Abstract
The purpose of this study was to reduce the non-specific renal uptake of Arg-Gly-Asp (RGD)-conjugated alpha-melanocyte stimulating hormone (α-MSH) hybrid peptide through structural modification or L-lysine co-injection. The RGD motif {cyclic(Arg-Gly-Asp-dTyr-Asp)} was coupled to [Cys3,4,10, d-Phe7, Arg11]α-MSH3-13 {(Arg11)CCMSH} through the Arg linker (substituting the Lys linker) to generate a novel RGD-Arg-(Arg11)CCMSH hybrid peptide. The melanoma targeting and pharmacokinetic properties of 99mTc-RGD-Arg-(Arg11)CCMSH were determined in B16/F1 melanoma-bearing C57 mice. The effect of L-lysine co-injection on the renal uptake was determined through the co-injection of L-lysine with 99mTc-RGD-Arg-(Arg11)CCMSH or 99mTc-RGD-Lys-(Arg11)CCMSH. Replacement of the Lys linker with an Arg linker exhibited a profound effect in reducing the non-specific renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH, as well as increasing the tumor uptake of 99mTc-RGD-Arg-(Arg11)CCMSH compared to 99mTc-RGD-Lys-(Arg11)CCMSH. 99mTc-RGD-Arg-(Arg11)CCMSH exhibited high tumor uptake (21.41 ± 3.74% ID/g at 2 h post-injection) and prolonged tumor retention (6.81 ± 3.71% ID/g at 24 h post-injection) in B16/F1 melanoma-bearing mice. The renal uptake values of 99mTc-RGD-Arg-(Arg11)CCMSH were 40.14-64.08% of those of 99mTc-RGD-Lys-(Arg11)CCMSH (p<0.05) at 0.5, 2, 4 and 24 h post-injection. Co-injection of L-lysine was effective in decreasing the renal uptakes of 99mTc-RGD-Arg-(Arg11)CCMSH by 27.7% and 99mTc-RGD-Lys-(Arg11)CCMSH by 52.1% at 2 h post-injection. Substitution of the Lys linker with an Arg linker dramatically improved the melanoma uptake and reduced the renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH, warranting the further evaluation of 188Re-labeled RGD-Arg-(Arg11)CCMSH as a novel MC1 receptor-targeting therapeutic peptide for melanoma treatment in the future.
Keywords: Arg-Gly-Asp, Alpha-melanocyte stimulating hormone hybrid peptide, Melanoma imaging and therapy
1. Introduction
High mortality of malignant melanoma is associated with the occurrence of metastatic melanoma due to its aggressiveness and resistance to current chemotherapy and immunotherapy regimens. 1 The survival times for patients with metastatic melanoma average 3-15 months. 2,3 Therefore, it is highly desirable to develop novel and effective therapeutic approaches for melanoma treatment. G protein-coupled melanocortin-1 (MC1) receptor is a distinct molecular target for developing diagnostic and therapeutic peptides 4-11 for melanoma due to its over-expression on human and mouse melanoma cells. 12-16 MC1 receptor-targeting α-MSH peptides have been used to specifically deliver the therapeutic radionuclides into melanoma cells for treatment. 9-11 Promising preclinical therapeutic results of 212Pb-, 177Lu-, 188Re-labeled metal-cyclized α-MSH peptides 9-11 in B16/F1 melanoma-bearing C57 mice demonstrated the potential of peptide-targeted radionuclide therapy as a novel effective approach for melanoma treatment.
Recently, we have reported a study on using a novel RGD-conjugated α-MSH hybrid peptide {RGD-Lys-(Arg11)CCMSH} for melanoma therapy. 17 The (Arg11)CCMSH peptide was used as an effective delivery vehicle to specifically transport the RGD motif (apoptosis inducer) into melanoma cells to induce apoptosis. Specifically, the RGD motif was conjugated to the (Arg11)CCMSH through the Lys linker to generate the RGD-Lys-(Arg11)CCMSH hybrid peptide. Technetium-99m-labeled RGD-Lys-(Arg11)CCMSH displayed rapid MC1 receptor-mediated internalization in B16/F1 melanoma cells. Furthermore, 99mTc-RGD-Lys-(Arg11)CCMSH exhibited rapid and high melanoma uptake (14.83 ± 2.94% ID/g at 2 h post-injection) and prolonged tumor retention (7.59 ± 2.04% ID/g at 24 h post-injection) in B16/F1 melanoma-bearing C57 mice. 17 RGD-Lys-(Arg11)CCMSH showed remarkable clonogenic cytotoxic effect in B16/F1 melanoma cells. Since 99mTc and 188Re are matched-pair diagnostic and therapeutic radionuclides sharing similar coordination chemistry, these results highlighted the potential of 188Re-labeled RGD-conjugated α-MSH hybrid peptides as a novel class of hybrid peptides for melanoma therapy.
Despite the high melanoma uptake, 99mTc-RGD-Lys-(Arg11)CCMSH also exhibited high non-specific renal uptake in B16/F1 melanoma-bearing mice in our previous report. 17 Reduction of non-specific renal uptake will facilitate further evaluation of the α-MSH hybrid peptide for melanoma therapy in melanoma-bearing mice. Both structural modification and co-injection of L-lysine were effective in decreasing the renal uptake of 188Re-labeled metal-cyclized α-MSH peptide. 15 For instance, the substitution of Lys with Arg at the 11th position of 188Re-(Arg11)CCMSH peptide dramatically reduced its renal uptake by 50% in B16/F1 melanoma-bearing mice. 15 Hence, we substituted the Lys linker in RGD-Lys-(Arg11)CCMSH with an Arg linker to examine whether such replacement could exhibit similar profound effect in reducing the renal uptake in this study. The RGD motif {cyclic(Arg-Gly-Asp-dTyr-Asp)} was coupled to [Cys3,4,10, D-Phe7, Arg11]α-MSH3-13 {(Arg11)CCMSH} through the Arg linker to generate a new RGD-Arg-(Arg11)CCMSH hybrid peptide. We determined melanoma targeting and pharmacokinetic properties, single photon emission computed tomography (SPECT)/CT imaging of 99mTc-RGD-Arg-(Arg11)CCMSH in B16/F1 melanoma-bearing mice. Meanwhile, we determined the effect of L-lysine co-injection on the renal uptakes of 99mTc-RGD-Arg-(Arg11)CCMSH and 99mTc-RGD-Lys-(Arg11)CCMSH in B16/F1 melanoma-bearing mice. Furthermore, we determined clonogenic cytotoxic effect of RGD-Arg-(Arg11)CCMSH in B16/F1 melanoma cells.
2. Materials and methods
2.1. Chemicals and reagents
Amino acids and resin were purchased from Advanced ChemTech Inc. (Louisville, KY) and Novabiochem (San Diego, CA). 99mTcO4- was purchased from Cardinal Health (Albuquerque, NM). 125I-Tyr2-[Nle4, D-Phe7]-α-MSH {125I-(Tyr2)-NDP-MSH} tracer was obtained from PerkinElmer, Inc. (Shelton, CT) for in vitro competitive binding studies. Cyclo(Arg-Gly-Asp-dPhe-Val) {RGD} peptide was purchased from Enzo Life Sciences (Plymouth Meeting, PA) for peptide blocking studies. All other chemicals used in this study were purchased from Thermo Fischer Scientific (Waltham, MA) and used without further purification. B16/F1 murine melanoma cells were obtained from American Type Culture Collection (Manassas, VA).
2.2. Peptide synthesis
RGD-Arg-(Arg11)CCMSH was synthesized according to our published procedure 17 with slight modification on Sieber amide resin by an Advanced ChemTech multiple-peptide synthesizer (Louisville, KY). Briefly, the linear peptide backbone was synthesized using standard 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry. Fmoc-Arg(pbf) was coupled between the RGD and (Arg11)CCMSH motifs to generate the Arg linker during the synthesis of linear peptide backbone. The protected linear peptide backbone was cyclized and all protecting groups were removed using the reagents described in our published procedure. 17 Finally, the peptide was purified by reverse phase-high performance liquid chromatography (RP-HPLC) and characterized by LC-MS.
2.3. In vitro receptor binding assay
The receptor binding affinity (IC50 value) of RGD-Arg-(Arg11)CCMSH was determined according to our previously published procedure 17 with slight modification. Briefly, B16/F1 cells were incubated at room temperature (25 °C) for 2 h with approximately 40,000 counts per minute (cpm) of 125I-(Tyr2)-NDP-MSH in the presence of increasing concentrations (10-12 to 10-5 M) of RGD-Arg-(Arg11)CCMSH in 0.3 mL of binding medium. The IC50 value of RGD-Arg-(Arg11)CCMSH was calculated using Prism software (GraphPad Software, La Jolla, CA).
2.4. Biodistribution studies
RGD-Arg-(Arg11)CCMSH was radiolabeled with 99mTc via a glucoheptonate transchelation reaction according to our previously published procedure 17 with slight modification. Firstly, the 99mTc7+ (as 99mTcO4- eluent) was easily reduced by SnCl2 to 99mTc5+ (as 99mTcO3+ metal center) at room temperature (25 °C). The 99mTcO3+ metal center was stabilized by glucoheptonate to form the 99mTc-glucoheptonate intermediate at room temperature (25 °C). Then, RGD-Arg-(Arg11)CCMSH was added to compete off glucoheptonate to yield 99mTc-RGD-Arg-(Arg11)CCMSH at 75 °C. The radiolabeled peptide was purified to a single species by Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse phase analytic column (Deerfield, IL). The stability of 99mTc-RGD-Arg-(Arg11)CCMSH was determined by incubation in mouse serum at 37 °C for up to 24 h according to our published procedure. 18
All of the animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. The pharmacokinetics of 99mTc-RGD-Arg-(Arg11)CCMSH was determined in B16/F1 melanoma-bearing C57 female mice (Harlan, Indianapolis, IN). B16/F1 melanoma-baring C57 mice were generated according to our published procedure. 17 Approximately 0.037 MBq of 99mTc-RGD-Arg-(Arg11)CCMSH was injected into each mouse via the tail vein. Groups of 5 mice were sacrificed at 0.5, 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the whole-body weight. To determine the receptor specificity of the tumor uptake at 2 h post-injection, 10 μg (6.1 nmol) of unlabeled NDP-MSH or 3.5 μg (6.1 nmol) of RGD peptide was co-injected with 0.037 MBq of 99mTc-RGD-Arg-(Arg11)CCMSH.
The effects of L-lysine co-injection on the renal uptakes of 99mTc-RGD-Arg-(Arg11)CCMSH and 99mTc-RGD-Lys-(Arg11)CCMSH was examined in B16/F1 melanoma-bearing C57 as well. Two groups of 5 mice were injected with an aqueous mixture of 0.037 MBq of 99mTc-RGD-Arg-(Arg11)CCMSH and 15 mg of L-lysine or an aqueous mixture of 0.037 MBq of 99mTc-RGD-Lys-(Arg11)CCMSH and 15 mg of L-lysine, respectively. The mice were sacrificed at 2 h post-injection, and tumors and kidneys were harvested, weighed and counted.
2.5. Imaging melanoma with 99mTc-RGD-Arg-(Arg11)CCMSH
Approximately 6.1 MBq of 99mTc-RGD-Arg-(Arg11)CCMSH was injected into a B16/F1 melanoma-bearing C57 mouse via the tail vein for melanoma imaging. The mouse was anesthetized with 1.5% isoflurane for small animal SPECT/CT (Nano-SPECT/CT®, Bioscan) imaging 2 h post-injection. It took 9 mins for a whole-body CT scan and 45 mins for the SPECT scan. Reconstructed SPECT and CT data were co-registered using InVivoScope (Bioscan, Washington DC).
2.6. Clonogenic cytotoxicity of RGD-Arg-(Arg11)CCMSH
To determine whether the replacement of the Lys linker with an Arg linker affected the clonogenic cytotoxicity of the hybrid peptide, we examined the clonogenic cytotoxic effect of RGD-Arg-(Arg11)CCMSH in B16/F1 melanoma cells according to our published procedure 17 with slight modification. Briefly, B16/F1 cells in a 6-well plate (200 cells/well) were incubated in the culture medium at 37 °C for 3 h with or without 0.1 μM of RGD-Arg-(Arg11)CCMSH, (Arg11)CCMSH or RGD, respectively. After the incubation, the cells were washed with PBS twice and returned to the culture medium to form colonies. After 6 days, the cells were fixed with methanol:glacial acetic acid (3:1), stained with hematoxylin and examined under a microscope for survivors. Colonies contained greater than 50 cells were determined to be survivors. The clonogenic survival percentages of peptide-treated groups were normalized taking the clonogenic survival percentage of untreated group (in culture medium) as 100%.
2.7. Statistical method
Student t-tests for unpaired data were conducted to determine the significant differences between the groups in the studies of biodistribution and clonogenic cytotoxicity. Differences at the 95% confidence level (p<0.05) were considered significant.
3. Results
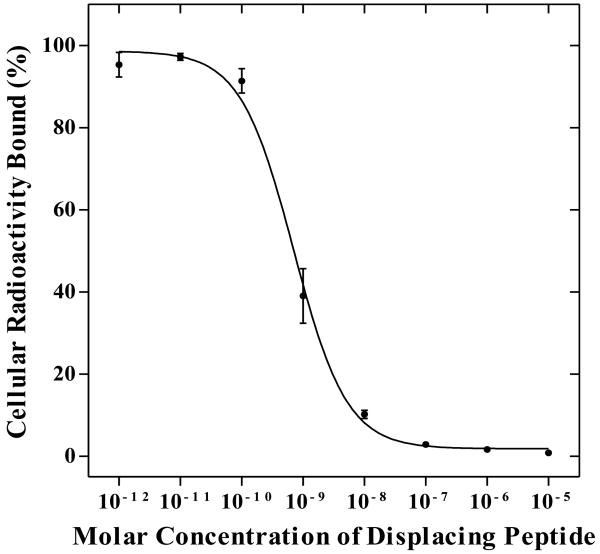
RGD-Arg-(Arg11)CCMSH was synthesized and purified by RP-HPLC. The identity of peptide was confirmed by electrospray ionization mass spectrometry (molecular weight, 2178.2; calculated molecular weight, 2177.9). RGD-Arg-(Arg11)CCMSH displayed greater than 95% purity with 30% overall synthetic yield. Figure 1 illustrates the schematic structure of RGD-Arg-(Arg11)CCMSH, as well as the schematic structure of RGD-Lys-(Arg11)CCMSH for comparison. The competitive binding curve of RGD-Arg-(Arg11)CCMSH is shown in Figure 2. The IC50 value of RGD-Arg-(Arg11)CCMSH was 0.7 nM in B16/F1 cells. The peptide was readily labeled with 99mTc in greater than 95% radiolabeling yield. 99mTc-RGD-Arg-(Arg11)CCMSH was completely separated from its excess non-labeled peptide by RP-HPLC. The retention times of 99mTc-RGD-Arg-(Arg11)CCMSH and its non-labeled RGD-Arg-(Arg11)CCMSH were 13.2 and 12.4 min, respectively. 99mTc-RGD-Arg-(Arg11)CCMSH was stable in mouse serum at 37 °C for 24 h.
Figure 1.
Schematic Structures of RGD-Arg-(Arg11)CCMSH and RGD-Lys-(Arg11)CCMSH.
Figure 2.
The competitive binding curve of RGD-Arg-(Arg11)CCMSH in B16/F1 melanoma cells. The IC50 value of RGD-Arg-(Arg11)CCMSH was 0.7 nM.
The melanoma targeting and pharmacokinetic properties of 99mTc-RGD-Arg-(Arg11)CCMSH are shown in Table 1. 99mTc-RGD-Arg-(Arg11)CCMSH exhibited rapid and high tumor uptake in melanoma-bearing mice. The tumor uptake value was 14.09 ± 2.42% ID/g at 0.5 h post-injection. 99mTc-RGD-Arg-(Arg11)CCMSH reached its peak tumor uptake value of 21.41 ± 3.74% ID/g at 2 h post-injection. There was 16.05 ± 2.00% ID/g of the 99mTc-RGD-Arg-(Arg11)CCMSH radioactivity remained in the tumors at 4 h post-injection. The tumor uptake value of 99mTc-RGD-Arg-(Arg11)CCMSH gradually decreased to 6.81 ± 3.71% ID/g at 24 h post-injection. In melanoma uptake blocking studies, the tumor uptake of 99mTc-RGD-Arg-(Arg11)CCMSH with 10 μg (6.1 nmol) of non-radiolabeled NDP-MSH co-injection was only 9.15% of the tumor uptake without NDP-MSH co-injection at 2 h after dose administration (P<0.01), demonstrating that the tumor uptake was specific and MC1 receptor-mediated. Compared to the tumor uptake of 99mTc-RGD-Arg-(Arg11)CCMSH, co-injection of 99mTc-RGD-Arg-(Arg11)CCMSH with 3.5 μg (6.1 nmol) of RGD decreased 21.2% of the tumor uptake value. Whole-body clearance of 99mTc-RGD-Arg-(Arg11)CCMSH was rapid, with approximately 68% of the injected radioactivity cleared through the urinary system by 2 h post-injection (Table 1). Normal organ uptakes of 99mTc-RGD-Arg-(Arg11)CCMSH were generally low (<3.6% ID/g) except for the kidneys after 2 h post-injection. High tumor/blood and tumor/muscle uptake ratios were demonstrated as early as 0.5 h post-injection (Table 1).
Table 1.
Biodistribution of 99mTc-RGD-Arg-(Arg11)CCMSH in B16/F1 melanoma-bearing C57 mice. The data was presented as percent injected dose/gram or as percent injected dose (mean ± SD, n=5).
| Tissue | 0.5 h | 2 h | 4 h | 24 h | 2 h NDP Blockade | 2 h RGD Blockade |
|---|---|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | ||||||
| Tumor | 14.09 ± 2.42 | 21.41 ± 3.74 | 16.05 ± 2.00 | 6.81 ± 3.71 | 1.96 ± 0.71* | 16.88 ± 2.69* |
| Brain | 0.16 ± 0.02 | 0.06 ± 0.02 | 0.04 ± 0.03 | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.04 ± 0.01* |
| Blood | 3.64 ± 0.69 | 0.55 ± 0.47 | 0.71 ± 0.41 | 0.24 ± 0.14 | 0.47 ± 0.29 | 0.04 ± 0.01* |
| Heart | 2.36 ± 0.37 | 0.73 ± 0.27 | 0.53 ± 0.19 | 0.34 ± 0.07 | 0.61 ± 0.21 | 0.55 ± 0.22 |
| Lung | 7.55 ± 1.29 | 2.05 ± 0.52 | 1.01 ± 0.41 | 0.76 ± 0.14 | 1.42 ± 0.31* | 1.21 ± 0.50* |
| Liver | 4.21 ± 1.04 | 3.58 ± 1.08 | 4.93 ± 0.38 | 1.89 ± 0.36 | 2.87 ± 0.34 | 2.30 ± 0.22* |
| Skin | 4.55 ± 1.01 | 1.29 ± 0.32 | 0.82 ± 0.22 | 0.75 ± 0.13 | 1.26 ± 0.14 | 0.95 ± 0.38 |
| Spleen | 2.73 ± 0.80 | 1.17 ± 0.57 | 1.75 ± 0.55 | 1.45 ± 0.37 | 1.53 ± 0.12 | 0.67 ± 0.38 |
| Stomach | 4.30 ± 1.78 | 2.75 ± 1.71 | 3.44 ± 0.83 | 0.47 ± 0.17 | 2.40 ± 0.49 | 2.03 ± 1.39 |
| Kidneys | 37.22 ± 4.72 | 43.01 ± 8.14 | 36.65 ± 11.68 | 16.16 ± 4.01 | 34.79 ± 8.46 | 38.48 ± 7.45 |
| Muscle | 1.79 ± 1.14 | 0.21 ± 0.12 | 0.32 ± 0.25 | 0.25 ± 0.05 | 0.50 ± 0.12* | 0.28 ± 0.20 |
| Pancreas | 1.02 ± 0.36 | 0.36 ± 0.20 | 0.37 ± 0.12 | 0.19 ± 0.09 | 0.37 ± 0.22 | 0.32 ± 0.17 |
| Bone | 2.01 ± 0.19 | 0.57 ± 0.15 | 0.97 ± 0.22 | 0.90 ± 0.39 | 0.98 ± 0.39 | 0.52 ± 0.42 |
| Percent injected dose (%ID) | ||||||
| Intestines | 3.19 ± 0.22 | 2.57 ± 0.58 | 2.62 ± 0.57 | 1.01 ± 0.11 | 2.34 ± 0.52 | 1.79 ± 0.58 |
| Urine | 43.10 ± 5.62 | 67.81 ± 6.28 | 75.13 ± 3.61 | 88.33 ± 1.64 | 76.85 ± 3.01 | 71.79 ± 5.07 |
| Uptake ratio of tumor/normal tissue | ||||||
| Tumor/Blood | 3.87 | 38.93 | 22.61 | 28.38 | 4.17 | 422.00 |
| Tumor/Kidneys | 0.38 | 0.50 | 0.44 | 0.42 | 0.06 | 0.44 |
| Tumor/Lung | 1.87 | 10.44 | 15.89 | 8.96 | 1.38 | 13.95 |
| Tumor/Liver | 3.35 | 5.98 | 3.26 | 3.60 | 0.68 | 7.34 |
| Tumor/Muscle | 7.87 | 101.95 | 50.16 | 27.24 | 3.92 | 60.29 |
p<0.05, significance comparison between 99mTc-RGD-Arg-(Arg11)CCMSH with or without peptide blockade at 2 h post-injection.
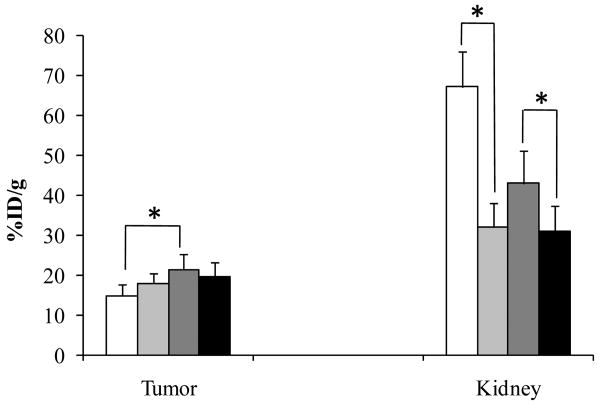
The renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH reached its peak value of 43.01 ± 8.14% ID/g at 2 h post-injection. The renal uptake decreased to 16.16 ± 4.01% ID/g at 24 h post-injection. The effects of L-lysine co-injection on the renal and tumor uptakes of 99mTc-RGD-Lys-(Arg11)CCMSH or 99mTc-RGD-Arg-(Arg11)CCMSH at 2 h post-injection are presented in Figure 3. Co-injection of 15 mg of L-lysine significantly (p<0.05) reduced the renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH from 43.01 ± 8.14% ID/g to 31.10 ± 6.42% ID/g without affecting the tumor uptake, as well as significantly (p<0.05) decreased the renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH from 67.12 ± 8.79% ID/g to 32.20 ± 5.98% ID/g without affecting the tumor uptake at 2 h post-injection.
Figure 3.
Effect of L-lysine co-injection on the tumor and kidney uptakes of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH at 2 h post-injection. The white (□) and light grey ( ) columns represented the tumor and renal uptakes of 99mTc-RGD-Lys-(Arg11)CCMSH with or without L-lysine co-injection. The heavy grey (
) columns represented the tumor and renal uptakes of 99mTc-RGD-Lys-(Arg11)CCMSH with or without L-lysine co-injection. The heavy grey ( ) and black (■) columns represented the tumor and renal uptakes of 99mTc-RGD-Arg-(Arg11)CCMSH with or without L-lysine co-injection. L-lysine co-injection significantly (*p<0.05) reduced the renal uptakes of 99mTc-RGD-Arg-(Arg11)CCMSH by 27.7% and 99mTc-RGD-Lys-(Arg11)CCMSH by 52.1% at 2 h post-injection without affecting the tumor uptakes. Meanwhile, the tumor uptake value of 99mTc-RGD-Arg-(Arg11)CCMSH was 1.44 times (*p<0.05) the tumor uptake value of 99mTc-RGD-Lys-(Arg11)CCMSH at 2 h post-injection.
) and black (■) columns represented the tumor and renal uptakes of 99mTc-RGD-Arg-(Arg11)CCMSH with or without L-lysine co-injection. L-lysine co-injection significantly (*p<0.05) reduced the renal uptakes of 99mTc-RGD-Arg-(Arg11)CCMSH by 27.7% and 99mTc-RGD-Lys-(Arg11)CCMSH by 52.1% at 2 h post-injection without affecting the tumor uptakes. Meanwhile, the tumor uptake value of 99mTc-RGD-Arg-(Arg11)CCMSH was 1.44 times (*p<0.05) the tumor uptake value of 99mTc-RGD-Lys-(Arg11)CCMSH at 2 h post-injection.
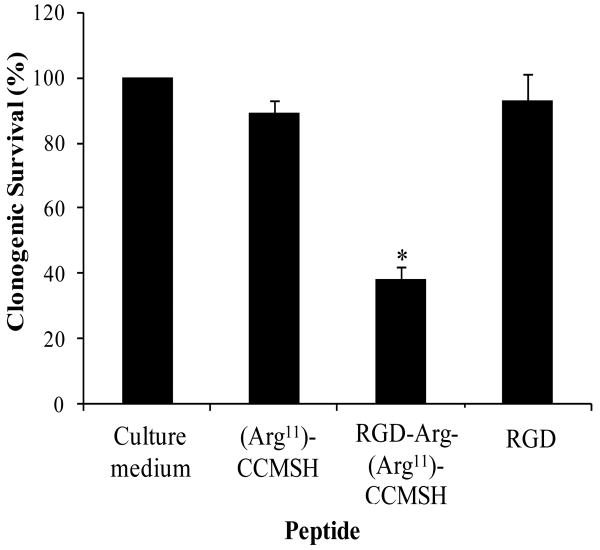
A whole-body SPECT/CT image of a B16/F1 melanoma-bearing mouse at 2 h post-injection is presented in Figure 4. Flank melanoma tumors were visualized clearly by SPECT using 99mTc-RGD-Arg-(Arg11)CCMSH as an imaging probe at 2 h post-injection. The SPECT image of tumor accurately matched its anatomical information from a corresponding CT image. 99mTc-RGD-Arg-(Arg11)CCMSH displayed high tumor to normal organ uptake ratios except for the kidneys, which was consistent with the biodistribution results. Figure 5 illustrates the clonogenic cytotoxic effect of RGD-Arg-(Arg11)CCMSH in B16/F1 melanoma cells. RGD-Arg-(Arg11)CCMSH exhibited remarkable cytotoxic effect in B16/F1 melanoma cells, with 62% decrease (p<0.05) in clonogenic survival in comparison with the untreated group. Although incubation with (Arg11)CCMSH and RGD peptides reduced 11% and 7% of clonogenic survival compared with the untreated group, the differences were not significant (p>0.05).
Figure 4.
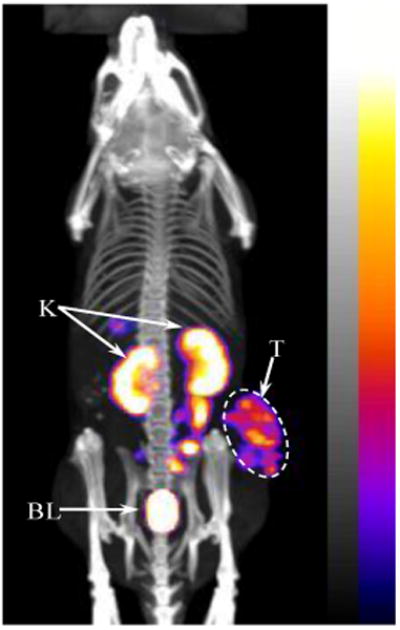
Whole-body SPECT/CT image of 99mTc-RGD-Arg-(Arg11)CCMSH in a B16/F1 melanoma-bearing C57 mouse at 2 h post-injection. Tumor (T), kidneys (K) and bladder (BL) were highlighted with arrows on the image.
Figure 5.
Clonogenic cytotoxic effect of RGD-Arg-(Arg11)CCMSH in B16/F1 melanoma cells. The cells were visually examined under microscope for survival. Colonies contained more than 50 cells were scored as survivors. * p<0.05, significance comparison between RGD-Arg-(Arg11)CCMSH treated cells and untreated cells (culture medium).
4. Discussion
Malignant melanoma is the most lethal form of skin cancer with an increasing incidence. It was predicted that 68,720 cases would be newly diagnosed and 8,650 fatalities would occur in the year 2009. 19 Unfortunately, there is no curative treatment available for metastatic melanoma patients. Clearly, novel and effective treatments are urgently needed to fulfill the desperate need for melanoma treatment. The RGD-containing peptide could induce cell apoptosis after entering the cells without any requirement for integrin-mediated cell clustering or signals, 20 indicating the potential of the RGD motif as an intracellular apoptosis inducer. The RGD motif was coupled to either a somatostatin peptide or a bombesin peptide to generate the hybrid peptides. 21-26 RGD-Lys(111In-DTPA)-Tyr3-Octreotate showed enhanced tumoricidal effects than 111In-DTPA-Tyr3-octreotate and 111In-DTPA-RGD due to elevated tumor cell apoptosis, 21 underscoring the feasibility of using the receptor-targeting peptides to transport the RGD motif (apoptosis inducer) into cancer cells to enhance the synergistic therapeutic effectiveness of the radiolabeled hybrid peptides. Recently, we have developed a novel RGD-Lys-(Arg11)CCMSH hybrid peptide with remarkable clonogenic cytotoxicity in B16/F1 melanoma cells. 17 Meanwhile, 188Re-(Arg11)CCMSH treatment effectively prolonged the mean survival times of mice bearing B16/F1 and TXM13 melanomas. 9 These results highlighted the potential of 188Re-RGD-Lys-(Arg11)CCMSH hybrid peptide as a novel therapeutic peptide for melanoma treatment. However, the relative high non-specific renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH (68.29 ± 14.34 %ID/g at 4 h post-injection) needs to be reduced to facilitate further evaluation of 188Re-labeled α-MSH hybrid peptides for melanoma treatment in melanoma mouse model.
A single amino acid change in the peptide sequence had a profound effect in reducing the non-specific renal uptake of the radiolabeled metal-cyclized α-MSH peptide. 15 The replacement of Lys with Arg at the 11th position of 188Re-(Arg11)CCMSH dramatically reduced its renal uptake by 50% (p<0.05) in B16/F1 melanoma-bearing C57 mice. 15 We demonstrated in our previous work 17 that the Lys linker between the RGD motif and the (Arg11)CCMSH moiety played an important role in the high non-specific renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH. Therefore, we replaced the Lys linker with an Arg linker to determine whether such single amino acid change could substantially decrease the renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH in this study. The replacement of the Lys linker with an Arg linker resulted in greater MC1 receptor binding affinity of the hybrid peptide. New RGD-Arg-(Arg11)CCMSH hybrid peptide displayed 0.7 nM MC1 receptor binding affinity in B16/F1 cells (Figure 2), whereas RGD-Lys-(Arg11)CCMSH exhibited 2.1 nM MC1 receptor binding affinity. 17 Favorable receptor binding results warranted the evaluation of 99mTc-RGD-Arg-(Arg11)CCMSH in B16/F1 melanoma-bearing C57 mice.
The replacement of the Lys linker with an Arg linker dramatically enhanced the tumor uptake of 99mTc-RGD-Arg-(Arg11)CCMSH. The tumor uptake values of 99mTc-RGD-Arg-(Arg11)CCMSH were 1.27, 1.44 and 1.28 times the tumor uptake values of 99mTc-RGD-Lys-(Arg11)CCMSH at 0.5, 2 and 4 h post-injection, respectively. The improved melanoma uptake of 99mTc-RGD-Arg-(Arg11)CCMSH was likely due to its stronger MC1 receptor binding affinity compared to 99mTc-RGD-Lys-(Arg11)CCMSH (0.7 nM vs. 2.1 nM). Meanwhile, the tumor uptake values of 99mTc-RGD-Arg-(Arg11)CCMSH were 1.44 and 2.05 times the tumor uptake values of 99mTc-(Arg11)CCMSH at 4 and 24 h post-injection. 7 Moreover, 99mTc-RGD-Arg-(Arg11)CCMSH displayed prolonged tumor retention. The tumor uptake value at 4 h post-injection (16.05 ± 2.00% ID/g) was 75% of the tumor uptake value at 2 h post-injection (21.41 ± 3.74% ID/g). From the therapeutic perspective, high melanoma uptake and prolonged retention of 99mTc-RGD-Arg-(Arg11)CCMSH could facilitate potential long-lasting synergistic therapeutic effects of apoptosis and targeted radiation from its 188Re-labeled RGD-Arg-(Arg11)CCMSH. Tumor uptake blocking studies with NDP-MSH or RGD in B16/F1 melanoma-bearing mice (Table 1) showed that 90.9% of the tumor uptake of 99mTc-RGD-Arg-(Arg11)CCMSH was blocked by 6.1 nmol of NDP-MSH, whereas 21.2% of the tumor uptake of 99mTc-RGD-Arg-(Arg11)CCMSH was blocked by 6.1 nmol of RGD, indicating that the majority of melanoma uptake of 99mTc-RGD-Arg-(Arg11)CCMSH was MC1 receptor-mediated.
Importantly, the replacement of the Lys linker with an Arg linker significantly (p<0.05) reduced the non-specific renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH as well. The renal uptake values of 99mTc-RGD-Arg-(Arg11)CCMSH were 53.65%, 64.08%, 52.89% and 40.14% of the renal uptake values of 99mTc-RGD-Lys-(Arg11)CCMSH at 0.5, 2, 4 and 24 h post-injection, respectively. Considering the structural difference between 99mTc-RGD-Arg-(Arg11)CCMSH and 99mTc-RGD-Lys-(Arg11)CCMSH, the reduced non-specific renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH was likely associated with the side chain of the Arg linker. The decreased renal uptake and improved tumor uptake of 99mTc-RGD-Arg-(Arg11)CCMSH increased the tumor/kidney uptake ratios of 99mTc-RGD-Arg-(Arg11)CCMSH. The tumor/kidney uptake ratios of 99mTc-RGD-Arg-(Arg11)CCMSH were 2.38, 2.27, 2.44 and 2.21 times the tumor/kidney uptake ratios of 99mTc-RGD-Lys-(Arg11)CCMSH at 0.5, 2, 4 and 24 h post-injection, respectively.
The strategy of infusing basic amino acids such as L-lysine has been successfully employed to decrease the renal uptakes of radiolabeled metal-cyclized α-MSH peptides by shielding the electrostatic interaction between positively-charged peptides and negatively-charged surface of tubule cells. 15,27,28 Hence, 15 mg of L-lysine was co-injected with either 99mTc-RGD-Lys-(Arg11)CCMSH or 99mTc-RGD-Arg-(Arg11)CCMSH at 2 h post-injection to determine whether the non-specific renal uptakes of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH were associated with the electrostatic interaction between the peptide and kidney cells. As we anticipated, co-injection of L-lysine reduced the renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH by 52.1% (p<0.05) and decreased the renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH by 27.7% (p<0.05) without affecting the tumor uptakes, demonstrating that the electrostatic interaction played an important role in the non-specific renal uptakes of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH. The effect of L-lysine co-injection in reducing the renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH demonstrated it as another effective approach to further decrease the renal uptake if needed.
The replacement of the Lys linker with an Arg linker maintained the clonogenic cytotoxicity of RGD-Arg-(Arg11)CCMSH hybrid peptide. RGD-Arg-(Arg11)CCMSH exhibited similar promising cytotoxic effects compared to RGD-Lys-(Arg11)CCMSH in B16/F1 cells. 17 Three-hour incubation with 0.1 μM of RGD-Arg-(Arg11)CCMSH and RGD-Lys-(Arg11)CCMSH decreased 62% and 65% of the clonogenic survival of B16/F1 cells compared to untreated control cells 6 days post the treatment. The cytotoxic effect of RGD-Arg-(Arg11)CCMSH hybrid peptide was due to the apoptotic effect of the RGD motif coupled to the hybrid peptide because neither treatment with 0.1 μM of (Arg11)CCMSH nor 0.1 μM of RGD peptide significantly (p>0.05) reduced the clonogenic survival of B16/F1 cells. The remarkable clonogenic cytotoxic effect of RGD-Arg-(Arg11)CCMSH warrants further evaluation of 188Re-labeled RGD-Arg-(Arg11)CCMSH for melanoma treatment.
Flank B16/F1 melanoma tumors were clearly visualized by SPECT/CT imaging using 99mTc-RGD-Arg-(Arg11)CCMSH as an imaging probe at 2 h post injection (Figure 4), demonstrating the feasibility of using 99mTc-RGD-Arg-(Arg11)CCMSH SPECT imaging to identify the MC1 receptor expression on human melanoma. The combination of using the matched-pair 99mTc-RGD-Arg-(Arg11)CCMSH and 188Re-RGD-Arg-(Arg11)CCMSH could potentially enhance the success of 188Re-RGD-Arg-(Arg11)CCMSH treatment. Imaging patients with 99mTc-RGD-Arg-(Arg11)CCMSH prior to the therapy would not only help the physicians to choose the right patients for effective treatments, but also allow the physicians to determine patient-specific dosimetries. Accurate patient-specific dosimetries would guide the physicians to determine the safe and efficacious doses for the patients. Furthermore, follow-up imaging with 99mTc-RGD-Arg-(Arg11)CCMSH during the therapy duration could monitor the response to the treatment, as well as provide the physicians critical information to modify the therapy regimens accordingly.
5. Conclusions
The replacement of the Lys linker with an Arg linker exhibited a profound effect in reducing the non-specific renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH, as well as increasing the tumor uptake of 99mTc-RGD-Arg-(Arg11)CCMSH. Co-injection of L-lysine was effective in decreasing the renal uptakes of 99mTc-RGD-Arg-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH. Compared to 99mTc-RGD-Arg-(Arg11)CCMSH, improved melanoma uptake and reduced renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH warrants the further evaluation of 188Re-labeled RGD-Arg-(Arg11)CCMSH as a novel MC1 receptor-targeting therapeutic peptide for melanoma treatment in the future.
Acknowledgments
We appreciate Dr. Fabio Gallazzi and Mr. Benjamin M. Gershman for their technical assistance. This work was supported in part by the Southwest Melanoma SPORE Developmental Research Program, the DOD grant W81XWH-09-1-0105 and the NIH grant NM-INBRE P20RR016480. The image in this article was generated by the Keck-UNM Small Animal Imaging Resource established with funding from the W.M. Keck Foundation and the University of New Mexico Cancer Research and Treatment Center (NIH P30 CA118100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marghood AA, Slade J, Salopek TG, Kopf AW, Bart RS, Rigel DS. Cancer. 1995;75:707. doi: 10.1002/1097-0142(19950115)75:2+<707::aid-cncr2820751415>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, Atkins MB, Thompson JA, Coit DG, Byrd D, Desmond R, Zhang Y, Liu PY, Lyman GH, Morabito A. J Clin Oncol. 2001;19:3622. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CM, Buzaid AC, Legha SS. Oncology. 1995;9:1149. [PubMed] [Google Scholar]
- 4.Giblin MF, Wang NN, Hoffman TJ, Jurisson SS, Quinn TP. Proc Natl Acad Sci USA. 1998;95:12814. doi: 10.1073/pnas.95.22.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. J Nucl Med. 2002;43:1699. [PubMed] [Google Scholar]
- 6.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. J Nucl Med. 2004;45:116. [PubMed] [Google Scholar]
- 7.Miao Y, Benwell K, Quinn TP. J Nucl Med. 2007;48:73. [PubMed] [Google Scholar]
- 8.Miao Y, Figueroa SD, Fisher DR, Moore HA, Testa RF, Hoffman TJ, Quinn TP. J Nucl Med. 2008;49:823. doi: 10.2967/jnumed.107.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao Y, Owen NK, Fisher DR, Hoffman TJ, Quinn TP. J Nucl Med. 2005;46:121. [PubMed] [Google Scholar]
- 10.Miao Y, Hylarides M, Fisher DR, Shelton T, Moore H, Wester DW, Fritzberg AR, Winkelmann CT, Hoffman TJ, Quinn TP. Clin Cancer Res. 2005;11:5616. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 11.Miao Y, Shelton T, Quinn TP. Cancer Biother Radiopharm. 2007;22:333. doi: 10.1089/cbr.2007.376.A. [DOI] [PubMed] [Google Scholar]
- 12.Tatro JB, Reichlin S. Endocrinology. 1987;121:1900. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- 13.Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, Eberle AN. Cancer Res. 1989;49:6352. [PubMed] [Google Scholar]
- 14.Chen J, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Cancer Res. 2000;60:5649. [PubMed] [Google Scholar]
- 15.Miao Y, Owen NK, Whitener D, Gallazzi F, Hoffman TJ, Quinn TP. Int J Cancer. 2002;101:480. doi: 10.1002/ijc.10640. [DOI] [PubMed] [Google Scholar]
- 16.Miao Y, Whitener D, Feng W, Owen NK, Chen J, Quinn TP. Bioconjug Chem. 2003;14:1177. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Guo H, Gallazzi F, Padilla RS, Berwick M, Miao Y. Bioconjug Chem. 2009;20:1634. doi: 10.1021/bc9001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aloj L, Panico M, Caraco C, Del Vecchio S, Arra C, Affuso A, Accardo A, Mansi R, Tesauro D, De Luca S, Pedone C, Visentin R, Mazzi U, Morelli G, Salvatore M. Cancer Biotherapy Radiopharm. 2004;19:93. doi: 10.1089/108497804773391739. [DOI] [PubMed] [Google Scholar]
- 19.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. CA Cancer J Clin. 2009;59:225. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 20.Buckley CD, Pilling D, Henriquez NV, Parsonage G, Threlfall K, Scheel-Toellner D, Simmons DL, Akbar AN, Lord JM, Salmon M. Nature. 1999;397:534. doi: 10.1038/17409. [DOI] [PubMed] [Google Scholar]
- 21.Capello A, Krenning EP, Bernard BF, Breeman WA, van Hagen MP, de Jong M. J Nucl Med. 2004;45:1716. [PubMed] [Google Scholar]
- 22.Bernard B, Capello A, van Hagen M, Breeman W, Srinivasan A, Schmidt M, Erion J, van Gameren A, Krenning E, de Jong M. Cancer Biother Radiopharm. 2004;19:173. doi: 10.1089/108497804323071940. [DOI] [PubMed] [Google Scholar]
- 23.Hofland LJ, Capello A, Krenning EP, de Jong M, van Hagen MP. J Nucl Med. 2005;46 1:191S. [PubMed] [Google Scholar]
- 24.Capello A, Krenning EP, Bernard BF, Breeman WA, Erion JL, de Jong M. J Nucl Med. 2006;47:122. [PubMed] [Google Scholar]
- 25.Li ZB, Wu Z, Chen K, Ryu EK, Chen X. J Nucl, Med. 2008;49:453. doi: 10.2967/jnumed.107.048009. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Yan Y, Liu S, Wang F, Chen X. Bioconjug Chem. 2009;20:1016. doi: 10.1021/bc9000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei L, Butcher C, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Lewis JS. J Nucl Med. 2007;48:64. [PubMed] [Google Scholar]
- 28.Miao Y, Fisher DR, Quinn TP. Nucl Med Biol. 2006;33:723. doi: 10.1016/j.nucmedbio.2006.06.005. [DOI] [PubMed] [Google Scholar]