Abstract
Microwave ablation is a relatively new technology under development and testing to treat the same types of cancer that can be treated with radiofrequency (RF) ablation. Microwave energy has several possible benefits over RF energy for tumor ablation but, because clinical microwave ablation systems are not widespread, the underlying principles and technologies may not be as familiar. The basic microwave ablation system contains many of the same components as an RF ablation system: a generator, a power distribution system, and an interstitial applicator. This manuscript will attempt to provide an overview of each of these components, outline their functions and roles, and provide some insight into what every potential microwave ablation user should know about systems in development.
INTRODUCTION
The rapid adoption of radiofrequency (RF) ablation has benefited many patients with primary and metastatic liver tumors (1, 2), and is being adapted for use in the lung, kidney and bone with some success (3–5). However, there are many drawbacks inherent to RF heating, including an inability to conduct current through charred tissue, a need for current return paths (ie, ground pads or additional interstitial electrodes), and a sizeable dependence on thermal conduction. For these reasons, RF energy creates relatively small zones of active heating, which can cause small ablations and poor results in areas near heat sinks such as large blood vessels (> 3 mm) and airways. These effects are amplified in high-impedance tissue like the lung. In addition, multiple electrodes have only been used effectively in a switched mode because of undue interactions between electrodes in close proximity (6).
Microwaves offer several advantages over RF energy for tumor ablation, including faster heating over a larger volume, less susceptibility to heat sinks or local perfusion, enhanced multiple-applicator support and no requirement for ground pads. Very few commercial devices are available but rapid commercial and academic development is aiming to make microwave ablation technology more accessible to physicians. However, the underlying technology of microwave ablation can often be unclear to the end user. The goal of this manuscript is to describe the main components of microwave ablation systems, including the power generation, distribution and delivery sub-systems, and explain how each might impact the efficacy, safety and cost of a microwave ablation system.
Microwave heating
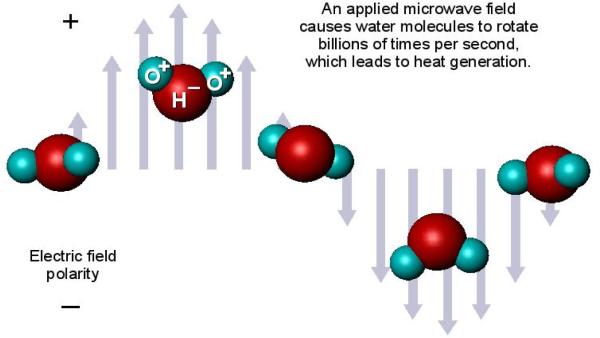
Microwaves generate heat through a process known as dielectric hysteresis: polar molecules (e.g., water) try to continuously realign with an applied electromagnetic (EM) field that alternates polarity billions of times per second (Figure 1). When the molecules fail to “keep up” with the alternating field, some of the microwave energy is absorbed by the material and converted to heat. The rate of heat generation (Qh) is proportional to the square of the applied electric field magnitude (E), or
| (1) |
where σ is effective conductivity (S/m), a measure of microwave absorption. Because of their high water content, most biological tissues have a relatively high conductivity and readily absorb microwave energy (7). For this reason, many insterstitial devices have been proposed to introduce microwave energy into the body (8–16). Limitations on field penetration and spatial resolution make interstitial antennas preferred for focal tumor ablation. The most general system design consists of an interstitial antenna (or array of antennas) receiving power from a microwave generator through a distribution system (Figure 2). The specifics of each of these subsystems, their technical specifications and most likely configurations for commercially available clinical systems will be discussed next.
Figure 1.
Microwave tissue heating relies on the interaction of an electromagnetic field with water molecules in the tissue.
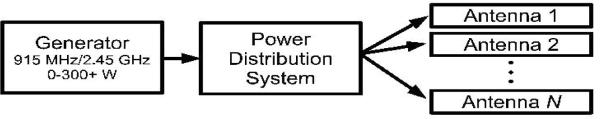
Figure 2.
Microwave ablation system schematic. The three basic components are the microwave generator, power distribution system and antenna. The generator frequencies allowed by the Federal Communications Commission (FCC) are, most commonly, 915 MHz or 2.45 GHz. The power distribution system may contain one or more of several components, including cables, power splitters, phase shifters and switches. The antenna delivery system may also contain one or more antennas of various designs, each with the goal of creating a large and reproducible zone of ablation.
Microwave generators
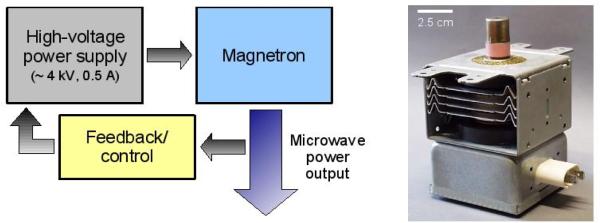
Microwave ablation generators utilize one of two basic power sources: a magnetron or solid-state amplifier. A magnetron generates electromagnetic energy by accelerating electrons through a magnetic field inside of a resonant cavity (Figure 3). The geometry of the cavity determines the output frequency. Magnetrons are characterized by relatively high efficiency (> 70%), high output powers (>10 kW is common), high reliability and low cost. Their extremely large “Watts-per-dollar” ratio has made the magnetron the source-of-choice for microwave ovens, plasma generators, food treatment and industrial heating applications. However, magnetrons require a high-voltage power supply (which often entails a large, heavy transformer) that can be precisely controlled. Monitoring and output control systems within the generator may also be large and bulky due to their need to handle high output powers. However, the high output powers available from magnetrons can be used to power several antennas from a single source (17).
Figure 3.
System schematic (left) and image of a typical 1000 W magnetron (right) generator. The advantages of using a magnetron are simple design, high power output, high efficiency and excellent robustness. The disadvantages include bulky power supplies and monitoring systems.
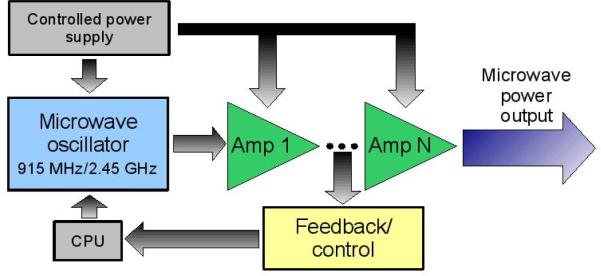
Solid-state generators create power in stages, with each stage consisting of a transistor-based amplifier that increases the power of the previous stage (Figure 4). Solid-state sources generally have a lower efficiency (< 30%), moderate output powers (< 150 W), high stability, good robustness and higher cost. However, they can be made smaller in size and are more controllable than magnetron devices. Due to their lower efficiency, solid-state sources generate large amounts of heat that need to be dissipated. Heating becomes more problematic as frequency increases or prolonged continuous-wave operation is required; 915 MHz solid-state sources are easier to come by and more reliable than their 2.45 GHz counterparts. One advantage to using solid state sources is that the pre-amplified signal can be monitored and controlled much easier than in magnetron sources. It remains to be seen how the competing interests of high output power and lightweight design will be resolved in clinical generator designs.
Figure 4.
Basic schematic of a solid-state generator. In contrast to a magnetron generator, power is generated in stages using solid-state devices. Advantages of solid-state generators include low-power (pre-amplified) control, a more stable output and often a smaller, lighter unit. Disadvantages include reduced efficiency and lower output power.
Regardless of power source, generators may also encompass some of the power distribution system. Current systems are capable of powering only one antenna per generator, but future generations may incorporate multiple-antenna operation into a single generator unit. Using separate generators makes advanced multiple-antenna capabilities all but impossible. Such capabilities have been investigated for microwave hyperthermia and are currently being researched for microwave ablation, but are likely several years from deployment (18–21). More information about power distribution options follows.
Power distribution
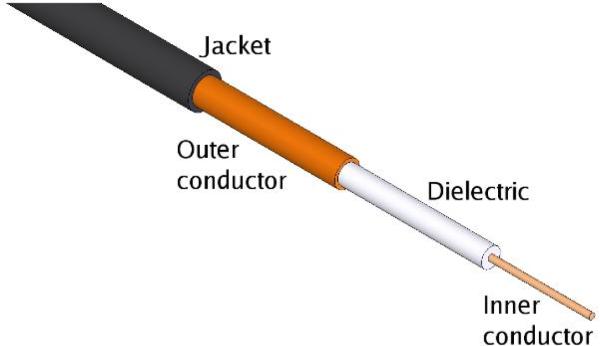
Electromagnetic energy is carried in transmission lines. One of the most popular is the coaxial transmission line (coaxial cable), which consists of an inner conductor, dielectric material and outer conductor (Figure 5). Coaxial cable is popular for many applications (e.g., cable television) because of its flexibility, compact size, excellent propagation characteristics and simplicity. For tumor ablation, coaxial cables can be used in various forms to carry power from the generator to the antenna. Antennas are typically built from a rigid form of coaxial cable as well.
Figure 5.
Basic coaxial cable. The inner and outer conductor are usually copper, but can be any good conductor. The inner conductor is often silver plated to improve performance. Solid conductors improve rigidity while braided conductors improve flexibility. The dielectric material is typically polytetrafluoroethylene (PTFE) or a similar polymer. The jacketing material is optional but helps to protect the cable from mechanical stress, especially if the outer conductor is braided.
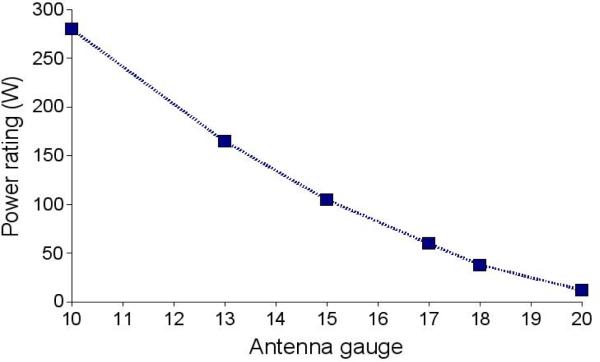
Despite their many strengths, coaxial cables are limited in their ability to carry large amounts of power at microwave frequencies. As cable diameter decreases, power handling ability decreases precipitously (Figure 6). This limits how small and flexible the distribution cables can be, though proper material selection can improve the flexibility of most cables. A bigger issue lies in the antenna cable diameter, as will be discussed later.
Figure 6.
Power ratings of common coaxial cables in air at 20 °C. Power rating increases for cables in tissue for several reasons, including the enhanced conductive heating offered by tissues and the increased convective cooling offered by blood flow. Actively cooling antennas can increase their power ratings as well.
Other components that may enter into the power distribution system include power splitters, phase shifters and amplitude modulation or switches. Power splitters use a specific transmission line geometry to divide an input power into a number of output channels, usually in equal proportions. While it is possible to split a single input into as many output channels as needed, a typical microwave ablation system would likely not have more than four output channels. Current current multiple-antenna RF systems support up to three electrodes and while cryoablation systems are capable of up to eight-probe operation, rarely are more than four used at a time.
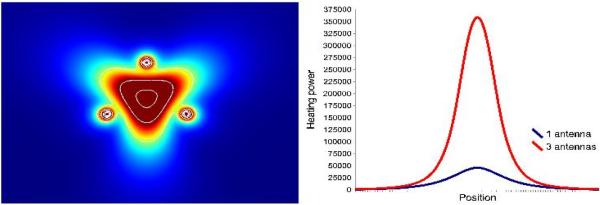
Phase shifters and amplitude modulation would be used on systems offering phased-array operation of multiple antennas. Phased arrays have been used for hyperthermia, but have only begun to be researched for tumor ablation (18–21). The goal of a phased array is to overlap the electromagnetic waves in a way that increases (or decreases) the amount of heating. When using constructive interference, heating in the array can be increased by a factor of by N2, where N is the number of antennas used in the array. For example, three coherently phased antennas can deliver 9× more power than a single antenna (Figure 7). Finally, switches or continuous amplitude modulation may also be used in the power distribution system to selectively activate antennas.
Figure 7.
Simulated heating pattern of a three-antenna array with constructive phase overlap at the array center (left) along with a plot comparing the heating power through the array center for one or three antennas (right). The use of phase shifters in the power distribution system could allow 9× more power deposition at the array center than with one antenna.
Microwave antennas
Antennas come in a variety of formats, but all antennas are linked by their ability to transfer energy from a source to a load (i.e., from the generator to the tissue). Unlike RF ablation electrodes, antennas radiate energy by virtue of their geometry without ground pads or other electrodes. Thus, microwave fields may propagate through and heat any normal or malignant tissue, desiccated tissue, blood, cystic masses, vessels, etc.
Several different designs have been proposed for interstitial microwave heating(8–16). Most of these designs have an essentially needle-like geometry, but some loop and deployable designs have been described (22). Each antenna design attempts to satisfy some basic requirements: the antenna should be minimally invasive, highly efficient and radiate deep into the tissue to create large zones of active heating. Two metrics are often used to describe the performance of an antenna:
Heating (SAR) pattern – an ideal heating pattern might be perfectly spherical around the end of the antenna, but most interstitial antennas create a more ellipsoidal or tear-drop-shaped pattern. Heating pattern changes with antenna geometry and many designs sacrifice invasiveness for improved heating pattern (Figure 8).
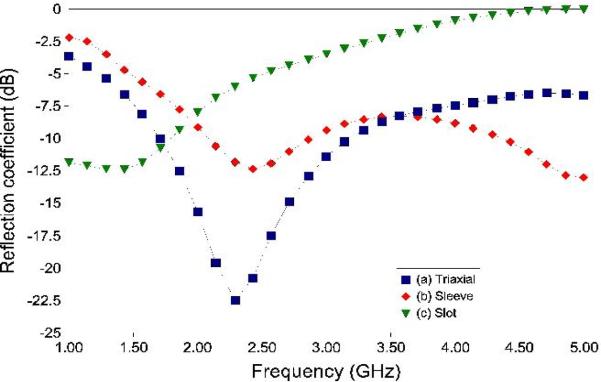
Reflection coefficient – the lower the reflection coefficient, the more efficiently the antenna transfers power into the tissue. Resonant designs such as monopolar, dipolar or triaxial antennas tend to be very efficient (Figure 9).
Generally, a balance exists between efficiency, heating pattern and invasiveness. For this reason, some antenna designs may be more suited to open or laparoscopic procedures, where invasiveness is not as much of a concern.
Figure 8.
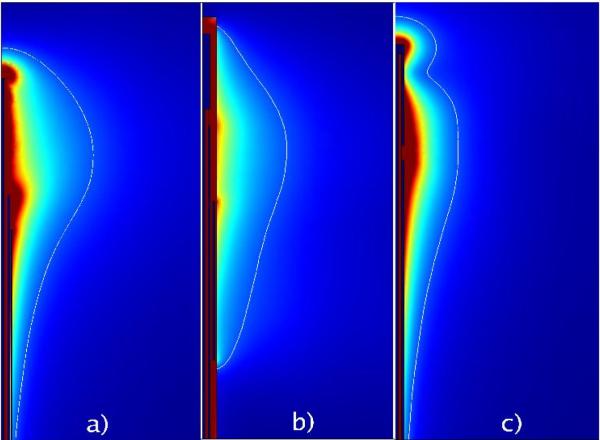
Comparison of the simulated electric fields produced by three microwave ablation antennas: a) a 17-gauge triaxial antenna (14), b) a 10-gauge floating sleeve antenna (16) and c) a 17-gauge coaxial slot antenna (8). The triaxial antenna sacrifices some localized heating for higher efficiency, while the sleeve antenna sacrifices invasiveness to reduce heating along the feedline.
Figure 9.
Comparison of simulated reflection coefficients of the antennas in Figure 8. The triaxial antenna is 99+ % efficient, the sleeve antenna is ~ 93 % efficient and the slot antenna is ~ 70 % efficient at the operating frequency (2.45 GHz). Tissue properties caused by heating and ablation will change the reflection coefficient of each antenna. Ideally, an ablation antenna would have a low reflection coefficient for a broad frequency range at and below the operating frequency to counteract tissue property changes.
From a clinical perspective, antenna design is not the only factor that determines the final zone of ablation. Changes in tissue properties that occur during an ablation tend to alter the impedance matching of an antenna and the way in which EM energy propagates through the tissue. For this reason, antenna performance at the beginning of an ablation is often very different than at the end of the ablation. Other factors, such as nearby blood vessels, airways, bile ducts, bowel, ureters, etc. will also influence the actual antenna performance. Finally, thermal conduction from the active heating zone – which is independent of antenna geometry – has the most impact on the final ablation zone when ablation times longer than 6–7 min are used (Schramm and Haemmerich, presented at the World Congress of Interventional Oncology, 2006).
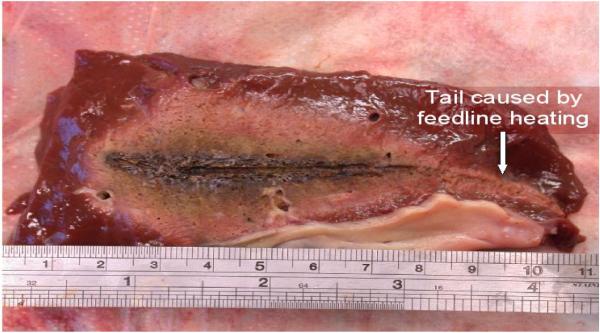
One major technical hurdle for microwave ablation is in antenna diameter. There is an inherent power handling-invasiveness trade-off with all microwave ablation antennas but it is known that increasing power is the easiest way to increase ablation zone size with a given antenna design (14, 23, 24). As power is increased, more heating of the feeding structure occurs, which can lead to unwanted ablation of the insertion track (Figure 10). One solution that is being researched to improve the power handling ability of small-diameter antennas is active cooling. Unlike cooled RF electrodes, which are designed to prevent char buildup at the electrode-tissue interface, microwave antennas are cooled to prevent overheating of the feeding structure and increase the power handling of the antenna. In a recent study, Kuang et al. found that cooling a 14-gauge antenna reduced unwanted heating along the feeding structure and allowed higher powers to be used, resulting in larger zones of ablation than a non-cooled antenna (25).
Figure 10.
Example of feedline heating created by uncooled antennas operated at high powers.
Conclusions
Three major components make up a microwave ablation system: the microwave generator, power distribution system and applicator antenna. Unlike RF ablation systems, microwave generators are capable of powering several antennas from the same source without the need for switching or bipolar techniques. The power distribution system may be a simple cable to transfer power directly to the antenna, or may contain components to control the phase, amplitude and duty cycle of multiple antennas. The antenna transfers energy into the tissue and could contain one of several designs, each having its own benefits and drawbacks for clinical applications. How a microwave ablation system will need to be configured for practical clinical use will depend on user feedback and clinical research with systems now coming into the marketplace.
Table 1.
Tissue properties at RF and microwave frequencies [1].
| Units | Liver | Lung (aerated) | Kidney | Bone | |
|---|---|---|---|---|---|
| Conductivity (480 kHz) | S/m | 0.148 | 0.122 | 0.226 | 0.022 |
| Dielectric constant (2.45 GHz) | 43.3 | 20.5 | 52.8 | 11.4 | |
| Conductivity (2.45 GHz) | S/m | 1.68 | 0.804 | 2.43 | 0.394 |
| Wavelength (2.45 GHz) | cm | 1.8 | 2.5 | 1.5 | 3.6 |
| Thermal conductivity | W/m K | 0.564 | 0.302 | 0.54 | 0.4 |
| Density | kg/m3 | 1050 | 260 | 1050 | 1990 |
| Specific heat capacity | J/kg K | 3600 | 2500 | 3890 | 1300 |
| Perfusion rate | ml/min kg | 1000 | 200 | 3000–4000 | 50 |
REFERENCES
- 1.Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 2.Solbiati L, Tiziana I, Michela B, Luca C. Radiofrequency Ablation of Liver Metastases of Colorectal Origin with Intention to Treat: Local Response Rate and Long-term Survival Over 7-year Follow-up. Radiological Society of North America Annual Meeting.2006. [Google Scholar]
- 3.Rose SC, Thistlethwaite PA, Sewell PE, Vance RB. Lung cancer and radiofrequency ablation. J Vasc Interv Radiol. 2006;17:927–51. doi: 10.1097/01.RVI.0000222707.44902.66. quiz 951. [DOI] [PubMed] [Google Scholar]
- 4.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 5.Callstrom MR, Charboneau JW. Percutaneous ablation: safe, effective treatment of bone tumors. Oncology (Williston Park) 2005;19(11 Suppl 4):22–26. [PubMed] [Google Scholar]
- 6.Haemmerich D, Lee FT, Jr, Schutt DJ, et al. Large-volume radiofrequency ablation of ex vivo bovine liver with multiple cooled cluster electrodes. Radiology. 2005;234:563–8. doi: 10.1148/radiol.2342031122. [DOI] [PubMed] [Google Scholar]
- 7.Duck FA. Physical properties of tissue: A comprehensive reference book. Academic Press; London: 1990. [Google Scholar]
- 8.Ito K, Hyodo M, Shimura M, Kasai H. Thin applicator having coaxial ring slots for interstitial microwave hyperthermia. Ant Prop Soc Int Sym. 1990;3:1233–36. [Google Scholar]
- 9.Hurter W, Reinbold F, Lorenz WJ. A dipole antenna for interstitial microwave hyperthermia. IEEE Trans Microwave Theory Tech. 1991;39:1048–54. [Google Scholar]
- 10.Labonte S, Blais A, Legault SR, Ali HO, Roy L. Monopole antennas for microwave catheter ablation. IEEE Trans Microwave Theory Tech. 1996;44:1832–1840. [Google Scholar]
- 11.Schaller G, Erb J, Engelbrecht R. Field simulation of dipole antennas for interstitial microwave hyperthermia. IEEE Trans Microwave Theory Tech. 1996;44:887–95. [Google Scholar]
- 12.Lin JC, Wang YJ. The cap-choke catheter antenna for microwave ablation treatment. IEEE Trans Biomed Eng. 1996;43:657–60. doi: 10.1109/10.495286. [DOI] [PubMed] [Google Scholar]
- 13.Longo I, Gentili GB, Cerretelli M, Tosoratti N. A coaxial antenna with miniaturized choke for minimally invasive interstitial heating. IEEE Trans Microwave Theory Tech. 2003;50:82–88. doi: 10.1109/TBME.2002.807320. [DOI] [PubMed] [Google Scholar]
- 14.Brace CL, Laeseke PF, van der Weide DW, Lee FT. Microwave ablation with a triaxial antenna: Results in ex vivo bovine liver. IEEE Trans Microw Theory Tech. 2005;53:215–220. doi: 10.1109/TMTT.2004.839308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn H, Lee K. Interstitial antennas tipped with reactive load. IEEE Microw Wireless Comp Lett. 2005;15:83–85. [Google Scholar]
- 16.Yang D, Bertram JM, Converse MC, et al. A floating sleeve antenna yields localized hepatic microwave ablation. IEEE Trans Biomed Eng. 2006;53:533–537. doi: 10.1109/TBME.2005.869794. [DOI] [PubMed] [Google Scholar]
- 17.Brace CL, Laeseke PF, Sampson LA, Frey TM, van der Weide DW, Lee FT., Jr. Microwave Ablation with Multiple Simultaneously Powered Small-gauge Triaxial Antennas: Results from an in Vivo Swine Liver Model. Radiology. 2007;244:151–156. doi: 10.1148/radiol.2441052054. [DOI] [PubMed] [Google Scholar]
- 18.Magin RL, Peterson AF. Noninvasive microwave phased arrays for local hyperthermia: a review. Int J Hyperthermia. 1989;5:429–450. doi: 10.3109/02656738909140470. [DOI] [PubMed] [Google Scholar]
- 19.Camart JC, Dubois L, Fabre JJ, Vanloot D, Chive M. 915 MHz microwave interstitial hyperthermia. Part II: Array of phase-monitored antennas. Int J Hyperthermia. 1993;9:445–454. doi: 10.3109/02656739309005043. [DOI] [PubMed] [Google Scholar]
- 20.Trembly BS, Douple EB, Ryan TP, Hoopes PJ. Effect of phase modulation on the temperature distribution of a microwave hyperthermia antenna array in vivo. International Journal of Hyperthermia. 1994;10:691–705. doi: 10.3109/02656739409022448. [DOI] [PubMed] [Google Scholar]
- 21.Lin JC, Hirai S, Chiang C, Hsu W, Su J, Wang Y. Computer simulation and experimental studies of SAR distributions of interstitial arrays of sleeved-slot microwave antennas for hyperthermia treatment of brain tumors. IEEE Transactions on Microwave Theory and Techniques. 2000;48:2191–2198. [Google Scholar]
- 22.Shock SA, Meredith K, Warner TF, et al. Microwave ablation with loop antenna: in vivo porcine liver model. Radiology. 2004;231:143–9. doi: 10.1148/radiol.2311021342. [DOI] [PubMed] [Google Scholar]
- 23.Strickland AD, Clegg PJ, Cronin NJ, et al. Experimental study of large-volume microwave ablation in the liver. Br J Surg. 2002;89:1003–1007. doi: 10.1046/j.1365-2168.2002.02155.x. [DOI] [PubMed] [Google Scholar]
- 24.Hines-Peralta AU, Pirani N, Clegg P, et al. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239:94–102. doi: 10.1148/radiol.2383050262. [DOI] [PubMed] [Google Scholar]
- 25.Kuang M, Lu MD, Xie XY, et al. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna--experimental and clinical studies. Radiology. 2007;242:914–924. doi: 10.1148/radiol.2423052028. [DOI] [PubMed] [Google Scholar]