rather than a compact, ovoid structure as observed in control hearts (Figure 3A through 3D), the node appeared as a twisted, Y-shaped structure (Figure 6C and 6D).
Figure 5.
Effects of procainamide in class III mutant hearts. vECG and activation maps before (A and C) and after (B and D) treatment with procainamide. In this particular heart, procainamide blocked conduction over the bypass tract, shifting conduction to the AV node pathway. E, From the posterior aspect of a control heart, the atria (RA) and coronary sinus (CS) were clearly visible. F, In the class III mutant heart shown in A through D, a connection (indicated by white arrows) was visible between the coronary sinus region and the ventricle. The remaining 5 class III mutant hearts showed no response to procainamide treatment. G and H, Representative vECGs of a nonresponsive class III mutant before (G) and after (H) a high dose (160 μg/mL) of procainamide. Impulse propagation continued through the accessory conduction pathway. P indicates atrial depolarization; QRS, ventricular depolarization; LV, left ventricle; RV, right ventricle; and A, Apex.
Figure 6.
Class III mutant hearts had defects in AV node morphology. Hearts were sectioned and stained with Masson's trichrome. B and D, Higher magnifications of boxed areas in A and C, respectively. Note the fibrosis (yellow arrowheads in B) within 1 AV node and the twisted Y shape of the other AV node (D) compared with the ovoid compact control node (Figure 3A and 3B). Ao indicates aorta; AVN, AV node; His, His bundle; LV, left ventricle; and RV, right ventricle. Scale bar 500 μm(A and C) and 200 μm (B and D).
Class IV: Severe AV Dysfunction
Class IV mutants had the most atypical activation patterns (n=6 of 28). In contrast to control hearts (Figure 7A), class IV mutant activation maps showed breakthroughs along the anterior septal surface rather than the apex (Figure 7C and 7E). These mutants had either normal (n=2) or prolonged PR intervals measured from vECGs (mean interval 54±1 ms; n=4), which indicates that the impulse was conducted through the node (Figure 7B, 7D, and 7F). In support of the notion that conduction was nodal and not through a slow-conducting bypass tract, adenosine treatment of the majority of mutant hearts led to block (n=4 of 5) at the same concentrations (10 to 40 μmol/L) as in controls. To test whether an abnormal activation pattern would arise from a defect in the ventricular muscle itself, conduction velocities were calculated from maps generated during pacing of the left ventricle and showed no significant difference between mutant and control hearts (data not shown). The entire study was conducted with mice aged 12 to 23 months. Interestingly, no evidence existed of increased-age–related abnormal activation pattern or heart block in control or mutant mice.
Figure 7.
Class IV mutants exhibited extremely altered activation pattern. A and B, Control anterior activation pattern and vECG. C through F, Activation maps and vECGs from class IV mutants showed breakthroughs along the anterior septal surface (C and E) with either normal (D; 37 ms) or prolonged (F; 53 ms) PR intervals. P indicates atrial depolarization; QRS, QRS depolarization; A, apex; RV, right ventricle; and LV, left ventricle.
Histological analysis revealed that all class IV mutant hearts had AV node abnormalities (n=3 of 3; Figure 8). In 1 class IV cGATA3-Cre/Alk3 heart, the AV node appeared stretched and with a loose morphology (Figure 8A and 8B) relative to control (Figure 3A through 3D). The other 2 class IV mutant hearts had twin AV nodes (Figure 8C through 8F). Intriguingly, 1 of these hearts had a relatively normal PR interval, whereas the other showed first-degree block.
Figure 8.
Abnormal AV node morphology in class IV mutant hearts. Hearts were sectioned and stained with Masson's trichrome. B, D, and F, Higher magnifications of boxed areas in A, C, and E, respectively. Note the abnormal shape and loose morphology of 1 AV node (A, B) and the twin or split AV nodes in the other 2 class IV mutant hearts (C through F) with either normal (C, D) or prolonged (E, F) PR intervals (corresponding to Figure 7D and 7F, respectively). Ao indicates aorta; AVN, AV node; LV, left ventricle; RV, right ventricle; RA, right atrium; and LA, left atrium. Scale bar 500 μm (A, C, and E) and 200 μm (B, D, and F).
Discussion
BMP signaling plays a key role in diverse aspects of cardiac differentiation and morphogenesis. Previous studies have indicated that Alk3 is required in cardiac myocytes subsequent to the onset of cardiac fate, playing an essential role in morphogenesis of the ventricular septum, trabeculae, compact myocardium, and endocardial cushion formation at mid gestation.13 More recently, Gaussin and colleagues14 targeted the inactivation of Alk3 more specifically to cardiac myocytes of the AV canal. These studies demonstrated a role for BMP signaling in tricuspid and mitral valve formation and development of the annulus fibrosus.14 In the present study, we tested the hypothesis that Alk3 is also required for normal formation and function of the AV node.
For this, cGATA6-Cre/Alk3 mice were analyzed with a combination of high-resolution optical mapping of cardiac impulse propagation, vECG, and correlative histological analysis. We identified a broad range of defects in AV conduction and associated abnormalities in AV nodal structure in these mutant mice. We defined 4 phenotypic classes of mutants in which the frequency of AV node conduction and morphological abnormalities increased from no detectable defects (class I) to severe defects (class IV).
Class I mutant hearts were indistinguishable from control hearts. Class II mutants were defined by the presence but intermittent use of accessory conduction pathways. In these hearts, impulses traveled through the AV node the majority of the time, and AV node morphology appeared normal. In contrast, class III cGATA6-Cre/Alk3 hearts rarely if ever conducted impulses through the AV node, even with procainamide treatment. The preference for bypass versus AV nodal conduction suggested a possible defect in the AV node itself. Histological data confirmed AV node anomalies in 3 of 4 class III hearts, including fibrosis within the node itself and abnormal node shape. Class IV mutants were the most striking, with extremely abnormal activation patterns and no evidence of a bypass tract as determined by AV conduction times. All class IV hearts exhibited AV node abnormalities. In particular, sections from 2 class IV hearts revealed twin AV nodes.
The age of mice used in the present study ranged from 12 to 23 months, and no correlation was detected between age and severity of defect. Surface ECG data, albeit limited in number, indicated that mice that fell into class III showed exclusive bypass conduction when sampled at multiple time points beginning as early as 2 months of age. Thus, currently, no evidence exists that the defects were progressive. Rather, it is very likely that the extent and/or location of Cre recombination during development was the determining factor in the extent of AV conduction dysfunction.15 This suggests that BMP signaling in AV myocardium is not required for maintenance of the AV node function in the adult but rather for proper development of the AV junction.
BMP Signaling in AV Canal Development
The different phenotypes in the 4 classes of mutants identified by optical mapping could have different developmental origins. The cGATA6 enhancer used in the present study is active in 2 posterior and lateral regions of the heart field at E7.5 through E8.5 and becomes restricted to the AV canal myocardium at E9.5.15 Lineage, anatomic, and expression studies have shown the AV myocardium contributes to the AV valves, AV node, His bundle, and the myocardium adjacent to the annulus fibrosus.1,14,15,20 Thus, the development of a number of structures could be affected by alterations in BMP signaling in the AV canal. Studies from chick embryos have revealed that early in development, myocytes of the AV canal dissociate, and the resulting space is filled with extracellular matrix. This coincides with the detection of slow AV conduction through the canal.21–23 If tight cellular connections were maintained between even a portion of AV myocytes, it could lead to the accessory pathways observed in class II and III hearts in the present study. Formation of the AV node and the His bundle occurs as the endocardial cushions fuse, the central fibrous body forms, and ventricular septation is complete, with only 1 pathway left for impulse conduction. In class IV hearts, AV conduction was perturbed without the presence of a bypass tract. The impulse breakthrough in these hearts occurred on the anterior septal surface, which suggests a disruption within the central conduction system of the node, bundle, and bundle branches. One possibility is that BMP signaling is required to maintain proper continuity between the AV node and His bundle or the His bundle and the bundle branches. No cGATA6-Cre–mediated recombination has been detected in the bundle branches or Purkinje fibers.15 Alternatively, disrupted BMP signaling could affect patterning of the AV canal, thereby impacting formation of the entire central conduction system.24–28 Detailed analysis of conduction system morphology and function during development may assist in resolving the origin of these AV defects in cGATA6-Cre mice.
Clinical Significance
Inherited cardiac conduction system defects, although rare, still pose a serious health risk for those afflicted. The abnormalities seen in cGATA6-Cre/Alk3 mice resemble those seen in human disorders such as Ebstein's anomaly and AV conduction disease.29,30 Both of these conditions in patients have been associated with mutations in the BMP target Nkx2.5.30–32 This suggests that BMP signaling through the Alk3 receptor and other downstream components may play a role in congenital conduction system defects. Furthermore, understanding the mechanisms of both normal and ectopic fibrous deposition in the heart is of great interest. Interruption of conduction system tissue by fibrosis is the most common cause of complete heart block.33 The cGATA6-Cre/Alk3 mouse provides a new avenue for investigation into congenital heart defects and underscores the importance of BMP signaling in AV canal myocardium for proper formation of the mature AV junction.
CLINICAL PERSPECTIVE.
A portion of mice with targeted inactivation of the type I bone morphogenetic protein receptor Alk3 in the atrioventricular (AV) canal develop a combination of defects similar to those observed in patients with Ebstein's anomaly. These include downward displacement of the posterior tricuspid valve, disruption of the annulus fibrosus, and ventricular preexcitation. In this study, we investigated the role of bone morphogenetic protein signaling in AV node development using both histology and high-resolution optical mapping. Mutants could be divided into 4 basic classes based on activation patterns and volume-conducted ECGs. Class I animals showed no detectable defects. Intermittent use of bypass tracts defined class II animals, whereas class III mutants conducted almost exclusively through accessory conduction pathways. Class IV mutants displayed severe AV conduction dysfunction with no evidence of a bypass tract. The frequency of altered AV node morphology increased from class II to class IV and included fibrosis within the node and twin AV nodes. The findings reveal that bone morphogenetic protein signaling is required in the AV canal for normal AV junction formation, including the node. Mutations in the bone morphogenetic protein target Nkx2.5 have been associated with Ebstein's anomaly and conduction disease, which lends support to arguments that components of the Alk3 signaling pathway may be involved in congenital conduction system disorders.
Acknowledgments
We thank L. Emile, J. Jetko, and P. Jetko for expert technical assistance.
Sources of Funding This work was supported by grants from the National Institutes of Health (HL76751 to Dr Morley, HL69020 and HL59139 to Dr Stephen F. Vatner), a National Research Service Award Fellowship (HL79796 to Dr Stroud), a grant from the American Heart Association (0555840T to Dr Gaussin), and the March of Dimes Birth Defects Foundation (1-FY06-375 to Dr Gaussin).
Footnotes
Disclosures None.
References
- 1.Wessels A, Markman MWM, Vermeulen JLM, Anderson RH, Moorman AFM, Lamers WH. The development of the atrioventricular junction in the human heart. Circ Res. 1996;78:110–117. doi: 10.1161/01.res.78.1.110. [DOI] [PubMed] [Google Scholar]
- 2.Viragh S, Challice CE. The development of the conduction system in the mouse embryo heart, II: histogenesis of the atrioventricular node and bundle. Dev Biol. 1977;56:397–411. doi: 10.1016/0012-1606(77)90279-2. [DOI] [PubMed] [Google Scholar]
- 3.Viragh S, Challice CE. The development of the conduction system in the mouse embryo heart, I: the first embryonic A-V conduction pathway. Dev Biol. 1977;56:382–396. doi: 10.1016/0012-1606(77)90278-0. [DOI] [PubMed] [Google Scholar]
- 4.Kondo RP, Anderson RH, Kupershmidt S, Roden DM, Evans SM. Development of the cardiac conduction system as delineated by minK-lacZ. J Cardiovasc Electrophysiol. 2003;14:383–391. doi: 10.1046/j.1540-8167.2003.02467.x. [DOI] [PubMed] [Google Scholar]
- 5.Rentschler S, Vaidya DM, Tamaddon H, Degenhardt K, Sassoon D, Morley GE, Jalife J, Fishman GI. Visualization and functional characterization of the developing murine cardiac conduction system. Development. 2001;128:1785–1792. doi: 10.1242/dev.128.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viragh S, Challice CE. The development of the conduction system in the mouse embryo heart, IV: differentiation of the atrioventricular conduction system. Dev Biol. 1982;89:25–40. doi: 10.1016/0012-1606(82)90290-1. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efimov IR, Nikolski VP, Rothenberg R, Greener ID, Li J, Dobrzynski H, Boyett M. Structure-function relationship in the AV junction. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:952–965. doi: 10.1002/ar.a.20108. [DOI] [PubMed] [Google Scholar]
- 9.Euler-Taimor G, Heger J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc Res. 2006;69:15–25. doi: 10.1016/j.cardiores.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 11.Eltyeb A, David R, Lauri JP, Eero J. Overlapping and differential localization of Bmp-2, Bmp-4, Msx-2 and apoptosis in the endocardial cushion and adjacent tissues of the developing mouse heart. Cell Tissue Res. 2001;305:67–78. doi: 10.1007/s004410100399. [DOI] [PubMed] [Google Scholar]
- 12.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 13.Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaussin V, Morley GE, Cox L, Zwijsen A, Vance KM, Emile L, Tian Y, Liu J, Hong C, Myers D, Conway SJ, Depre C, Mishina Y, Behringer RR, Hanks MC, Schneider MD, Huylebroeck D, Fishman GI, Burch JBE, Vatner SF. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res. 2005;97:219–226. doi: 10.1161/01.RES.0000177862.85474.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis DL, Edwards AV, Juraszek AL, Phelps A, Wessels A, Burch JBE. A GATA-6 gene heart-region-specific enhancer provides a novel means to mark and probe a discrete component of the mouse cardiac conduction system. Mech Dev. 2001;108:105–119. doi: 10.1016/s0925-4773(01)00500-7. [DOI] [PubMed] [Google Scholar]
- 16.Gutstein DE, Danik SB, Lewitton S, France D, Liu F, Chen FL, Zhang J, Ghodsi N, Morley GE, Fishman GI. Focal gap junction uncoupling and spontaneous ventricular ectopy. Am J Physiol Heart Circ Physiol. 2005;89:H1091–H1098. doi: 10.1152/ajpheart.00095.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamaddon HS, Vaidya D, Simon AM, Paul DL, Jalife J, Morley GE. High-resolution optical mapping of the right bundle branch in connexin40 knockout mice reveals slow conduction in the specialized conduction system. Circ Res. 2000;87:929–936. doi: 10.1161/01.res.87.10.929. [DOI] [PubMed] [Google Scholar]
- 18.Yee R, Klein GJ, Prystowsky E, Zipes DP, Jalife J. Cardiac Electrophysiology: From Cell to Bedside. Vol 3. Pa: Saunders; Philadelphia: 2006. The Wolff-Parkinson-White syndrome and related variants; pp. 845–861. [Google Scholar]
- 19.Patel VV, Arad M, Moskowitz IPG, Maguire CT, Branco D, Seidman JG, Seidman CE, Berul CI. Electrophysiologic characterization and postnatal development of ventricular pre-excitation in a mouse model of cardiac hypertrophy and Wolff-Parkinson-White syndrome. J Am Coll Cardiol. 2003;42:942–951. doi: 10.1016/s0735-1097(03)00850-7. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Viragh S, Moorman AFM, Anderson RH, Lamers WH. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circ Res. 2001;88:395–402. doi: 10.1161/01.res.88.4.395. [DOI] [PubMed] [Google Scholar]
- 21.Arrechedera H, Strauss M, Arguello C, Ayesta C, Anselmi G. Ultra-structural study of the myocardial wall of the atrio-ventricular canal during the development of the embryonic chick heart. J Mol Cell Cardiol. 1984;16:885–895. doi: 10.1016/s0022-2828(84)80025-5. [DOI] [PubMed] [Google Scholar]
- 22.Arguello C, Alanis J, Pantoja O, Valenzuela B. Electrophysiological and ultrastructural study of the atrioventricular canal during the development of the chick embryo. J Mol Cell Cardiol. 1986;18:499–510. doi: 10.1016/s0022-2828(86)80915-4. [DOI] [PubMed] [Google Scholar]
- 23.Martinsen BJ. Reference guide to the stages of chick heart embryology. Dev Dyn. 2005;233:1317–1327. doi: 10.1002/dvdy.20468. [DOI] [PubMed] [Google Scholar]
- 24.Yamada M, Revelli JP, Eichele G, Barron M, Schwartz RJ. Expression of chick Tbx-2, Tbx-3, and Tbx-5 genes during early heart development: evidence for BMP2 induction of Tbx2. Dev Biol. 2000;228:95–105. doi: 10.1006/dbio.2000.9927. [DOI] [PubMed] [Google Scholar]
- 25.Christoffels VM, Burch JBE, Moorman AFM. Architectural plan for the heart: early patterning and delineation of the chambers and the nodes. Trends Cardiovasc Med. 2004;14:301–307. doi: 10.1016/j.tcm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Christoffels VM, Hoogaars WMH, Tessari A, Clout DE, Campione M. T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Dev Dyn. 2004;229:763–770. doi: 10.1002/dvdy.10487. [DOI] [PubMed] [Google Scholar]
- 27.Hoogaars WMH, Tessari A, Moorman AFM, de Boer PAJ, Hagoort J, Soufan AT, Campione M, Christoffels VM. The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovasc Res. 2004;62:489–499. doi: 10.1016/j.cardiores.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Rutenberg JB, Fischer A, Jia H, Gessler M, Zhong TP, Mercola M. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Development. 2006;133:4381–4390. doi: 10.1242/dev.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf CM, Berul CI. Inherited conduction system abnormalities: one group of diseases, many genes. J Cardiovasc Electrophysiol. 2006;17:446–455. doi: 10.1111/j.1540-8167.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 30.Benson DW. Genetics of atrioventricular conduction disease in humans. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:934–939. doi: 10.1002/ar.a.20099. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda Y, Hiroi Y, Hosoda T, Utsunomiya T, Matsuo S, Ito T, Inoue J, Sumiyoshi T, Takano H, Nagai R, Komuro I. Novel point mutation in the cardiac transcription factor CSX/NKX2.5 associated with congenital heart disease. Circ J. 2002;66:561–563. doi: 10.1253/circj.66.561. [DOI] [PubMed] [Google Scholar]
- 32.Jay PY, Harris BS, Maguire CT, Buerger A, Wakimoto H, Tanaka M, Kupershmidt S, Roden DM, Schultheiss TM, O'Brien TX, Gourdie RG, Berul CI, Izumo S. Nkx2–5 mutation causes anatomic hypoplasia of the cardiac conduction system. J Clin Invest. 2004;113:1130–1137. doi: 10.1172/JCI19846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suarez-Penaranda JM, Munoz JI, Rodriguez-Calvo MS, Ortiz-Rey JA, Concheiro L. The pathology of the heart conduction system in congenital heart block. J Clin Forensic Med. 2006;13:341–343. doi: 10.1016/j.jcfm.2006.06.010. [DOI] [PubMed] [Google Scholar]