Abstract
A diagnosis of dementia is devastating at any age but diagnosis in younger patients presents a particular challenge. The differential diagnosis is broad as late presentation of metabolic disease is common and the burden of inherited dementia is higher in these patients than in patients with late-onset dementia. The presentation of the common degenerative diseases of late life, such as Alzheimer's disease, can be different when presenting in the fifth or sixth decade. Moreover, many of the young-onset dementias are treatable. The identification of causative genes for many of the inherited degenerative dementias has led to an understanding of the molecular pathology, which is also applicable to later-onset sporadic disease. This understanding offers the potential for future treatments to be tailored to a specific diagnosis of both young-onset and late-onset dementia.
Introduction
Dementia is a major public health concern that is a growing burden owing to an ageing society. However, the high prevalence of dementia in the elderly can overshadow the importance of its occurrence in younger patients. Young-onset dementias can present a substantial diagnostic challenge but can also provide important biological insights that might also be applicable to the more common presentation in older patients. For example, the high prevalence of inherited dementias in younger age-groups has led to the identification of causative genes and subsequent molecular pathology of direct relevance to the more common sporadic disease seen in older patients. The prospect of future treatments targeted at the specific molecular pathological changes of the different dementias makes precise diagnosis essential. In this Review, we discuss the differences between young onset and late onset for the four major dementia diseases: Alzheimer's disease, vascular disease, frontotemporal lobar degeneration (FTLD), and dementia with Lewy bodies. We also suggest a structured approach to the choice of investigations, building on the “dementia plus” concept; this concept exploits the fact that many of the diseases that cause dementia in young adults also cause additional neurological or systemic features, and the identification of these features can aid diagnosis. A diagnosis of dementia often attracts therapeutic nihilism and so we also include examples of treatable dementias that commonly present to young-onset dementia clinics.
Definitions
The term “presenile dementia”, used widely in the published literature until about 10 years ago, is no longer favoured and the terms “young-onset dementia”, “younger-onset dementia”, and “younger people with dementia” are now commonly used. In this paper, we use the term “young-onset dementia”.
Young-onset dementia is conventionally thought to include patients with onset before 65 years of age. This cutoff point is indicative of a sociological partition in terms of employment and retirement age, but this age has no specific biological significance and there is a range of disease features across this arbitrary divide.
The term dementia as currently defined presents two particular challenges. The first is that standard criteria for dementia require that cognitive impairment is sufficiently severe to compromise social and occupational functioning.1 The second is that memory must be specifically impaired. A consequence of the first challenge is a delay in a specific diagnosis of the cause of the dementia. This is evident in the diagnostic criteria for Alzheimer's disease from the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS),2 which first require that the patient fulfils the criteria for dementia. Thus, before a patient with Alzheimer's disease can be diagnosed using these criteria, the disease will be well advanced (table 1). Although patients who present with the mild but consistent cognitive decline that accompanies ageing should not be categorised as having dementia, it is increasingly important to make a specific early diagnosis of the cause of cognitive impairment when appropriate, particularly with the possibility of disease-modifying treatments becoming available in the future. The use of the term mild, or minimum, cognitive impairment to describe patients with cognitive impairment that is not of sufficient severity to fulfil criteria for dementia has found widespread support.3 This term is perhaps most useful when different forms of mild cognitive impairment are recognised (eg, amnestic versus non-amnestic; single versus multi-domain) because these have some value in helping to identify precursor states of specific dementia syndromes.5 In recently proposed criteria for Alzheimer's disease, both the importance of early diagnosis and the role of biomarkers irrespective of severity are acknowledged4 (table 1).
Table 1.
Clinical criteria for dementia and Alzheimer's disease
| Amnestic MCI3 | Dementia DSM-IV-TR1 | Alzheimer's disease NINCDS-ADRDA2 | Alzheimer's disease4* | |
|---|---|---|---|---|
| History | ||||
| Age | · · | · · | 40–90 years | · · |
| Progression | · · | Yes | Yes | Yes |
| Onset | · · | Gradual | Gradual | Gradual |
| Memory complaint | Yes | Yes | Yes | Core: early and significant >6 months |
| Activities of daily living | Normal | Impaired | Impaired | · · |
| Other CNS/general conditions | · · | Absent | Absent | Absent |
| Incoordination/gait disturbance | · · | · · | Absent | Absent |
| Early seizures | · · | · · | Absent | Absent |
| Examination | ||||
| Memory impairment | Core | Core | Core | Core: can be isolated |
| General cognitive function | Normal | One or more of: aphasia, apraxia, agnosia, disturbance in executive function | Deficits in at least two areas of cognition | Can be intact or deficits in areas of cognition other than memory |
| Delirium | · · | Absent | Absent | · · |
| General neurological function | · · | · · | Normal | Normal |
| Investigation | ||||
| MRI | · · | · · | · · | Medial temporal lobe atrophy |
| CSF | · · | · · | · · | Low amyloid, increased tau or phosphorylated tau |
| PET | · · | · · | · · | Bilateral temporoparietal hypometabolism on FDG-PET or abnormal PiB study |
| Genetics | · · | · · | · · | Proven FAD mutation in immediate family |
··=not addressed in that set of criteria. Early criteria for Alzheimer's disease had low specificity and could be used only to diagnose late in the course of the disease. Attempts to refine and accelerate diagnosis included describing a pre-Alzheimer's disease group: amnestic MCI. However, transition from MCI to Alzheimer's disease remained poorly defined. Recently published criteria amalgamate MCI and Alzheimer's disease and are the first to suggest use of biomarkers in diagnosis, reflecting changes in clinical practice.
MCI=mild cognitive impairment. DSM-IV-TR=Diagnostic and Statistical Manual of Mental Disorders, 4th edition text revision. NINCDS=National Institute of Neurological and Communicative Disorders and Stroke. ADRDA=Alzheimer's Disease and Related Disorders Association. PiB=Pittsburgh B compound. FDG=fluorodeoxyglucose. FAD=familial Alzheimer's disease. core=core features.
Probable Alzheimer's disease=core memory impairment plus one or more supportive investigations.
The second challenge, that most criteria for dementia require impairment of episodic memory, has come about because Alzheimer's disease is both the most common cause of dementia in the elderly and the most studied. The concern here is that patients with progressive cognitive decline without memory impairment (eg, posterior variant Alzheimer's disease) or with other focal impairments (eg, semantic dementia) might be excluded from the dementia diagnostic algorithm. Thus, the clinical approach should emphasise analysis of the cognitive and neurological syndrome in any patient who presents with cognitive decline, and this is particularly the case for young-onset dementia, for which the differential diagnosis is often broad.
Epidemiology
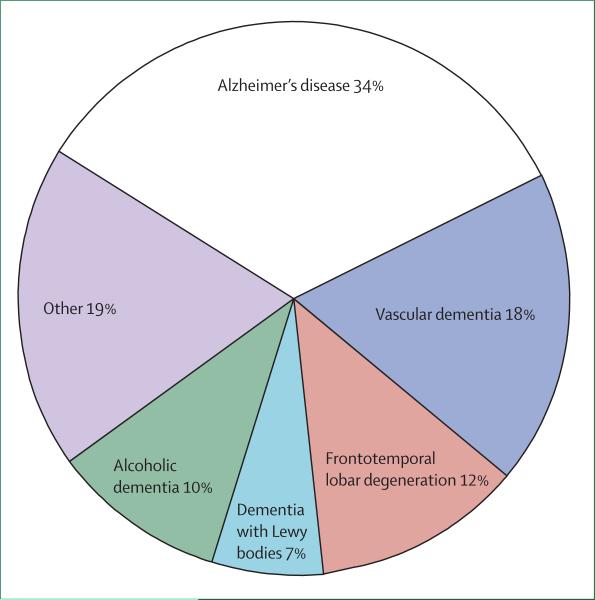
There are few population-based studies on the epidemiology of young-onset dementia and we are not aware of any on cognitive impairment more generally. Harvey and colleagues6 estimated that, in two London boroughs in the UK, the prevalence of dementia with onset between the ages of 30 and 65 years was 54 per 100 000 (95% CI 45–64) and 98 per 100 000 (81–118) between the ages of 45 and 65 years. Alzheimer's disease was the most common single diagnosis followed by vascular disease and FTLD (figure 1). Using a two-stage postal survey, Ikejima and colleagues7 found a broadly similar prevalence of 42 per 100 000 (39–45) in the Ibaraki prefecture in Japan between the ages of 18–65 years. By contrast with the UK study, the most common cause in the Japanese study was vascular disease, followed by Alzheimer's disease. There have been several series of convenience samples (eg, groups of patients attending specialist clinics or those in care registries) reported,8–10 which all lend support to the view that Alzheimer's disease, dementia with Lewy bodies, and vascular cognitive impairment comprise a smaller proportion of cases in younger patients than in the older population. Kelley and colleagues11 analysed a group of patients who were younger than 45 years and found that patients with an onset age of less than 35 years mainly had metabolic causes with substantial overlap with diseases more commonly seen in children and adolescents. By contrast, in individuals who had an onset age of between 35 years and 45 years, most dementias were caused by degenerative diseases.
Figure 1. Epidemiology of young-onset dementia.
Data from the community study of Harvey and colleagues.6
The extent of overlap between the causes of late-onset and young-onset dementia increases with increasing age, with Alzheimer's disease, vascular dementia, FTLD, and dementia with Lewy bodies being the most common causes. The younger the onset of dementia, the more likely it is that the patient has a genetic or metabolic disease.12
The major causes of dementia
Alzheimer's disease, vascular disease, FTLD, and dementia with Lewy bodies are the most common diseases that cause dementia both in the elderly and in younger patients, although not in those who are younger than 35 years.11 However, the clinical features of these diseases in younger patients can differ from those seen at a later age.
Alzheimer's disease
Alzheimer's first patient was only 51 years at the time of presentation and,13 for the next 50 years, Alzheimer's disease was referred to as a presenile dementia. It was the work of Blessed and colleagues14 that led to the recognition of the importance of the disease in the elderly: these authors showed that the brains of patients who had so-called senile dementia when they died had senile plaques and neurofibrillary tangles that were qualitatively the same as those seen in presenile Alzheimer's disease. This finding led to the view that the disease was the same regardless of age and the term Alzheimer's disease has since been used to include all ages. This has been a valuable advance but has masked important differences. One obvious difference is that, compared with elderly individuals, younger patients have fewer comorbidities such as renal disease and heart disease, and lower medication use, which can exacerbate cognitive impairment. Co-existent cerebro vascular disease is also less common in younger patients, whereas there is frequent co-existence of Alzheimer's disease with vascular disease in the older population.
Autosomal dominant familial Alzheimer's disease is also more common in individuals with younger onset; sporadic Alzheimer's disease in individuals younger than 50 years is rare. Although mutations in the amyloid precursor protein (APP) and presenilin-1 and presenilin-2 (PSEN1 and PSEN2) genes, which are associated with familial Alzheimer's disease, are seen in older patients with Alzheimer's disease, most patients present below the age of 65 years.15,16 In general, individuals with familial Alzheimer's disease present with features similar to individuals with later-onset sporadic Alzheimer's disease with prominent episodic memory impairment. This similarity has meant that the insights gained from the studies of pre-manifest and early familial Alzheimer's disease17 can be generalised to the more common older-onset sporadic disease.18 However, unlike patients with sporadic disease, patients with familial Alzheimer's disease generally have myoclonus, relative preservation of naming, and, in some cases, prominent speech production deficits.19 Rarely, some patients have features that are not seen in late-onset sporadic disease. In patients with PSEN1 deletions and some point mutations, spastic paraparesis can be a prominent and even a presenting feature years before the onset of cognitive impairment;20,21 rarely, a cerebellar ataxia is seen.22 Mutations in the prion protein (PRNP) gene might cause a clinical syndrome that closely resembles familial Alzheimer's disease.23
There are also important phenotypic variants within the group of patients with younger-onset sporadic Alzheimer's disease: patients who have non-amnestic deficits collectively comprise about a third of cases who present with young onset compared with about 5% of later-onset presentations.24 Within the range of non-amnestic disorders, presentations with executive behavioural or language dysfunction are well documented; however, the most frequent phenotype is a biparietal or more posterior biparieto-occipital presentation, so-called posterior cortical atrophy, particularly in those with onset between 50 years and 65 years.25–27 The cortical nature of the visual impairment might not be identified, and these patients often have many appointments with opticians and ophthalmologists because of the difficulty they have with locating and perceiving objects. Such patients might not fulfil criteria for Alzheimer's disease, having relatively preserved episodic memory. The association with the ApoEε4 genotype seen with amnestic Alzheimer's disease might not be observed in patients with posterior cortical atrophy, hinting at neurobiological differences between these groups of patients.28
The ApoEε4 genotype might contribute to a more aggressive clinical disease course in younger patients.29 A language variant of Alzheimer's disease, so-called logopenic progressive aphasia, characterised by prolonged word-finding pauses, anomia, and impaired sentence processing, is also more common in younger patients. The extensive anatomical overlap between the logopenic and posterior cortical atrophy syndromes underlines the posterior emphasis of cortical involvement in the younger-onset Alzheimer's disease variants.30
There is an important association between early-onset Alzheimer's disease and Down's syndrome. From a neurobiological perspective, recognition of the role of APP over-production due to increased gene dosage with trisomy 21 was an important clue to the identification of the APP gene and the amyloid hypothesis of Alzheimer's disease pathogenesis. Clinically, people with Down's syndrome have a substantially increased risk of developing younger-onset dementia after the age of 35 years. Alzheimer's disease changes at post mortem are essentially universal, while the prevalence of clinical dementia in individuals with Down's syndrome has been estimated as 15–25% overall and increases steeply with increasing age.31 Clinical assessment is particularly challenging in this population, particularly as indices of executive and social and emotional functioning might be more important than tests of memory in indicating the onset of clinical dementia.
Vascular dementia and vascular cognitive impairment
The term vascular dementia has been problematic for the same reasons as the term dementia, and the term vascular cognitive impairment is preferable.32 Impairment of episodic memory is less prominent in vascular dementia than in Alzheimer's disease, particularly in patients with small vessel disease in whom impairment of executive function and cognitive slowing (subcortical dementia) are more common.33 White matter changes indicative of small vessel disease and lacunar infarcts are commonly seen on MRI scans in elderly individuals and are particularly common in association with Alzheimer's disease, often indicating “mixed dementia”.34 In younger patients there is usually, but not invariably, an association with vascular risk factors but intensive investigation might identify rarer causes, including mitochondrial disease or cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).35 Amyloid angiopathy is important to recognise as some patients might have an inflammatory component that could be responsive to steroids;36 lobar microhaemorrhages seen on T2*-weighted MRI might help detection. APP duplications are commonly associated with a prominent amyloid angiopathy with cerebral haemorrhages and seizures.37 Treatable causes such as cerebral vasculitis are also more commonly found in younger patients than in the elderly.
Frontotemporal lobar degeneration
The broad descriptive term frontotemporal lobar degeneration (FTLD) refers to the regional atrophy of a group of non-Alzheimer's disease degenerative dementias. These dementias are associated with three classic clinical syndromes: behavioural variant FTLD, semantic dementia (a fluent aphasia with loss of word meaning), and progressive non-fluent aphasia (a disorder typified by effortful, non-fluent speech). FTLD accounts for a greater proportion of dementias in younger patients than in elderly patients6 (figure 1), although this might be partly attributable to ascertainment bias: patients with young-onset dementia are more likely to be studied post mortem and elderly patients with FTLD might be misdiagnosed. This bias might also be associated with the high heritability of FTLD: about 20–40% of FTLD cases are familial in series from specialist referral centres.38–41 Among the FTLD syndromes, the behavioural variant is the most heritable and semantic dementia the least heritable, perhaps accounting for recent evidence suggesting that a high proportion of patients with semantic dementia present over the age of 65 years.42 In cases of autosomal dominantly inherited FTLD in which a mutation has been determined, microtubule-associated protein tau (MAPT) and progranulin (GRN) gene mutations are approximately equally represented.43 Age at onset tends to be younger in patients with MAPT-associated FTLD than in patients with GRN-associated FTLD, but onset age can be highly variable even within a family. Other rare gene mutations have recently been described44–46 (table 2). The extent to which age at onset affects FTLD phenotype is unclear: for the behavioural variant, early-onset and later-onset forms all show a similar range of phenotypes.47
Table 2.
Autosomal dominant primary dementia syndromes
| Gene | Chromosome | Onset age (years) | Clinical features | MRI features | Pathological changes | |
|---|---|---|---|---|---|---|
| Alzheimer's disease | PSEN1 >150 mutations | 14 | Usually <60; typically 35–55 | ~70% of familial Alzheimer's disease; usually resembles sporadic Alzheimer's disease; can have behavioural presentation; variants associated with spastic paraparesis and ataxia | Disproportionate symmetrical hippocampal atrophy; can have white matter change | Often prominent tau pathology; mutations above residue 200 associated with increased Aβ deposition; exon 8 and 9 mutations associated with ‘cotton wool’ plaques; can have associated Lewy bodies |
| PSEN2 | 1 | Variable, usually 45–65 | Very rare (association with Volga German ancestry) | Limited information | Can have associated Lewy bodies | |
| APP | 21 | Usually <65; typically 45–60 | ~10–15% of familial Alzheimer's disease; APP duplications recently recognised, associated with early seizures | Disproportionate symmetrical hippocampal atrophy; often prominent white matter change | APP duplications associated with increased Aβ deposition | |
| Prion disease | PRNP | 20 | Highly variable | Rarely presents with an amnestic phenotype resembling Alzheimer's disease; can have rapid course; can be associated with familial fatal insomnia, GSS, or CJD phenotypes | Variable; can have cerebellar atrophy, altered basal ganglia signal on FLAIR | Deposition of amyloid-containing abnormal prion protein; Alzheimer's disease-like phenotype associated with prominent vascular involvement |
| Behavioural variant FTLD, CBS, PSP | MAPT >40 mutations | 17 | 25–65 | 5–15% of FTLD; presentation can be cognitive, parkinsonian disorder, or combination; phenotypic variation within families; can have semantic impairment | Frontotemporal atrophy; can have relatively symmetrical anterior mesial temporal lobe atrophy | Tau-positive inclusions in neurons and glia |
| Behavioural variant FTLD, PA, CBS | GRN >50 mutations | 17 | 35–90 | 5–15% of FTLD; wide phenotypic variation within families, often prominent parietal signs; shorter duration of disease than for MAPT mutations | Frontal, temporal, and parietal atrophy on MRI: often strikingly asymmetric | TARDBP-positive inclusions in neurons |
| Behavioural variant FTLD | VCP | 9 | 20–65 | Very rare; association with inclusion body myopathy and Paget's disease, which can precede cognitive complaints; can have sphincteric disturbance, semantic impairment | Frontal or temporal atrophy (can be asymmetric), can have prominent white matter change | TARDBP-positive intranuclear inclusions in neurons |
| Behavioural variant FTLD | TARDBP (encodes TARDBP) | 1 | 50–75 | Very rare (French families); associated with MND; can have semantic impairment | Unclear (frontotemporal hypoperfusion on SPECT) | Not described (anticipated to have TARDBP-positive inclusions) |
| Behavioural variant FTLD | Not known | 9 | 40–70 | Rare; associated with MND | Frontal atrophy | TARDBP inclusions (MND type) in neurons and glia |
| Behavioural variant FTLD | CHMP2B | 3 | >50 | Very rare (Danish family); can have extrapyramidal features, MND | Generalised atrophy | Ubiquitin-positive, TARDBP-negative cytoplasmic inclusions in neurons |
Key features of the major familial dementias with autosomal dominant inheritance are summarised. Cognitive dysfunction is generally the hallmark of these diseases at presentation; however, other neurological features can supervene during the course of the disease. CBS=corticobasal syndrome. CJD=Creutzfeldt-Jakob disease. FLAIR=fluid-attenuated-inversion-recovery sequence. FTLD=frontotemporal lobar degeneration. GSS=Gerstmann-Straussler-Scheinker syndrome. MND=motor neuron disease. PA=progressive aphasia. PSP=progressive supranuclear palsy. SPECT=single-photon emission computed tomography. APP=amyloid precursor protein. PSEN=presenilin. PRNP=prion protein. MAPT=microtubule-associated protein tau. GRN=granulin. VCP=valosin-containing protein. TARDBP=TAR-DNA binding protein (also known as TDP-43). CHMP=chromatin-modifying protein. Aβ=amyloid β.
FTLD is pathologically heterogeneous and prediction of the underlying pathological process on the basis of the clinical phenotype is generally difficult. Two broad histopathological groupings collectively account for most cases (whether sporadic or inherited): diseases with tau-positive cellular inclusions and diseases with tau-negative, ubiquitin-positive cellular inclusions containing TAR-DNA binding protein (TARDBP; also known as TDP-43). Morphological features of deposition of TARDBP have recently been linked to particular clinical syndromes,48 suggesting a possible pathogenetic framework that might help resolve the current nosological confusion surrounding FTLD. Among the major FTLD subtypes, the characteristic clinico-anatomical syndrome of semantic dementia has the closest pathological correspondence with tau-negative TARDBP pathology seen in more than 75% of cases;42 behavioural variant FTLD has wide anatomical and pathological heterogeneity. Non-fluent speech breakdown, particularly in the context of parkinsonism, is more frequently associated with tau pathology, including progressive supranuclear palsy, corticobasal degeneration and Pick's disease;49 however, these associations are of limited predictive value in individual patients. The presence of lower motor neuron signs are associated with ubiquitin pathology.48
A recently identified small subgroup of patients with fused-in-sarcoma (FUS)-positive, TARDBP-negative ubiquitinated inclusions commonly presents with a behavioural syndrome before the age of 40 years; substantial caudate atrophy can be a consistent feature in this group.50
Dementia with Lewy bodies and Parkinson's disease dementia
Dementia with Lewy bodies, the second most common cause of dementia in the elderly, is typically associated with the development of a cognitive syndrome with frontal/parietal involvement, well formed visual hallucinations, and fluctuations, followed by the development of parkinsonism.51 Dementia with Lewy bodies is characterised pathologically by Lewy bodies, senile plaques, and variable tangle formation. A rare pure form comprises only Lewy bodies with a much younger onset; in a series of nine cases from Japan, eight had an onset before 40 years of age.52 In other patients, dementia is increasingly recognised as a common feature of advancing Parkinson's disease,53 but develops less frequently and with a longer latency in patients with young-onset disease.54 Patients with early-onset parkinsonism are more likely to have an underlying genetic cause. Of these, mutations in the parkin (PARK2) gene are not typically associated with dementia, but α-synuclein triplications55 and mutations in the glucocerebrosidase gene56 can be associated with prominent cognitive impairment, in some cases resembling classic dementia with Lewy bodies.
Dementia plus syndromes
There are many causes of young-onset dementia, and detailed coverage of them all is beyond the scope of this Review. However, the clinical concept of dementia plus syndromes (ie, the dementia syndromes in which cognitive impairment is accompanied by additional neurological or systemic features) can be useful to guide a structured approach to clinical diagnosis and investigations. The pattern of cognitive domains that are impaired can be relatively specific in terms of the underlying molecular pathology; for example, posterior cortical atrophy is most frequently associated with Alzheimer's disease pathology and a verbal semantic memory impairment with ubiquitin-positive, tau-negative inclusions. However, many diseases present with a non-specific memory or frontosubcortical impairment. In these cases, the additional neurological features or systemic features (dementia plus) can be very informative.57 Careful examination is mandatory as presence of one of these additional features narrows the differential diagnosis. For example, the presence of a gaze palsy restricts the differential diagnosis and the additional presence of splenomegaly makes a diagnosis of Niemann-Pick disease type C very likely. These dementia plus syndromes are summarised in panels 1 and 2.
Panel 1: Dementia plus syndromes and associated diseases—neurological features.
Ataxia
Spinocerebellar ataxia (particularly types 2, 12, and 17), paraneoplastic diseases, prion diseases (particularly familial forms and variant CJD), DRPLA (common in Japan), fragile x-associated tremor ataxia syndrome,58 familial British and Danish dementias, mitochondrial disorders, superficial siderosis, neuronal ceroid lipofuscinosis (Kuf's disease), Niemann-Pick disease type C, multiple system atrophy (dementia usually mild, if present), Alexander's disease, and multiple sclerosis
Pyramidal signs
Multiple sclerosis, frontotemporal lobar degeneration with motor neuron disease, Alzheimer's disease (some presenilin mutations), spinocerebellar ataxias, phenylketonuria, familial British and Danish dementias, hereditary spastic paraparesis (SPG4), adrenoleukodystrophy, vanishing white matter disease, polyglucosan body disease, polycystic lipomembranous sclerosing leukoencephalopathy (Nasu-Hakola disease)
Dystonia/chorea
Huntington's disease (and Huntington's disease-like syndromes 1–3), Kuf's disease (characteristic facial dyskinesia), Wilson's disease, neuroacanthocytosis, pantothenate kinase-associated neurodegeneration (neurodegeneration with brain iron accumulation), Lesch-Nyhan syndrome, DRPLA, corticobasal degeneration, neuroferritinopathy, anti-NMDA receptor-mediated limbic encephalitis, variant CJD
Bucco-lingual mutilation
Neuroacanthocytosis, Lesch-Nyhan syndrome
Akinetic-rigid syndrome
Lewy body disease (dementia with Lewy bodies and Parkinson's disease dementia), progressive supranuclear palsy, multiple system atrophy (dementia usually mild, if present), Huntington's disease (particularly juvenile onset), corticobasal degeneration, dementia pugilistica, Wilson's disease, pantothenate kinase-associated neurodegeneration (neurodegeneration with brain iron accumulation), frontotemporal lobar degeneration with parkinsonism-17, Alzheimer's disease (usually advanced)
Peripheral neuropathy
Neuroacanthocytosis, cerebrotendinous xanthomatosis, HIV infection, giant axonal neuropathy, alcohol-related diseases, metachromatic leukodystrophy, porphyria, adrenoleukodystrophy, GM2 gangliosidosis, polyglucosan body disease, Krabbe's disease, sialidosis, Fabry's disease, mitochondrial disorders, spinocerebellar ataxias (particularly type 3)
Myoclonus or early seizures
Prion disease, Alzheimer's disease, Lewy body disease, DRPLA, mitochondrial disorders, Gaucher's disease, GM2 gangliosidosis, neuroserpinopathy, polycystic lipomembranous sclerosing leukoencephalopathy, subacute sclerosing panencephalitis, progressive myoclonic epilepsy syndromes, Kuf's disease, Lafora body disease, sialidosis
Gaze palsy
Niemann Pick disease type C (vertical supranuclear; early downgaze loss), Gaucher's disease (horizontal supranuclear), progressive supranuclear palsy (vertical supranuclear), mitochondrial disorders, spinocerebellar ataxias (particularly type 2), paraneoplastic disorders, Whipple's disease
Deafness
Superficial siderosis, mitochondrial disorders, familial Danish dementia, alpha mannosidosis, sialidosis
Dysautonomia
Lewy body disease, multiple system atrophy, prion disease (fatal familial insomnia), porphyria, adrenoleukodystrophy, anti-NMDA receptor-mediated limbic encephalitis
The dementia plus syndromes describe patterns of cognitive impairment (dementia) plus additional neurological or systemic features that aid investigation and diagnosis of the underlying disease process. This list cannot be comprehensive. Note that vascular disease, structural disorders, and (para) neoplastic disease can be associated with a wide range of presentations. DRPLA=dentatorubral-pallidoluysian atrophy. CJD=Creutzfeldt-Jakob disease.
Diagnoses not to be missed
A diagnosis of dementia tends to attract therapeutic nihilism and, although treatment is only symptomatic for many patients with degenerative dementias, many other non-degenerative diseases that can present with cognitive impairment or in which dementia is the major or only feature can be successfully treated. The following are examples of diseases that can mimic Alzheimer's disease and often present in young-onset dementia clinics.
Sleep apnoea
Patients with sleep apnoea can present with memory complaints and, depending on when they are examined, can show cognitive deficits. This presentation is not uncommon: sleep apnoea was reported in 8% of patients presenting to a young-onset dementia clinic.8 In addition to the typical constellation of snoring, morning headache, and daytime somnolence, more subtle clues can include refractory nocturnal seizures or cerebrovascular disease. The mechanism of cognitive dysfunction might be intermittent hypoxaemia or sleep deprivation.59 Cognitive improvement, particularly in executive functioning, can be achieved by treating patients with obstructive sleep apnoea, although the effects might not always be substantial.
Transient epileptic amnesia
The syndrome of transient epileptic amnesia is characterised by fluctuations in cognitive function associated with episodes of anterograde amnesia or retrograde amnesia for discrete time periods (eg, vacations or other salient events for which the patient has essentially no recollection). Exacerbation of deficits after sleep is a characteristic feature. Other clinical features of temporal lobe seizures often coexist but these are variable and pure amnestic seizures can occur. Temporal lobe spikes can be evident on a standard electroencephalogram (EEG), but prolonged recording might be needed. There might be evidence of hippocampal damage (altered signal or subtle volume loss) on MRI.60 Although treatment with anticonvulsants might prevent seizures, and some patients have improvements in cognition, complete resolution of cognitive complaints is unusual.
Limbic encephalitis
The past few years have seen substantial advances in our understanding of limbic encephalitis, a rare syndrome associated with subacute onset of cognitive impairment attributable to medial temporal lobe, amygdala, insula, and orbitofrontal cortical involvement, often accompanied by seizures, psychiatric features, and temporal lobe signal change on MRI. Once infectious causes, including herpes viruses, have been excluded, an immune-mediated process is most likely. In addition to well established paraneoplastic antibodies (including Hu, Ma2, CV2/CRMP5) targeted against intraneuronal antibodies, a range of antibodies directed against cell-surface antigens (including voltage-gated potassium channels,61 the NMDA receptor,62 the GABAB receptor, 63 and the AMPA glutamate receptor64) are now associated with a limbic encephalitis phenotype. Certain clinical features can give clues to the causative antibody (eg, older onset with hyponatraemia with voltage-gated potassium channels antibodies; younger female adult/child with dyskinesias in anti-NMDA receptor-mediated limbic encephalitis). In cases of immune-mediated limbic encephalitis, an underlying tumour should be sought and treated if identified; best available evidence suggests that tumour-negative cases should be treated promptly with immunosuppression.61,62 Several antibody-negative patients might also respond to immune modulation and it is likely that further causative antibodies are yet to be determined; Hashimoto's encephalopathy might fall into this category, with the involvement of thyroid antibodies as an epiphenomenon.65
Clinical assessment
The clinical assessment of a patient with cognitive impairment should be the same regardless of age but the breadth of the differential diagnosis in younger patients, which includes many rare diseases, demands a structured approach. The first objective is to determine the pattern of cognitive and behavioural deficit. The second objective is to determine the involvement of the nervous system more generally. Finally, the general physical examination should not be overlooked because clues to the cause of cognitive dysfunction might lie outside the nervous system. Clinicians should be aware that a patient might initially be referred to non-cognitive specialist clinics; thus, patients with Kuf's disease or Wilson's disease might present to a movement disorders specialist at a stage when the cognitive impairment is mild or even absent. However, all patients can present with cognitive impairment when other features are either less salient or absent; for example, the cognitive presentation of multiple sclerosis.66
Cognitive assessment
Much information can be obtained from the bedside assessment of different domains of cognitive function, although widely used instruments such as the mini-mental state examination tend to focus on memory, language, and literacy skills, at the expense of non-dominant hemisphere skills. Testing of executive function at the bedside is often difficult to interpret, but reduced verbal fluency might be a useful non-specific indicator of cognitive dysfunction.67 Dyspraxia and apperceptive agnosia are suggestive of organic disease, implicating dominant and non-dominant parietal function, respectively.
A useful bedside distinction can be made between cortical dementia and subcortical dementia. Cortical dementia is characterised by clear errors in specific domains with relative preservation of cognitive speed, and is exemplified by Alzheimer's disease and semantic dementia.68 By contrast, subcortical dementia68 is characterised by profound slowing of cognition, with a frontal dysexecutive syndrome and impairment of memory retrieval, and is exemplified by progressive supranuclear palsy. In general, the cognitive pattern of dementia plus syndromes is predominantly subcortical. The association of cognitive slowing and behavioural changes has long been associated with disorders of the basal ganglia but similar syndromes are seen with diseases that affect subcortical white matter pathways or ascending projections linking the brainstem to the cortex. These ascending systems are vulnerable to many metabolic disturbances and to drugs and disturbances to these systems account for the pattern of cognitive dysfunction most widely seen with systemic disease.
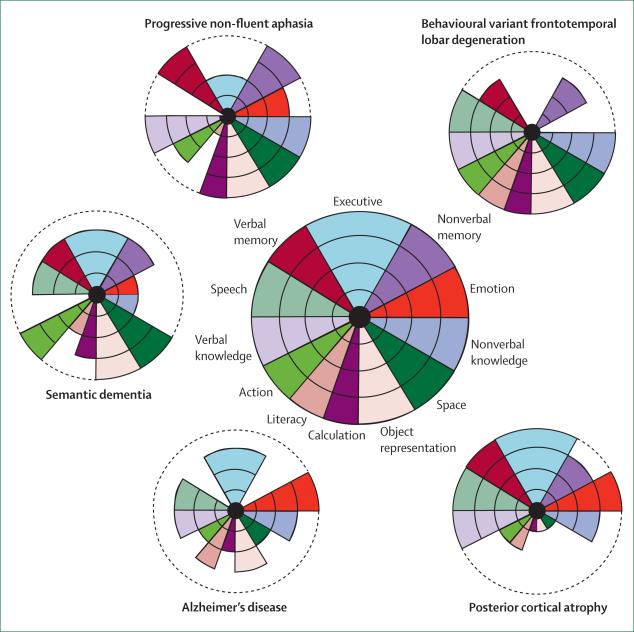
Although bedside cognitive examination can be informative, a formal neuropsychological assessment is necessary to characterise the patient's cognitive syndrome in detail. The neuropsychologist uses tests of graded difficulty with well established, age-related normative data so that cognitive performance can be compared across specific domains. It is important to determine premorbid function as far as possible, by use of details of educational attainment, employment history, and reading skills. However, serial assessment is often necessary to be confident of a decline in cognition: neuropsychometry offers the further advantage of quantification of any interval change in performance, bearing in mind that practice effects lead to an improvement in some scores in healthy individuals (eg, IQ scores). The association of cognitive syndromes with particular degenerative dementias is shown in figure 2.69
Panel 2: Dementia plus syndromes and associated diseases—systemic features.
Cataracts
Myotonic dystrophy, cerebrotendinous xanthomatosis, mitochondrial disorders, familial Danish dementia
Splenomegaly
Niemann-Pick disease type C, Gaucher's disease
Tendon xanthomas
Cerebrotendinous xanthomatosis
Bone cysts
Polycystic lipomembranous sclerosing leucoencephalopathy
Paget's disease
Valosin-associated frontotemporal lobar degeneration
Renal impairment
Fabry's disease, Lesch-Nyhan syndrome, mitochondrial disorders
Hepatic dysfunction
Wilson's disease, Gaucher's disease, mitochondrial disorders
Respiratory failure
Frontotemporal lobar degeneration and motor neuron disease, Perry syndrome, mitochondrial disease (eg, POLG), anti-NMDA receptor-mediated limbic encephalitis
Gastrointestinal dysfunction
Coeliac disease, Whipple's disease, porphyria
Anaemia
Vitamin B12 deficiency, neuroacanthocytosis (McLeod's syndrome), Wilson's disease, Gaucher's disease
Skin lesions
Behcet's disease, systemic vasculitides and connective tissue disease, Fabry's disease
Metabolic or infectious crises
Vanishing white matter disease, Alexander's disease, ornithine transcarbamylase deficiency, alpha mannosidosis, porphyria
Hyponatraemia
VGKC limbic encephalitis
The dementia plus syndromes describe patterns of cognitive impairment (dementia) plus additional neurological or systemic features that aid investigation and diagnosis of the underlying disease process. This list cannot be comprehensive. Note that vascular disease, structural disorders, and (para) neoplastic disease can be associated with a wide range of presentations. POLG=polymerase (DNA directed), gamma. VGKC=voltage-gated potassium channel.
Figure 2. Neuropsychological signatures of young-onset dementia.
The target diagrams (based on McFie69) show neuropsychological profiles in a healthy individual (centre) and in patients with typical profiles from the range of young-onset dementias. Each sector of the target shows a particular cognitive domain. The distance along the radial dimension indicates the level of functioning, and the concentric lines indicate the percentile scores relative to a healthy age-matched population. Normal function in a cognitive domain is shown by colour extending to the perimeter of the target; loss of function is indicated by reduction of the coloured sector that corresponds to that cognitive domain. The neuropsychological profile of a particular disease is evident in the pattern of decline of cognitive functions: the differential loss of function across cognitive domains. These profiles define clinical syndromes but the correspondence with tissue pathology is variable between diseases; for example, whereas semantic dementia is closely associated with TARDBP-positive cellular inclusions, other tissue pathologies such as cerebrovascular disease or dementia with Lewy bodies have more variable clinical syndromes that overlap with those shown here. TARDBP=TAR-DNA binding protein (also known as TDP-43).
Behavioural and psychiatric assessment
The behavioural examination begins during history-taking, with an assessment of the patient's bearing, their interactions with others, and their spontaneous conversation. This assessment is particularly important in patients with behavioural variant FTLD, who might not have deficits on formal cognitive testing but who might make fatuous remarks, perseverate, or have environmental dependency (eg, spontaneously attempting to write with the examiner's pen). Patients with loss of emotional reactivity might appear inexplicably aloof or hostile. The patient's approach to testing might also be informative (impulsive in frontal cortical syndromes, slow in subcortical syndromes). Conversely, patients with Alzheimer's disease generally have a well preserved social appearance but might appear passive during the interview, turning often to their partner to answer questions (the “head turning” sign). In addition to close observation of the patient, it is important to record a history of behavioural and psychiatric (including major mood or psychotic) symptoms, emphasising the need for a corroborating history from an informant who knows the patient well. Some dementias (commonly dementia with Lewy bodies and occasionally FTLD) might present with prominent delusions or other psychotic features;70 conversely, the profound apathy of negative symptom schizophrenia might mimic a degenerative frontal lobe syndrome.71 Some patients who present with a static behavioural syndrome and normal imaging might have non-degenerative FTLD phenocopies.72 Features of REM sleep behaviour disorder should also be sought in the history as this favours a diagnosis of dementia with Lewy bodies.
Neurological examination
The additional neurological features of a dementia plus syndrome are frequently mild. Careful clinical examination is therefore essential to narrow the large range of potential underlying pathological changes (panels 1 and 2). The range and speed of pursuit and saccadic eye movements can be useful in refining the differential diagnosis (eg, to identify the supranuclear gaze palsy of progressive supranuclear palsy, Niemann Pick type C disease, and Gaucher's disease). The presence of a jaw jerk or pout, brisk tendon reflexes, and gait apraxia can indicate an underlying vascular disorder. Fasciculations, which can be the clue to lower motor neuron involvement in some types of FTLD, can be restricted to the deltoids and triceps in the absence of long tract signs. Fine myoclonus of the hands in familial Alzheimer's disease might emerge only when the patient is relaxed or distracted.
General examination
Although systemic ill health (eg, renal or hepatic dysfunction) can be readily apparent from the history or on routine blood tests, a careful general examination is needed to ensure that important clues are not overlooked. Examination of the fundi and blood pressure are mandatory for the identification of vascular disease and vascular risk factors. Inspection of the skin might reveal stigmata of a vasculitic or connective tissue disorder. Subtle splenomegaly in the absence of hepatomegaly is found in adult-onset Niemann-Pick disease type C, and Achilles tendon xanthomata are found in cerebrotendinous xanthomatosis. Systemic findings might also point to an underlying neoplasm; in particular, the breasts and testes should be examined if a paraneoplastic syndrome is a possibility. The patient's history could be suggestive of obstructive sleep apnoea with loud snoring or daytime sleepiness, which can be supplemented by examining for a crowded oropharynx or large collar size.
Laboratory investigations
The investigations commonly undertaken in older patients with dementia also apply to young-onset dementia but the broader differential diagnosis mandates a full investigation. The order of investigation follows the general rule of the simplest test first and the most complex and invasive last, which is the order we have detailed below. The extent of the blood tests will, however, depend on the individual patient. For example, neurogenetics is usually confined to those with a positive family history or additional features such as Paget's disease, which is suggestive of FTLD associated with valosin-containing protein (VCP) mutations (table 2).
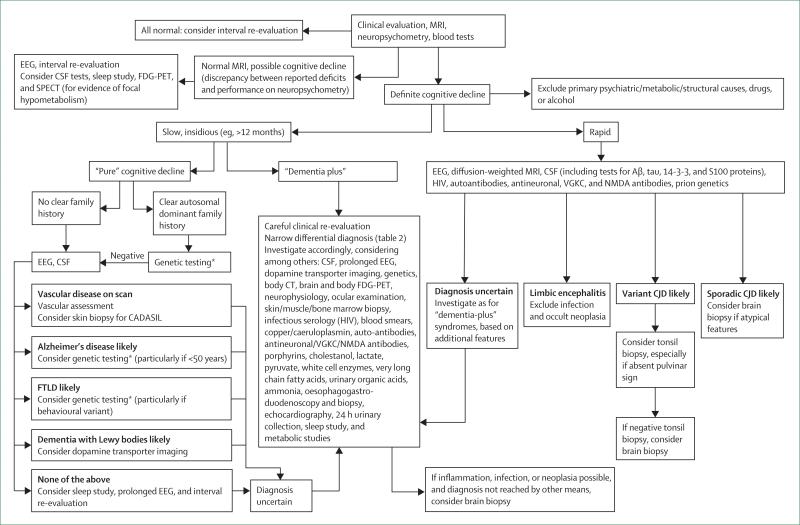
All patients with young-onset dementia should have structural neuroimaging and CSF examination as recommended by the American Academy of Neurology and European Federation of Neurological Societies guidelines.73,74 Decisions about whether to undertake tissue biopies are based on the clinical phenotype and usually confined to the dementia plus syndromes. Cerebral biopsy is occasionally needed to diagnose cerebral vasculitis. A flowchart for investigations is shown in figure 3, but, because of the wide differential diagnosis, this is not exhaustive. When a diagnosis cannot be established, observation and repeat investigations are often informative.
Figure 3. Flow chart for assessment and investigation of young-onset dementia.
This algorithm provides an overview of the diagnostic approach to patients with young-onset dementia. Given the many causes, this can only act as a general guide.*In amnestic young-onset dementia, first-line genetic testing is for APP, PSEN1, PSEN2, and prion. In behavioural cases, first-line testing is for MAPT (particularly if symmetrical atrophy on MRI) and GRN (particularly if asymmetric pattern of atrophy). EEG=electroencephalogram. FDG=fluorodeoxyglucose. SPECT=single photon emission computed tomography. Aβ=amyloid β. VGKC=voltage-gated potassium channel. FTLD=frontotemporal lobar degeneration. CADASIL=cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. CJD=Creutzfeldt-Jakob disease.
Blood tests
Routine haematological and biochemical blood tests are more useful for detecting comorbidity than for establishing the underlying cause, although metabolic encephalopathies are more likely to occur in younger patients than in older patients. The choice of tests depends on the background and age of the patient, and testing for syphilis or HIV is more relevant in certain settings and, although not routine, should always be considered. Auto-antibodies, antineuronal antibodies, and antibodies implicated in limbic encephalitis should be screened for in patients with rapid-onset dementias or in patients with signs of systemic disease. White cell enzyme and very long chain fatty acid assays are relevant for detection of various metabolic disorders that present in early adulthood, whereas multiple blood films might be necessary to substantiate a diagnosis of neuroacanthocytosis.
Neurogenetics
Neurogenetics has transformed our ability to make precise diagnoses and has extended our understanding of the phenotype of many diseases (eg, leading to the recognition that spastic paraparesis can be associated with some PSEN1 mutations; table 2). Genotyping is often labour-intensive and expensive, although it is anticipated to become less so with novel technologies. Moreover, at present, screening is impractical in diseases in which many mutations, some family-specific, might be causative. Thus, for some diseases, it is still preferable to establish the diagnosis on the basis of a metabolic profile (eg, increased copper excretion and low ceruloplasmin in Wilson's disease), and many metabolic disorders can best be determined by direct enzyme assay. Rational use of a neurogenetics service relies on an accurate and complete family history in all patients presenting with young-onset dementia, with the caveat that a family history might not always be apparent because of censoring by premature death, non-paternity, or de-novo mutations.
Imaging
Historically, the main role of neuroimaging was to exclude a space-occupying lesion, and a CT scan is usually adequate for that purpose. However, MRI offers substantial advantages in enabling assessment of signal change and diagnostic patterns of regional brain atrophy. Signal change, particularly in the white matter, best seen with T2 or fluid-attenuated inversion recovery (FLAIR) acquisitions, is an important clue to underlying inflammatory disorders such as multiple sclerosis, vasculitis, limbic encephalitis, or CADASIL (in which there is a characteristic anterior temporal lobe white matter change). Specific patterns of altered signal on FLAIR or diffusion imaging can suggest a prion disease; diffusion imaging is particularly sensitive and should be included if this diagnosis is suspected. MRI sequences sensitive to iron deposition can provide specific clues to several metabolic and genetic disorders (eg, neuroferritinopathy and pantothenate kinase-associated neurodegeneration). Specific patterns of atrophy, best seen with a volumetric MRI acquisition, can be invaluable in differential diagnosis, reflecting the characteristic patterns of selective neuronal vulnerability (eg, bilateral hippocampal atrophy in Alzheimer's disease; asymmetric antero-inferior temporal lobe atrophy in semantic dementia; figure 4). Longitudinal imaging enables changes over time to be visualised and quantified.18 Metabolic and molecular imaging have an emerging role in the assessment of patients with young-onset dementia. Although fluorodeoxyglucose PET imaging (or single photon emission computed tomography imaging) can show temporo-parietal hypometabolism in Alzheimer's disease, this rarely adds to the visualisation of hippocampal atrophy on structural MRI. By contrast, evidence of frontal hypometabolism can be useful to identify patients with early FTLD (particularly behavioural variant FTLD) and minimum atrophy. PET imaging with ligands such as Pittsburgh B compound (PiB) can show the presence of amyloid, and is likely to emerge as an important diagnostic adjunct in Alzheimer's disease75 and amyloid angiopathy. Extensive systemic imaging including CT and whole body PET might be needed to search for primary tumours in suspected paraneoplastic syndromes.
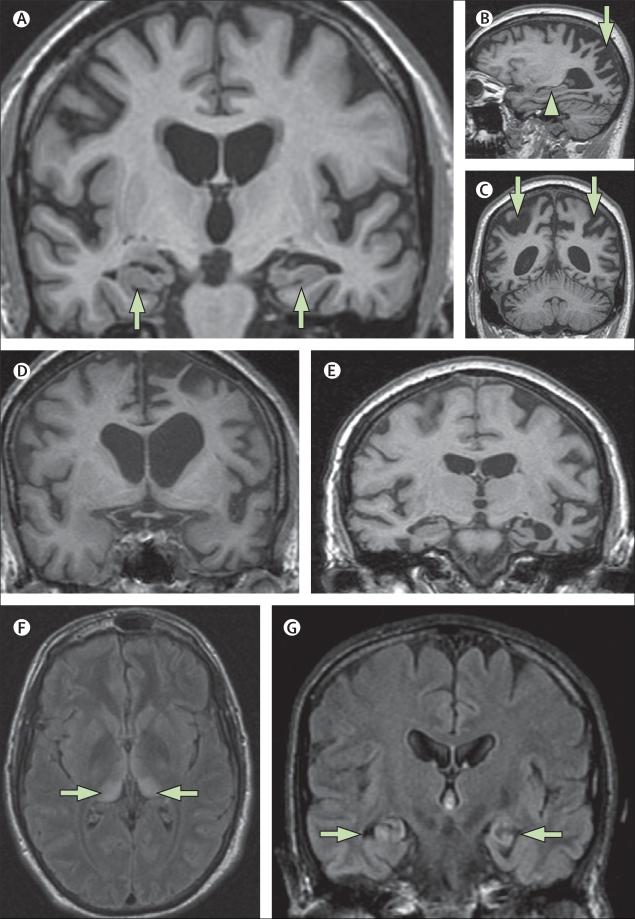
Figure 4. The value of MRI in investigation of young-onset dementia.
(A) Mild Alzheimer's disease in a 60-year-old individual with sporadic Alzheimer's disease (T1-weighted MRI): atrophy of hippocampi (arrows) is the earliest feature in amnestic Alzheimer's disease but hippocampi might appear normal, particularly in younger patients with Alzheimer's disease. (B, C) Posterior cortical atrophy in a 58-year-old individual (T1-weighted MRI). The sagittal view (B) shows a relatively well preserved hippocampus (arrow head); parieto-occipital atrophy (arrows) is seen on sagittal (B) and coronal (C) views. (D) T1-weighted MRI of a 58-year-old individual with progressive non-fluent aphasia who had pathologically proven Pick's disease. (E) T1-weighted MRI of a 63-year-old woman with semantic dementia who had tau-negative, TARDBP-positive inclusions at autopsy. (F) A 21-year-old individual with increased signal bilaterally in the pulvinar (arrows) on axial FLAIR MRI. The pulvinar sign is indicative of variant Creutzfeldt-Jakob disease and is best seen on axial FLAIR (or T2-weighted) MRI where the postero-medial thalami are brighter than the basal ganglia. Variant Creutzfeldt-Jakob disease was subsequently confirmed on tonsillar biopsy. (G) By use of FLAIR MRI, bilateral hippocampal high signal and atrophy is shown in a 57-year-old man with voltage-gated potassium channel antibody limbic encephalitis (arrows). FLAIR=fluid attenuated inversion recovery. TARDBP=TAR-DNA binding protein (also known as TDP-43).
Neurophysiology
EEG has tended to fall out of favour in the assessment of cognitive impairment, but the characteristic EEG changes of periodic complexes in some prion diseases and in subacute sclerosing panencephalitis are valuable.76 Early slowing or loss of alpha rhythm is a feature of Alzheimer's disease but there is relative preservation of this alpha rhythm in the FTLDs.77 The EEG can also be used to detect covert epileptiform changes in amnestic syndromes due to partial seizures. Electromyography and nerve conduction studies can be used to identify neuropathies or myopathy in the dementia plus syndromes and can help to establish anterior horn cell dysfunction in patients with FTLD and motor neuron disease.
CSF
A lumbar puncture is recommended by both the American Academy of Neurology and the European Federation of Neurological Society guidelines on investigation of younger patients with dementia.73,74 Examination of the CSF can help with the identification of inflammatory causes such as multiple sclerosis, vasculitides, and infections and, together with PCR, might help to identify specific chronic infections such as subacute sclerosing panencephalitis, human herpesvirus 6, Whipple's disease, cryptococcosis, and tuberculosis. Increasingly, however, the assay of specific proteins has diagnostic value for particular neurodegenerative diseases. The presence of 14-3-3 protein in the CSF is supportive of a diagnosis of Creutzfeldt-Jakob disease and forms part of diagnostic guidelines for dementia;73 Creutzfeldt-Jakob disease is also characterised by high concentrations of CSF tau. Decreased amyloid β1–42 and increased tau concentrations have good sensitivity and specificity for Alzheimer's disease and are also predictive at the mild cognitive impairment stage,78 and therefore have been incorporated into recent diagnostic criteria for Alzheimer's disease.4
Tissue biopsies
Tissue biopsy might be needed to establish the diagnosis in a few cases, as directed by the clinical features. Skin biopsy (including apocrine sweat glands) might be used to detect abnormal accumulations in storage diseases and in CADASIL, whereas culture of skin fibroblasts can confirm the diagnosis of Niemann-Pick disease type C. Muscle biopsy including histochemistry and respiratory enzyme analysis can confirm a mitochondrial disorder. Tonsillar biopsy can confirm the diagnosis in patients with suspected variant Creutzfeldt-Jakob disease in the absence of a pulvinar sign on MRI.79 Brain biopsy, however, might be necessary in exceptional cases of young-onset dementia if there is suspicion of a treatable (inflammatory or infectious) process,80 the diagnosis cannot be made by other means, or if potentially toxic treatment is contemplated. Unless a focal lesion is present, this is necessarily a “blind” procedure, generally from the non-dominant frontal lobe. A full thickness open biopsy including cortex, white matter, and meninges should be done by a neurosurgical team experienced in the technique. Disposable instruments should be used in cases in which prion risk is thought to be clinically significant. A specific diagnosis can be anticipated in more than 50% of cases and a treatable process in about 10% of cases,81 while the procedure itself carries an about a 10% risk of significant morbidity.
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed with the search terms “young onset”, “early onset”, “presenile“, and “dementia” from 1990 until April, 2010. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
Conclusions
In this Review, we have focused on the clinical approach to young-onset dementia, emphasising the breadth of the differential diagnosis and the need for a structured approach based on all clinical features. The management of young-onset dementia presents challenges that differ from those of older patients. By definition, all are of employable age; many might be the main earner and will often be a parent of young children. The burden of genetic disease in young-onset dementia is also higher than in late-onset dementia. The general issues of management of challenging behaviours, non-pharmacological strategies, and carer support and education all apply in young-onset dementia. However, within the many diseases that can present as young-onset dementia, many demand specific treatment and some curative treatments, necessitating careful investigation. Furthermore, with the dissection of the molecular pathology of the degenerative dementias, specific diagnosis will be essential as disease-modifying therapies become available. Much of our understanding of the pathogenesis of the degenerative dementias has been driven by the identification of genetic mutations causing early-onset familial disease. Thus, the development of transgenic mouse models for Alzheimer's disease followed directly from the discovery of mutations in the APP and PSEN genes. The extent to which early-onset familial Alzheimer's disease is typical of late-onset sporadic disease is an important area of future research.
Acknowledgments
NCF receives research support from the Medical Research Council (G0801306 and G0601846) the National Institutes of Health (U01 AG024904), the Alzheimer Research Trust (ART/RF/2007/1), and the National Institute for Health Research. This work was undertaken at UCL and University College London Hospitals, which received a proportion of funding from the Departments of Health National Institute for Health Research Biomedical Research Centre funding scheme. The Dementia Research Centre is an Alzheimer Research Trust Co-ordinating Centre.
Footnotes
Conflicts of interest
NCF has served on the scientific advisory boards of Alzheimer's Research Forum, Alzheimer's Society and Alzheimer's Research Trust. He holds a patent for QA Box that might accrue revenue. In the past 5 years, his research group has received payment for consultancy or for conducting studies from Abbott Laboratories, Elan Pharmaceuticals, Eisai, Eli Lilly, GE Healthcare, IXICO, Lundbeck, Pfizer Inc, Sanofi-Aventis, and Wyeth Pharmaceuticals. MNR, CJM, JMS, and JDW have no conflicts of interest.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edn. American Psychiatric Association; Washington DC, USA: 2000. text revision (DSM-IV-TR). [Google Scholar]
- 2.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characteristics and outcome. Arch Neurol. 1999;56:303–08. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman H, Jacova C, et al. Research criteria for the diagnosis of alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 5.Gauthier S, Reisberg B, Zaudig M, et al. International Psychogeriatric Association Expert Conference on mild cognitive impairment. Mild cognitive impairment. Lancet. 2006;367:1262–70. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 6.Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatr. 2003;74:1206–09. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikejima C, Yasuno F, Mizukami K, Sasaki M, Tanimukai S, Asada T. Prevalence and causes of early-onset dementia in Japan: a population-based study. Stroke. 2009;40:2709–14. doi: 10.1161/STROKEAHA.108.542308. [DOI] [PubMed] [Google Scholar]
- 8.Panegyres PK, Frencham K. Course and causes of suspected dementia in young adults: a longitudinal study. Am J Alzheimers Dis Other Demen. 2007;22:48–56. doi: 10.1177/1533317506295887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley BJ, Boeve BF, Josephs KA. Cognitive and noncognitive neurological features of young-onset dementia. Dement Geriatr Cogn Disord. 2009;27:564–71. doi: 10.1159/000228258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley BJ, Boeve BF, Josephs KA. Rapidly progressive young-onset dementia. Cogn Behav Neurol. 2009;22:22–27. doi: 10.1097/WNN.0b013e318192cc8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley BJ, Boeve BF, Josephs KA. Young-onset dementia: demographic and etiologic characteristics of 235 patients. Arch Neurol. 2008;65:1502–08. doi: 10.1001/archneur.65.11.1502. [DOI] [PubMed] [Google Scholar]
- 12.Sampson EL, Warren JD, Rossor MN. Young onset dementia. Postgrad Med J. 2004;80:125–39. doi: 10.1136/pgmj.2003.011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer's disease. Lancet. 1997;349:1546–49. doi: 10.1016/S0140-6736(96)10203-8. [DOI] [PubMed] [Google Scholar]
- 14.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 15.Janssen JC, Beck JA, Campbell TA, et al. Early onset familial Alzheimer's disease: mutation frequency in 31 families. Neurology. 2003;60:235–39. doi: 10.1212/01.wnl.0000042088.22694.e3. [DOI] [PubMed] [Google Scholar]
- 16.Kumar-Singh S, Theuns J, Van Broeck B, et al. Mean age-of-onset of familial Alzheimer disease caused by presenilin mutations correlates with both increased Abeta42 and decreased Abeta40. Hum Mutat. 2006;27:686–95. doi: 10.1002/humu.20336. [DOI] [PubMed] [Google Scholar]
- 17.Fox NC, Warrington EK, Seiffer AL, Agnew SK, Rossor MN. Presymptomatic cognitive deficits in individuals at risk of familial Alzheimer's disease. A longitudinal prospective study. Brain. 1998;121:1631–39. doi: 10.1093/brain/121.9.1631. [DOI] [PubMed] [Google Scholar]
- 18.Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci USA. 2002;99:4703–07. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan NS, Rossor MN. Correlating familial Alzheimer's disease gene mutations with clinical phenotype. Biomarkers Med. 2010;4:99–112. doi: 10.2217/bmm.09.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assini A, Terreni L, Borghi R, et al. Pure spastic paraparesis associated with a novel presenilin 1 R278K mutation. Neurology. 2003;60:150. doi: 10.1212/01.wnl.0000040252.43269.83. [DOI] [PubMed] [Google Scholar]
- 21.Verkkoniemi A, Somer M, Rinne JO, et al. Variant Alzheimer's disease with spastic paraparesis: clinical characterization. Neurology. 2000;54:1103–09. doi: 10.1212/wnl.54.5.1103. [DOI] [PubMed] [Google Scholar]
- 22.Anheim M, Hannequin D, Boulay C, Martin C, Campion D, Tranchant C. Ataxic variant of Alzheimer's disease caused by Pro117Ala PSEN1 mutation. J Neurol Neurosurg Psychiatr. 2007;78:1414–15. doi: 10.1136/jnnp.2007.123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mead S, Poulter M, Beck J, et al. Inherited prion disease with six octapeptide repeat insertional mutation—molecular analysis of phenotypic heterogeneity. Brain. 2006;129:2297–317. doi: 10.1093/brain/awl226. [DOI] [PubMed] [Google Scholar]
- 24.Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA. Early-versus late-onset Alzheimer's disease: more than age alone. J Alzheimers Dis. 2010;19:1401–08. doi: 10.3233/JAD-2010-1337. [DOI] [PubMed] [Google Scholar]
- 25.Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45:789–93. doi: 10.1001/archneur.1988.00520310107024. [DOI] [PubMed] [Google Scholar]
- 26.McMonagle P, Deering F, Berliner Y, Kertesz A. The cognitive profile of posterior cortical atrophy. Neurology. 2006;66:331–38. doi: 10.1212/01.wnl.0000196477.78548.db. [DOI] [PubMed] [Google Scholar]
- 27.Tang-Wai, Tang-Wai DF, Graff-Radford NR, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–74. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- 28.Schott JM, Ridha BH, Crutch SJ, et al. Apolipoprotein E genotype modifies the phenotype of Alzheimer disease. Arch Neurol. 2006;63:155–56. doi: 10.1001/archneur.63.1.155. [DOI] [PubMed] [Google Scholar]
- 29.van der Vlies AE, Koedam EL, Pijnenburg YA, Twisk JW, Scheltens P, van der Flier WM. Most rapid cognitive decline in APOE epsilon4 negative Alzheimer's disease with early onset. Psychol Med. 2009;39:1907–11. doi: 10.1017/S0033291709005492. [DOI] [PubMed] [Google Scholar]
- 30.Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndrome associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73:1571–78. doi: 10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieuwenhuis-Mark RE. Diagnosing Alzheimer's dementia in Down syndrome: problems and possible solutions. Res Dev Disabli. 2009;30:827–38. doi: 10.1016/j.ridd.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurol. 2008;7:246–55. doi: 10.1016/S1474-4422(08)70040-1. [DOI] [PubMed] [Google Scholar]
- 33.Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2:538–47. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- 34.Zekry D, Hauw JJ, Gold G. Mixed dementia; epidemiology, diagnosis and treatment. J Am Geriatr Soc. 2002;50:1431–38. doi: 10.1046/j.1532-5415.2002.50367.x. [DOI] [PubMed] [Google Scholar]
- 35.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. CADASIL. Lancet Neurol. 2009;8:643–53. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 36.Kinnecom C, Lev MH, Wendell L, et al. Course of cerebral amyloid angiopathy-related inflammation. Neurology. 2007;68:1411–16. doi: 10.1212/01.wnl.0000260066.98681.2e. [DOI] [PubMed] [Google Scholar]
- 37.Cabrejo L, Guyant-Maréchal L, Laquerrière A, et al. Phenotype associated with APP duplication in five families. Brain. 2006;129:2966–76. doi: 10.1093/brain/awl237. [DOI] [PubMed] [Google Scholar]
- 38.Chow TW, Miller BL, Hayashi VN, Geschwind DH. Inheritance of frontotemporal dementia. Arch Neurol. 1999;56:817–22. doi: 10.1001/archneur.56.7.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–21. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 40.Seelaar H, Kamphorst W, Rosso SM, et al. Distinct genetic forms of frontotemporal dementia. Neurology. 2008;71:1220–26. doi: 10.1212/01.wnl.0000319702.37497.72. [DOI] [PubMed] [Google Scholar]
- 41.Rohrer JD, Guerreiro R, Vandrovcova J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;734:1451–56. doi: 10.1212/WNL.0b013e3181bf997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodges JR, Mitchell J, Dawson K, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;1332:300–06. doi: 10.1093/brain/awp248. [DOI] [PubMed] [Google Scholar]
- 43.Pickering-Brown SM, Rollinson S, Du Plessis D, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008;131:721–31. doi: 10.1093/brain/awm331. [DOI] [PubMed] [Google Scholar]
- 44.Skibinski G, Parkinson NJ, Brown JM, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–08. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 45.Bradshaw CB, Davis RL, Shrimpton AE, et al. Cognitive deficits associated with a recently reported familial neurodegenerative disease: familial encephalopathy with neuroserpin inclusion bodies. Arch Neurol. 2001;58:1429–34. doi: 10.1001/archneur.58.9.1429. [DOI] [PubMed] [Google Scholar]
- 46.Borroni B, Bonvicini C, Alberici A, et al. Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Hum Mutat. 2009;30:E974–83. doi: 10.1002/humu.21100. [DOI] [PubMed] [Google Scholar]
- 47.Borroni B, Agost C, Bellelli G, Padovani A. Is early-onset clinically different from late-onset frontotemporal dementia? Eur J Neurol. 2008;15:1412–15. doi: 10.1111/j.1468-1331.2008.02338.x. [DOI] [PubMed] [Google Scholar]
- 48.Rademakers R, Rovelet-Lecrux A. Recent insights into the molecular genetics of dementia. Trends Neurosci. 2009;032:451–61. doi: 10.1016/j.tins.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–98. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seelaar H, Klijnsma KY, de Koning I, et al. Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol. 2010;257:1432–59. doi: 10.1007/s00415-009-5404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 52.Kosaka K. Diffuse Lewy body disease in Japan. J Neurol. 1990;237:197–204. doi: 10.1007/BF00314594. [DOI] [PubMed] [Google Scholar]
- 53.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 54.Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson's disease. Brain. 2009;132:2947–57. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 55.Fuchs J, Nilsson C, Kachergus J, et al. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology. 2007;68:916–22. doi: 10.1212/01.wnl.0000254458.17630.c5. [DOI] [PubMed] [Google Scholar]
- 56.Goker-Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008;65:1353–57. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamont PJ. Cognitive decline in a young adult with pre-existing developmental delay—what the adult neurologist needs to know. Pract Neurol. 2004;4:70–87. [Google Scholar]
- 58.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–30. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 59.Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 2007;7:161–66. doi: 10.1007/s11910-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 60.Butler CR, Bhaduri A, Acosta-Cabronero J, et al. Transient epileptic amnesia: regi0onal brain atrophy and its relationship to memory deficits. Brain. 2009;132:357–68. doi: 10.1093/brain/awn336. [DOI] [PubMed] [Google Scholar]
- 61.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–12. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 62.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–98. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–34. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol. 2003;60:164–71. doi: 10.1001/archneur.60.2.164. [DOI] [PubMed] [Google Scholar]
- 66.Zarei M, Chandran S, Compston A, Hodges J. Cognitive presentation of multiple sclerosis: evidence for a cortical variant. J Neurol Neurosurg Psychiatry. 2003;74:872–77. doi: 10.1136/jnnp.74.7.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Griffiths TD, Welch JL. How to do it: use a diagnostic neuropsychology service properly. Pract Neurol. 2003;3:170–75. [Google Scholar]
- 68.Albert ML, Feldman RG, Willis AL. The ‘subcortical dementia’ of progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 1974;37:121–30. doi: 10.1136/jnnp.37.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McFie J. Assessment of organic intellectual impairment. Academic Press; London: 1975. [Google Scholar]
- 70.Omar R, Sampson EL, Loy CT, Mummery CJ, Fox NC, Rossor MN, Warren JD. Delusions in frontotemporal lobar degeneration. J Neurol. 2009;256:600–07. doi: 10.1007/s00415-009-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrara M, Freda F, Massa R, Carratelli TJ. Frontal lobe syndrome or adolescent-onset schizophrenia? A case report. Acta Psychiatr Scand. 2006;114:375–77. doi: 10.1111/j.1600-0447.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 72.Kipps CM, Hodges JR, Fryer TD, Nestor PJ. Combined magnetic resonance imaging and positron emission tomography brain imaging in behavioural variant frontotemporal degeneration: refining the clinical phenotype. Brain. 2009;132:2566–78. doi: 10.1093/brain/awp077. [DOI] [PubMed] [Google Scholar]
- 73.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–53. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 74.Waldemar G, Dubois B, Emre M, Scheltens P, Tariska P, Rossor M. Diagnosis and management of Alzheimer's disease and other disorders associated with dementia. The role of neurologists in Europe. European Federation of Neurological Societies. Eur J Neurol. 2000;7:133–44. doi: 10.1046/j.1468-1331.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 75.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 76.Smith SJ. EEG in neurological conditions other than epilepsy: when does it help, what does it add? J Neurol Neurosurg Psychiatry. 2005;76(suppl 2):ii8–12. doi: 10.1136/jnnp.2005.068486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan D, Walters RJ, Sampson EL, Schott JM, Smith SJ, Rossor MN. EEG abnormalities in frontotemporal lobar degeneration. Neurology. 2004;62:1628–30. doi: 10.1212/01.wnl.0000123103.89419.b7. [DOI] [PubMed] [Google Scholar]
- 78.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–93. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 79.Heath CA, Cooper SA, Murray K, et al. Validation of diagnostic criteria for variant Creutzfeldt-Jakob disease. Ann Neurol. 2010;67:761–70. doi: 10.1002/ana.21987. [DOI] [PubMed] [Google Scholar]
- 80.Scolding NJ. Central nervous system vasculitis. Semin Immunopathol. 2009;31:527–36. doi: 10.1007/s00281-009-0183-2. [DOI] [PubMed] [Google Scholar]
- 81.Warren JD, Schott JM, Fox NC, et al. Brain biopsy in dementia. Brain. 2005;128:2016–25. doi: 10.1093/brain/awh543. [DOI] [PubMed] [Google Scholar]