Abstract
Objective
The phenotype of the Chromogranin A(Chga) null (knockout) mouse is hypertensive. However, hypertensive humans and spontaneously hypertensive rats display elevated CHGA expression. This study addresses the paradox that both ablation and elevation of CHGA result in hypertension.
Methods
Mice with varying copy-number of the CHGA gene were generated. In these mice CHGA, catecholamine and blood pressure (BP) was measured. Also a cohort of healthy human subjects was stratified into tertiles based on plasma CHGA expression and phenotyped for characteristics including their BP response to environmental (cold) stress.
Results
The mice displayed a direct CHGA gene-dose (0 to 4 copies/genome) - dependent activation of CHGA expression in both plasma and adrenal gland, yet the BP dependence of CHGA gene dose was U-shaped: maximal at 0 and 4 copies of the gene, while minimal at 2 copies (i.e., the wild-type gene dosage). Plasma catecholamine showed a parallel U-shaped dose/response in mice, while adrenal epinephrine exhibited a reciprocal (inverted) U-shaped response, suggesting dysregulated neurotransmission at both extremes of CHGA expression. The human subjects also showed a non-linear relationship between CHGA expression and pressor responses to environmental (cold) stress, that were maximal in the highest and lowest tertiles, though basal BPs did not differ among the groups. The human CHGA tertiles also differed in epinephrine secretion as well as degree of CHGA processing to catestatin (catecholamine release-inhibitory peptide derived from CHGA processing).
Conclusions
Thus, across mammalian species, an optimal amount of CHGA may be required to establish appropriate catecholamine storage and release, and hence BP homeostasis.
Keywords: “Humanized”CHGA transgenic mice, hypertension, catecholamine, cold-stress blood pressure response
Introduction
Chromogranin A (CHGA) is an acidic-soluble pro-hormone in secretory granules of chromaffin cells and post-ganglionic sympathetic neurons, where it is co-stored and co-released by exocytosis along with catecholamine [1, 2]. The extensive phenotypes of Chga null (knockout) mice indicate roles for CHGA in biogenesis of secretory granules as well as regulation of BP [3]. In humans, CHGA expression is heritable and elevated in essential hypertension [4, 5]. Spontaneously hypertensive rats (with hereditary hypertension) also have augmented adrenal medullary CHGA stores [6]. Thus, both elevation and diminution of CHGA expression have been associated with hypertension. The processing of CHGA yields several biologically active polypeptides [2] including catestatin, a nicotinic-cholinergic antagonist that diminishes catecholamine release [7], and whose plasma concentration may be diminished in hypertension [8]. Catestatin may also constitute as an early or “intermediate phenotype” in assessing genetic risk for cardiovascular disease [9].
In the present study, we varied CHGA gene dosage using genetically engineered mouse models with stable expression of CHGA over a range of 0-4 copies (per genome) of the gene. These mice were then used to systematically study the effect of CHGA expression on BP. We then extended our observations to human subjects stratified by degrees of circulating CHGA expression. This study aimed to probe seemingly paradoxical earlier findings in rodents [3, 6] and humans [4, 5] that: Chga-/- mice lacking CHGA are hypertensive, as are humans and spontaneously hypertensive rats with elevated CHGA.
Methods
Mice with varying copy number of the chromogranin A gene
The BAC transgenesis protocol employed to generate the ‘humanized’ CHGA mice has been described in detail before [3]. These 2-copy, 4-copy and 0-copy mice have the background 50% each C57BL6 and 129SvJ. The schematic in Figure 1 describes the protocol for deriving these mice. While 0, 2 and 4-copy mice are inbred lines, 1 and 3-copy are F1 pups of specific matings. However, in each case (inbred or F1; 0, 1, 2, 3, or 4-copy) the final genetic background was ∼50% C57BL6 and ∼50%129SvJ. PCR analysis [3] of genomic DNA extracted from tail snips of mice confirmed the presence of CHGA and the number of copies of Chga per diploid genome. Mice of age 12-14 weeks were used to measure BP and catecholamines.
Figure 1. Schematic for generating mice with varied copy numbers of chromogranin A gene.
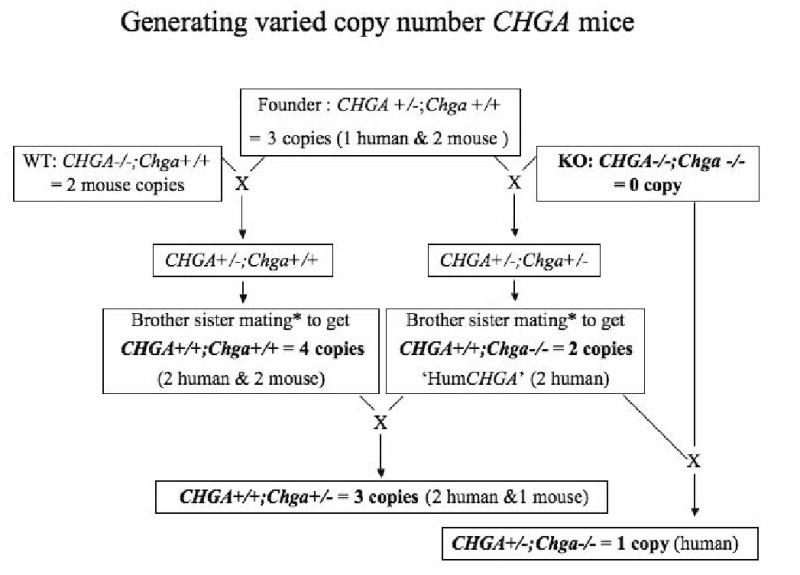
The transgenic founder has a single copy of the human CHGA BAC clone RP11862G15 stably integrated in its genome. The “humanized” CHGA (HumCHGA) line, was derived by mating a (symbolized as X) transgenic founder with Chga knockout (KO) as described earlier [3]. They have 2-copies of the human CHGA gene and lack the mouse allele. Similarly, the founder was mated with wild-type (WT, Chga+/+) mice to generate 4-copy chromogranin A mice (2 alleles each, of human and mouse). The F1 pups of the mating between 4-copy mice with 2-copy HumCHGA mice generated the 3-copy mice. Also the F1 pups of mating between 2-copy HumCHGA and KO mice produced the 1-copy CHGA mice (1 human copy). The mice with 0, 1, 2, 3 or 4 copies of chromogranin A gene used in subsequent experiments have been highlighted in bold.
CHGA ELISA and immunoblotting
Sandwich ELISA between monoclonal anti-chromogranin A (mAb 5A8) antibody and rabbit polyclonal anti-human CHGA antiserum has previously been described in detail [10]. The rabbit polyclonal anti-CHGA was raised against recombinant human CHGA. Recombinant human vasostatin-1 (STA-CgA1-78) was used as antigen to generate mAb 5A8, which recognizes the epitope located within residues 53-57 and in particular Arg53, His54 and Leu57 (likely an alpha-helix) [11]. Plasma samples of each animal were assayed in duplicate. For immunoblots, adrenal glands were homogenized, the protein concentration was determined using Biorad/Bradford assay reagent (Hercules, CA) and 5 μg of total protein was loaded per well. The primary polyclonal antibody used in the immunoblot analysis was generated against the catestatin domain of human CHGA352-372 using the vendor Strategic Biosolutions, ME.
Measurement of blood pressure in mice
BP of mice was measured using the BP-2000 Blood Pressure Analysis System (Visitech Systems Inc., Cary, NC). The instrument employs a non-invasive tail-cuff method. Mice were placed in individual rodent restraint holders on a pre-heated specimen platform at 38°C. BP values were recorded 10 times in rapid succession and the results were averaged. This process was repeated 3–4 times daily between 10:00 and 12:00 hours, for 5 consecutive days. Over the first week the readings were not collected, so as to allow the mice to adapt. Over the following week, the data were analyzed. During measurements, data were continuously stored in a notebook computer running the BP Analysis software package (Visitech Systems Inc.) via a PCMCIA data acquisition card. Averaged readings of systolic BP (SBP) having standard deviation of less than 10 mmHg were accepted for further analysis. Non-invasive BP measurements in WT versus KO mice have been validated previously by intra-arterial pressure transducers [3].
Catecholamine measurements in mice
Catecholamine was measured in plasma drawn from mice euthanized by deep anesthesia in isoflurane (Baxter, IL) chambers, followed by cervical dislocation. Adrenals were also collected from these animals. A volume of 300 μl plasma was supplemented with sodium meta-bisulfite to a final concentration of 0.125 mM and 1 ng of 3,4-dihydroxybenzylamine (DHBA) and mixed. The mixture was then added to an Eppendorf tube containing 15 mg alumina (aluminium oxide, activity grade: super 1 Type WA-4, Sigma). The pH was raised to pH 8.6 by adding Tris-HCl buffer, pH 8.9.
Using a Tissumizer, adrenals were homogenized in 0.3 ml cold 1× phosphate buffered saline, pH 7.4. An aliquot was assayed for protein concentration using the Biorad/Bradford colorimetric assay. To 250 μl of the adrenal lysate, an equal volume of 0.8 N perchloric acid was added and vortexed, whereupon and the mixture was centrifuged at 6800 × g to settle the debris. The supernatant was supplemented sodium meta-bisulfite to a final concentration of 0.125 mM and 10 ng DHBA. The mixture was then transferred to a tube with 40 mg alumina.
Both plasma and adrenal samples were allowed to mix with alumina for 30 min, then centrifuged (2000 × g, 5 min), the supernatant discarded and the beads washed with 1 ml water. The wash was repeated and finally the bound catecholamine was eluted with 80 μl (plasma) or 400 μl (adrenal) of 0.1 N HCl supplemented with 100 mM sodium metabisulfite, and injected for analysis.
Catecholamines were measured by high performance liquid chromatography coupled to an electrochemical detector (Waters 600E Multisolvent Delivery system and Waters 2465 Electrochemical Detector, MA). Separation was performed on an Atlantis dC18 column (2.1×150 mm, 3 μm) from Waters. The mobile phase used was a mixture composed of phosphate-citrate buffer (2 mM NaH2PO4, 268 μM Na2EDTA, 50 mM sodium citrate, 10 mM diethylamine hydrochloride, 0.072% 1-octanesulfonic acid, pH 3.1 adjusted using phosphoric acid, and 2.2% N, N-dimethylacetamide) and acetonitrile at 95:5 (v/v). A flow rate of 0.25 ml/min was used with an isocratic mobile phase. The electrode potential was set at +0.6 V. The data were analyzed using Empower software from Waters, and catecholamine values normalized according to the recovery of DHBA standard.
Environmental (cold) stress test and biochemical assays in human subjects
BP responses to cold stress (immersion of one hand in ice water for one minute) were evaluated non-invasively, with radial artery applanation tonometry (Colin Pilot; Colin Instruments, San Antonio, TX) and thoracic EKG electrodes, as described earlier [8, 12]. 497 individuals were studied, 124 male and 373 female, each subject was self-identified as of white (European) ancestry and 89.7% were normotensive. Subjects were derived from 228 nuclear families: 192 based on twinships (monozygotic or dizygotic), and 28 additional nuclear families with sib ships of 3 or higher order. Ethylenediamine tetraaceticacid anticoagulated plasma was assayed by methods established earlier for CHGB, CHGA, catestatin [13, 14] and catecholamine [15]. The glomerular filtration rate (GFR) was estimated from plasma creatinine using the NIDDK Modification in Diet and Renal Disease (MDRD) equation [16].
Statistics
Data are presented as the mean value ± one SEM. Multiple comparisons were made using one- or two-way ANOVA followed by Bonferroni post hoc test. Statistical significance was concluded at p<0.05.
Results
Generating mouse models with varying copy number of the chromogranin A gene
Transgenic Chga-/- (0-copy) and humanized CHGA (2-copy) mouse models were generated as described earlier [3]. By employing a series of matings schematized in Figure 1, additional categories of mice with 1, 3 or 4 copies of chromogranin A were generated. In both plasma (Figure 2) and adrenal gland (Figure 3), CHGA expression increased in proportion to chromogranin A gene dosage. The lower molecular weight bands in the adrenal immunoblot may represent proteolytic fragments of CHGA bearing the catestatin epitope.
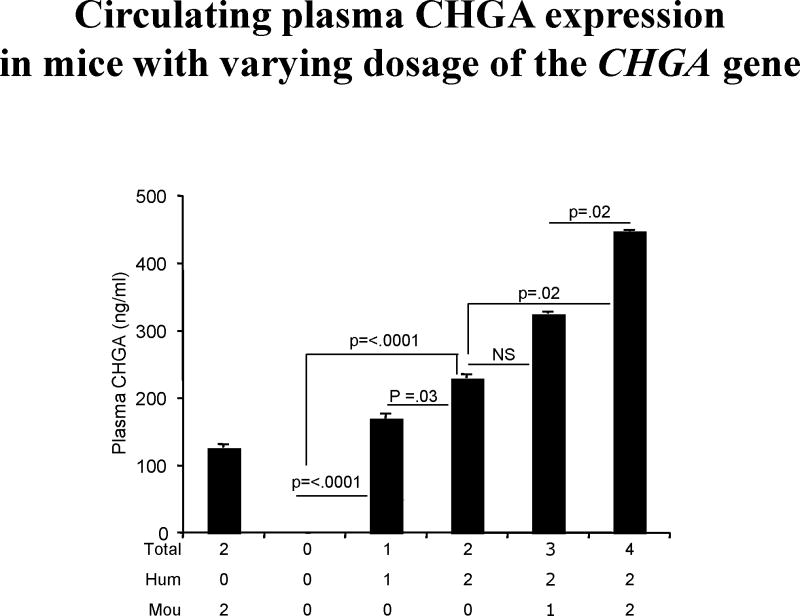
Figure 2. ELISA assaying CHGA levels in plasma of mice with varying copies of the chromogranin A gene.
The capturing mouse monoclonal antibody 5A8, raised against the amino-terminal region of human CHGA, differs in its affinity for both mouse and human CHGAs, with a stronger affinity for the latter [10]. 5A8 binds to the epitope in the CHGA53-57 region. Therefore full-length and fragments of CHGA with the epitope are detected by the assay. Both mouse (Mou) and/or human (Hum) alleles contribute towards the total number of chromogranin A alleles. Plasma from each group of mice (n = 8) was assayed. Increasing levels of circulating CHGA with boost in copy number is observed.
Figure 3. Expression of chromogranin A in adrenal glands.
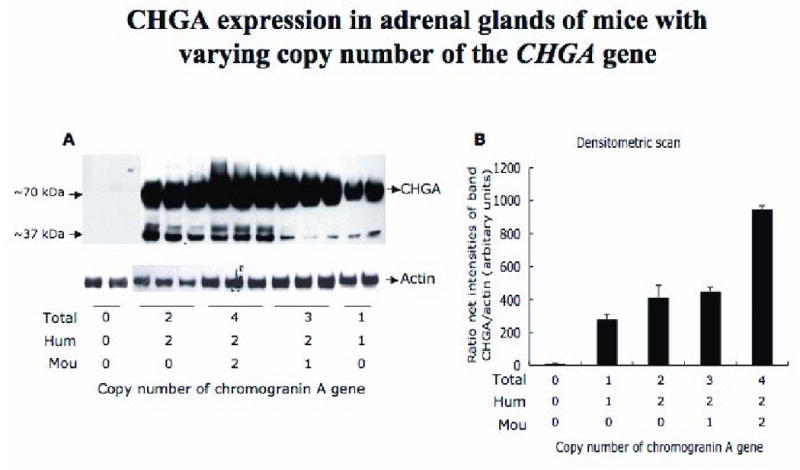
A. Immunoblot demonstrating that an increase in copy number of chromogranin A alleles augments expression of CHGA protein in the adrenal gland. The primary antibody is rabbit polyclonal raised against human catestatin (human CHGA352-372). A total of 5 μg adrenal protein was loaded per lane. The antibody detects the full length and the processed fragments of CHGA and cross-reacts with mouse and human.
B. Densitometric scan of the same immunoblot, normalized to actin levels.
The 5A8 ELISA (detecting both mouse and human CHGA, Figure 2) showed a roughly linear increase in levels of plasma CHGA despite the 5A8 antibody's low affinity for the mouse CHGA [10]. Thus an increase in copy number of the chromogranin A gene results in an increase in level of CHGA expression.
Biphasic effect of chromogranin A gene dosage on BP levels and catecholamine secretion in mice
Mice with 2 human CHGA alleles (but zero mouse Chga alleles, for a total of two alleles) displayed normal SBP/DBP (at 104.2± 0.9/77.8±1.2 mmHg), comparable to values found in WT mice (with 2 mouse but no human CHGA alleles), at SBP/DBP 105.3±2.6/80.5±3.8 mmHg) (Figure 4). Since CHGA exerts a powerful influence towards regulating BP [3], it was measured in the groups of mice with zero to 4 copies of chromogranin A. Both SBP and DBP exhibit U-shaped responses as a function of gene dosage (Figure 4). Thus CHGA's effect BP seems to be optimal at the mid-range between lower and higher gene dosages, specifically at the 2-copy dosage. The highest BP was observed in mice with 0-copies of the chromogranin A gene. Mice expressing lower or higher CHGA than 2-copy mice, as in 1, 3 or 4-copy animals, develop higher BP levels.
Figure 4. Biphasic effect of chromogranin A gene dosage on blood pressure.
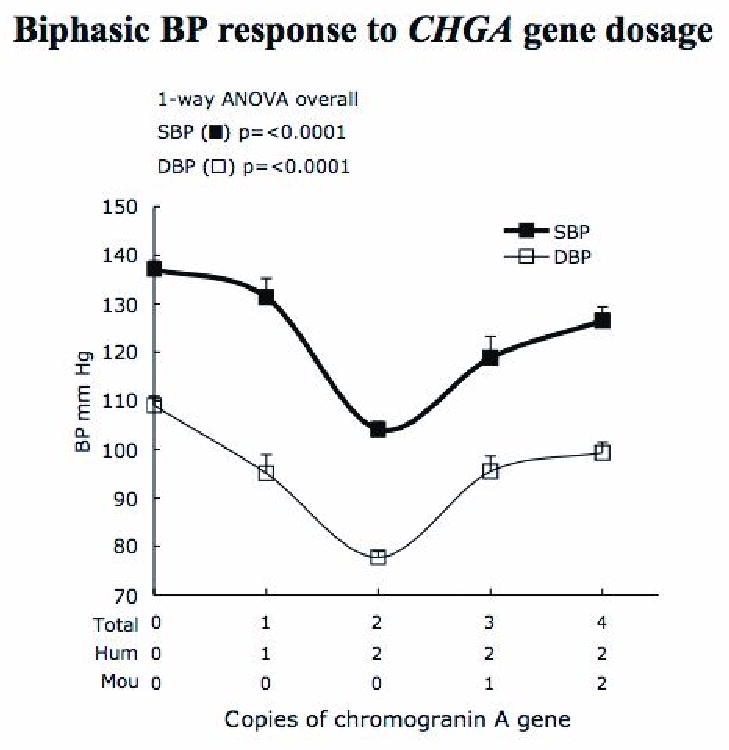
Mice with 2 copies of human CHGA display normal BP (104.5/77.8 mm Hg). Both an increase and decrease in gene dosage of chromogranin A results in BP amplification ranging between ∼15 to 33 mmHg (systolic) and ∼17 to 31 mmHg (diastolic). The BP was recorded by tail-cuff measurements in n = 86, 18, 85, 28 and 28 mice of the groups 0, 1, 2, 3 and 4-copy, respectively. The age of the mice is 12-14 weeks. Comparing 2-copy (*) mice vs. each of the other groups (◆) p <0.0001, and vs. (:) p=0.0008 by student t-test analysis.
Since CHGA also regulates catecholamine storage and release [3], we evaluated both plasma catecholamine concentrations and adrenal catecholamine storage in the 5 mouse groups. A biphasic response mirroring that of BP was observed for the plasma catecholamine (Figure 5 A and B). The 0-copy mice have elevated plasma norepinephrine (NE) and epinephrine (E) compared to 2-copy mice, while mice with 4-copies appear to have the greatest release of both catecholamine. Mice with 2-copies of the chromogranin A gene exhibited the lowest BP and plasma catecholamine responses.
Figure 5. Catecholamine response to chromogranin A gene dosage. A, B & C.
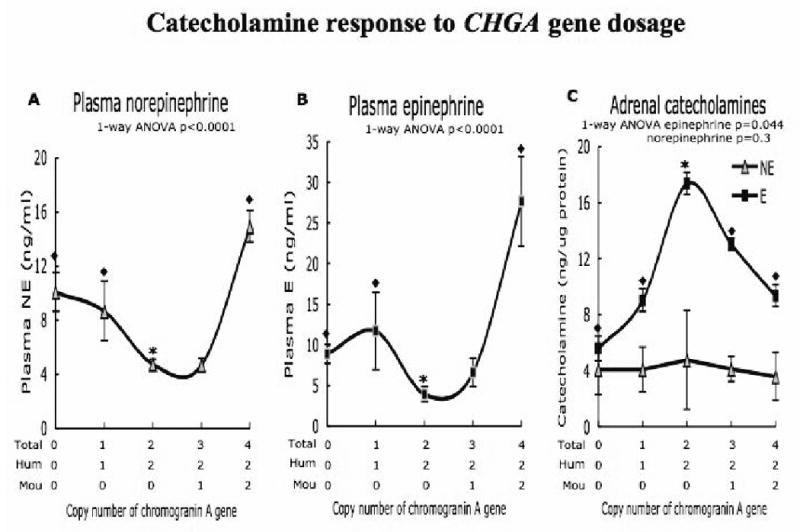
Group 0, 1, 2, 3 and 4 copy mice: n = 5, 7, 8, 8 and 8 of age 12 weeks were used for the experiment.
A. & B. Copy number of chromogranin A gene significantly elevates both plasma NE and E in 0, 1 and 4-copy mice (◆) compared to 2-copy mice (*). The student t-test analysis of NE levels, 2-copy vs. 0-copy p = 0.001, 2-copy vs. 1-copy p = 0.03, 2-copy vs. 3-copy p = .91, 2-copy vs. 4-copy p < .0001. Circulating E concentration 2-copy vs. 0-copy p = .0007, 2-copy vs. 1-copy p = 0.04, 2-copy vs. 3-copy p = 0.06, 2-copy vs. 4-copy p = 0.0005. C. Adrenals of 2-copy mice express the highest levels of E. Comparatively, 0 and 4-copy mice show depressed levels of E. 2-copy vs. 0-copy p = 0.006, 2-copy vs. 1-copy 0.03, 2 vs. 3-copy p = 0.014, 2-copy vs. 4-copy p=0.019. The NE levels do not vary significantly with chromogranin A gene dosage.
The adrenal gland displayed a reciprocal pattern of catecholamine contents, especially for epinephrine (Figure 5C), with the lowest levels of epinephrine observed in animals with the highest BPs.
Biological and physiological phenotypes in humans correlated with degree of CHGA expression
In the human study, a carefully phenotyped cohort of 497 predominantly (∼89.7%) normotensive individuals, was subdivided into three tertiles based on their plasma CHGA concentration (Table 1), which spanned a range of ∼2.4-fold from the first to third tertile.
Table 1. Physiological and biochemical traits in individuals stratified by circulating chromogranin A levels.
Individuals in a cohort were grouped into three tertiles based on their circulating CHGA116-439 protein concentration. Tertile 1 represents individuals in <33.3rd percentile, with lowest CHGA116-439 concentration. Tertile II includes individuals in the 33.3 - 66.6 percentile range, with intermediate concentration. Individuals with the highest concentration of CHGA (above the 66.7th percentile) are in tertile III. The table lists the demographics of the studied population, their physiological and biochemical traits.
| Circulating CHGA stratification into tertiles in humans: Effects on multiple physiological and biochemical traits | |||||
|---|---|---|---|---|---|
| Tertile | 1 (CHGA ≤3.11 nM) | 2 (3.11<CHGA≤3.99 nM) | 3 (CHGA>3.99 nM) | ANOVA | |
| N | 166 | 166 | 165 | (adjusted by age, sex) | |
| Mean±SEM (n) | Mean±SEM (n) | Mean±SEM (n) | F | p | |
| Demographic | |||||
| Age, years | 38.4±1.3 | 39.2±1.3 | 44.2±1.3 | 6.163 | 0.002 |
| Sex, M/F | (43/123) | (42/124) | (39/126) | - | 0.88 (by c2) |
| Ethnicity | White | White | White | - | - |
| Physiological | |||||
| Cold pressor test | |||||
| Basal SBP, mmHg | 119.1±1.2 | 117.2±1.2 | 115.0±1.2 | 2.703 | 0.068 |
| Basal DBP, mmHg | 64.3±0.9 | 63.4±0.9 | 62.0±0.9 | 1.624 | 0.19 |
| Post cold SBP, mmHg | 132.7±1.8 | 126.8±1.8 | 128.6±1.9 | 2.806 | 0.061 |
| Post cold DBP, mmHg | 75.9±1.2 | 70.9±1.2 | 73.7±1.2 | 4.23 | 0.015 |
| Change in SBP, mmHg | 15.2±1.9 | 8.7±1.8 | 15.5±1.7 | 3.79 | 0.023 |
| Change in DBP, mmHg | 11.2±1.0 | 8.0±1.0 | 11.3±0.9 | 4.694 | 0.01 |
| GFR (by NIDDK/MDRD), mL/min | 91.9±2.2 (102) | 96.2±2.1 (112) | 88.2±2.2 (101) | 3.499 | 0.03 |
| Biochemical (plasma) | |||||
| Chromogranin/secretogranins | |||||
| CHGA116-439, nM | 2.56±0.27 | 3.57±0.27 | 6.03±0.27 | 39.61 | <0.001 |
| CHGB439-451, nM | 0.32±0.02 | 0.37±0.02 | 0.37±0.01 | 3.202 | 0.042 |
| Catecholamines | |||||
| Epinephrine, pg/ml | 22.6±1.9 (112) | 26.6±1.9 (117) | 31.9±1.9 (118) | 5.727 | 0.004 |
| Norepinephrine, pg/ml | 324.3±15.8 (112) | 323.9±15.2 (117) | 340.8±15.4 (118) | 0.379 | 0.68 |
Physiologically, the 3 tertiles had similar mean basal BP values at rest (Table 1), but individuals in the mid-tertile exhibited substantially diminished BP responses to environmental (cold) stress, by ∼44% for SBP (p=0.023) and ∼29% for DBP (p=0.01). By contrast, the mid-CHGA-tertile displayed elevated basal eGFR, by ∼8%.
Biochemically, as the CHGA precursor rose progressively across tertiles (p<0.001), and the plasma concentration of catestatin also fell progressively (p<0.001), as did estimated cleavage of CHGA to catestatin (p<0.001; Table 1, Figure 7); indeed, estimated catestatin processing (as the ratio of catestatin: CHGA in plasma) fell by ∼61%. In the same tertile groups, plasma epinephrine (though not norepinephrine) concentration rose by ∼41% (p=0.004). Finally, CHGB also rose progressively across the CHGA tertile groups, by ∼16% (Table 1).
Figure 7. Chromogranin A levels in plasma of human subjects.
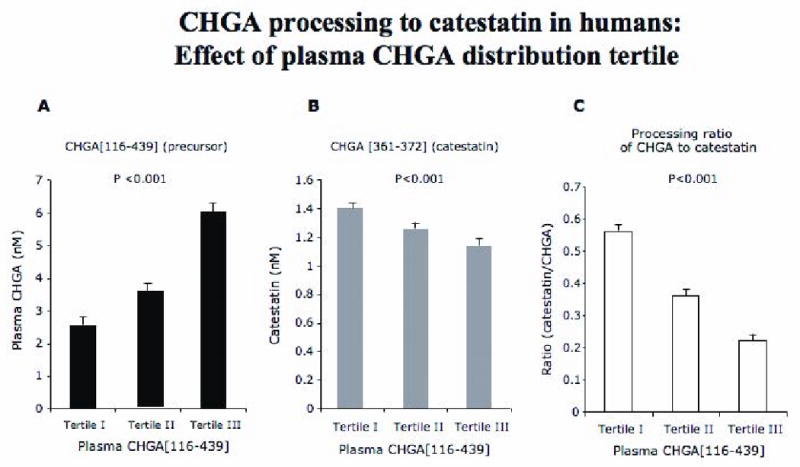
The plasma CHGA precursor was accessed using radioimmunoassay based on the large fragment CHGA116-439. Processing to the catestatin fragment (human CHGA352-372) was assayed by a synthetic peptide epitope corresponding to CHGA amino acids 361-372.
A. A linear increase in the precursor CHGA116-439 is seen in the 3 tertiles as expected based on the stratification.
B. Catestatin, the nicotinic-cholinergic antagonist peptide derived by processing full-length CHGA was assayed in the plasma of these individuals, surprisingly the least catestatin is observed in tertile III individuals that have the most precursor.
C. Ratio of catestatin: full-length CHGA again indicates least efficient processing of CHGA to catestatin in tertile III individuals.
The 165 individuals in the highest CHGA tertile were older by 4-5 years than the other two tertiles (Table 1). Since age [17]and sex [18] may influence the sympathetic responses (BP and plasma NE) to cold stress, our ANOVA analyses in Table 1 were adjusted by age and sex, and the effect of CHGA tertile on BP response to cold was independent of either age or sex during such covariate adjustment. In other analyses of the 497 phenotyped individuals, age did influence SBP (p=0.0001), DBP (p=0.005), plasma NE (p=0.026), and plasma CHGA116-439 (p=0.001), though not plasma E (p=0.075), or the SBP (p=0.209) or DBP (p=0.341) changes in response to cold stress. Since family history (genetic risk) of hypertension may influence the BP response to cold stress [19],we also evaluated the effect of family history on the trait in these predominantly normotensive subjects (Figure 6), but family history did not predict cold-induced change in either SBP (p=0.584) or DBP (p=0.574).
Figure 6. Environmental (cold) stress test BP responses in individuals with varying plasma CHGA.
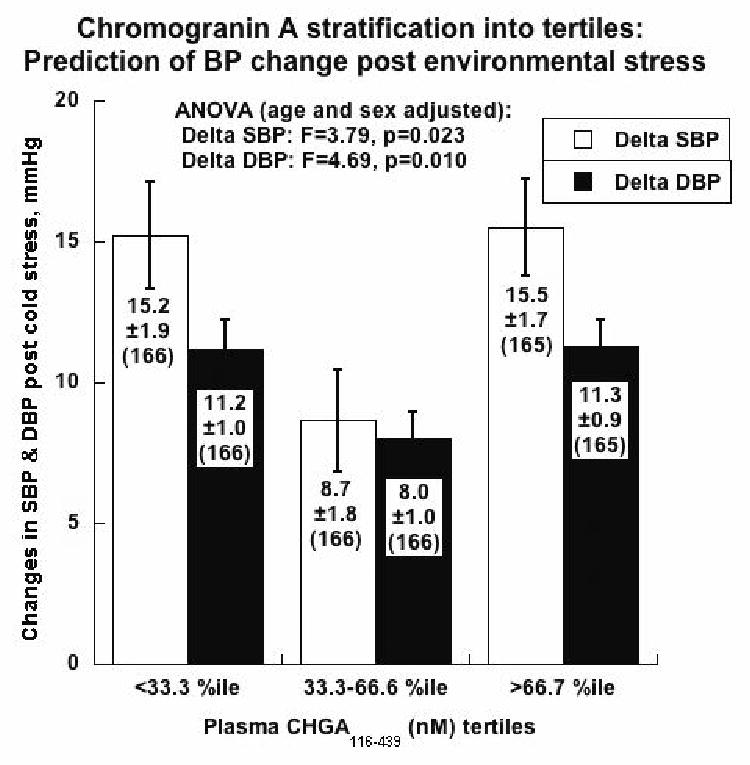
The change in BP was measured in individuals belonging to the 3 tertiles upon being subjected to systematic cold stress. The cold stress final SBP and DBP values were significantly higher in all three groups compared to basal level. However the tertile (II) had the smallest change in BP compared to both tertiles I and III, indicating that this group of individuals, has the least efferent sympathetic outflow. This biphasic response to CHGA protein dosage in humans mimics the BP response to chromogranin A gene dosage in mice.
Discussion
Overview
In this report we find that the relationship between CHGA expression and BP is distinctly non-linear, and even biphasic (or U-shaped), in mice with systematic variation in the copy number (0, 1, 2, 3, or 4 copies) of CHGA alleles (Figure 4). In such mice, the expression of plasma catecholamine (both E and NE) was also U-shaped as a function of CHGA gene dose (Figure 5), with a reciprocal pattern of adrenal epinephrine stores (in an inverted U-shape) suggesting dysregulated storage and release of catecholamine as the underpinning of the BP trait pattern (Figure 4). When a large, intensively phenotyped group of predominantly normotensive humans was profiled for both CHGA expression and the BP response to environmental stress, we also observed a U-shaped response of both SBP and DBP reactivity to increasing CHGA expression (Figure 6). The results indicate nonlinear coupling of CHGA expression and BP across mammalian species. Since CHGA gene expression is important for BP control in humans, the results may have implications for cardiovascular disease.
Transgenic mice
In mice with increasing numbers of CHGA alleles (zero to 4), CHGA expression in plasma (Figure 2) and adrenal gland (Figure 3) directly paralleled copy number, but the BP response was decidedly non-linear and in fact U-shaped (Figure 4). The U-shaped BP response was paralleled by plasma catecholamine (Figure 5), suggesting that elevated catecholamine secretion underlined the increased BP at extremes of CHGA expression. A reciprocal (inverted U-shaped) response of adrenal epinephrine contents, the major adrenomedullary catecholamine, suggests dysregulation of catecholamine storage and release at the extremes of CHGA expression (Figure 5).
How might catecholamine storage and release be disturbed as CHGA expression changes? CHGA has both intracellular/intragranular effects on catecholamine storage and release. Within chromaffin cells, CHGA participates in the formation of catecholamine storage vesicles; the biogenesis of such granules is impaired if CHGA expression is diminished, either in isolated chromaffin cells when CHGA is depleted by antisense RNA [20, 21] or siRNA [18], or by targeted ablation (knockout) of the CHGA locus in vivo [3]. CHGA itself physically interacts with catecholamine in a process driven principally by favorable enthalpy (ΔH), resulting in not only binding of catecholamine but also aggregation/precipitation of CHGA [22]. Chromaffin cells derived from CHGA knockout mice [23] display impaired catecholamine accumulation within chromaffin granules, leading to diminished amine content per granule. CHGA is also proteolytically cleaved to its catecholamine release-inhibitory fragment catestatin [7], which blocks the physiological (nicotinic cholinergic) signal towards catecholamine secretion.
Thus, both CHGA excess and CHGA deficiency might be predicted to augment catecholamine release: CHGA excess can augment releasable catecholamine stores [18], while CHGA deficiency results in unstable storage [3, 23] as well as exaggerated release in the face of catestatin deficiency [7]. CHGA seems to play a major role in the process of both transmitter storage and release from chromaffin granules. The cargo capacity of catecholamine in chromaffin vesicles and their exocytotic release is impaired in the absence of CHGA in Chga-/- mice [23]. In the case of increased CHGA expression (Table 1 and Figure 6, right panels), elevated CHGA may augment adrenal chromaffin granule catecholamine storage, while the reciprocal defect in catestatin formation (Figure 7) may unleash epinephrine secretion, a prediction consistent with elevation of plasma epinephrine in the higher CHGA tertiles (Table 1). By contrast, circulating NE is predominantly derived from storage vesicles in post-ganglionic sympathetic axons; while Large Dense core Vesicles (LDVs) do contain both norepinephrine and CHGA in such cells, CHGA is a relatively minor component of LDVs [24], and Smaller Dense core Vesicles (SDVs, which do not contain soluble core peptides such as chromogranins) play a more prominent role in catecholamine storage and release.
Human “intermediate phenotypes”
In predominantly normotensive humans, although basal BP did not differ across 3 tertiles of CHGA expression, the BP response to environmental (cold) stress, an “intermediate phenotype” for the later development of established hypertension [25-27] was exaggerated in individuals in the lowest and highest tertiles of CHGA expression (Table 1, Figure 6).
“Intermediate traits” [25-27] have been employed in the study of complex disease traits such as essential hypertension, a disease state with contributions from both genes and environment; late penetrance; and genetic heterogeneity [26, 28]. Two such “intermediate traits” include the hemodynamic response to environmental (cold) stress [29-31] and the circulating concentration of catestatin [9]. Plasma CHGA is elevated and its processing to catestatin reduced in essential hypertension [32]. In this study both the precursor CHGA and catestatin levels were estimated in plasma of human subjects as previously described by Stridsberg [14], showing an inverse relationship. Tertile-III, with the greatest CHGA expression but least catestatin processing (Table 1, Figure 7), also displayed the highest plasma epinephrine concentration; thus augmented catecholamine release may underlie the elevated stress BP response in this group (Figure 6). Tertile-I, with the lowest CHGA expression but highest processing to catestatin, does not display a catecholamine excess phenotype at baseline; the exaggerated pressor response in this group might represent catecholamine excess exclusively during stress-stimulated release, or there may be another pressor mechanism not yet well defined in this group. Although we did not observe an elevation in BP across the CHGA tertiles (Table 1), these 497 phenotyped subjects are predominantly (∼89.3%) normotensive; when we evaluate subjects with established hypertension, we typically observe elevations of both CHGA and catecholamine secretion [18, 33].
The blood pressure response to cold stress (cold pressor test, or CPT) is predictive of future changes in basal blood pressure and hence development of hypertension [29-31]. While we did not evaluate additional neurohumoral responses to such stress in the current study, previously we noted that cold stress-induced increments in SBP and DBP are associated with a coordinate elevation in systemic vascular resistance [8], as well as elevations in plasma norepinephrine [17].
Mechanistic implications for hypertension
This study yields insight into earlier observations that both increased CHGA expression, as previously observed in humans [18, 34] and rats [35] and CHGA absence in mice [3] are associated with BP elevation.
Might extremes of catestatin itself be involved in generating the elevated human pressor responses at both ends of the CHGA expression spectrum (Tertiles I and III; Table 1, Figure 6)? As CHGA expression increases, its degree of conversion to catestatin decreases (Table 1, Figure 7). In Tertile-III with the lowest catestatin coupled with highest epinephrine, unleashed catecholamine secretion in the face of diminished catestatin constitutes a plausible mechanism for the enhanced pressor response. However, catestatin also acts to blunt nicotinic cholinergic receptor desensitization [36], and the relative excess of catestatin in Tertile-I could thus result in augmentation of pressor responses in a setting of prolonged or repeated exposure to agonist. In addition, catestatin seems to exert central effects upon autonomic tone that may enhance rather than diminish sympathetic outflow [37]. Other biological responses, such as the antihypertensive effect of the alpha-2-adrenergic agonist clonidine [38], anti-tumor activity of endostatin [39, 40], or inhibition of angiogenesis by IFN-α [41] exhibit U-shaped dose/response curves.
Conclusions and perspectives
A biphasic relationship between BP and CHGA expression seems to occur across mammalian species, in both experimental (Figure 4) and clinical (Figure 6) settings. The phenomenon may suggest alternative mechanisms of regulating BP, and may provide a window into interpretation of seemingly irreconcilable previous observations in human and rodents [3-5].
Acknowledgments
The CHGA ELISA reagents were a gift from Prof. Angelo Corti.
Sources of funding: This work was supported by grants from the National Institutes of Health (DK069613 to SMV; R01 DA011311 to SKM; DK 60702 to DTOC; P01 HL58120 to SKM & DTOC), and the Department of Veterans Affairs (SKM & DTOC).
Abbreviations
- CHGA
Chromogranin A protein
- BP
Blood pressure
- CHGA
Human Chromogranin A gene
- Chga
Mouse Chromogranin A gene
- KO
Chromogranin A null or knockout mice
- CHGA+/+Chga-/-
“humanizedCHGA” mice
Footnotes
Disclosures: The authors declare no conflicts of interest.
References
- 1.O'Connor DT, Frigon RP. Chromogranin A, the major catecholamine storage vesicle soluble protein. Multiple size forms, subcellular storage, and regional distribution in chromaffin and nervous tissue elucidated by radioimmunoassay. J Biol Chem. 1984;259:3237–3247. [PubMed] [Google Scholar]
- 2.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 3.Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connor DT. Chromogranin A: implications for hypertension. J Hypertens Suppl. 1984;2:S147–150. [PubMed] [Google Scholar]
- 5.Takiyyuddin MA, Cervenka JH, Hsiao RJ, Barbosa JA, Parmer RJ, O'Connor DT. Chromogranin A. Storage and release in hypertension Hypertension. 1990;15:237–246. doi: 10.1161/01.hyp.15.3.237. [DOI] [PubMed] [Google Scholar]
- 6.Schober M, Howe PR, Sperk G, Fischer-Colbrie R, Winkler H. An increased pool of secretory hormones and peptides in adrenal medulla of stroke-prone spontaneously hypertensive rats. Hypertension. 1989;13:469–474. doi: 10.1161/01.hyp.13.5.469. [DOI] [PubMed] [Google Scholar]
- 7.Mahapatra NR, Mahata M, Mahata SK, O'Connor DT. The chromogranin A fragment catestatin: specificity, potency and mechanism to inhibit exocytotic secretion of multiple catecholamine storage vesicle co-transmitters. J Hypertens. 2006;24:895–904. doi: 10.1097/01.hjh.0000222760.99852.e0. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, et al. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin a: implications for secretion and blood pressure. Circulation. 2008;118:247–257. doi: 10.1161/CIRCULATIONAHA.107.709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo B, Curnis F, Foglieni C, Monno A, Arrigoni G, Corti A. Chromogranin A expression in neoplastic cells affects tumor growth and morphogenesis in mouse models. Cancer Res. 2002;62:941–946. [PubMed] [Google Scholar]
- 11.Ratti S, Curnis F, Longhi R, Colombo B, Gasparri A, Magni F, et al. Structure-activity relationships of chromogranin A in cell adhesion. Identification of an adhesion site for fibroblasts and smooth muscle cells. J Biol Chem. 2000;275:29257–29263. doi: 10.1074/jbc.M003796200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Rao F, Wessel J, Kennedy BP, Rana BK, Taupenot L, et al. Functional allelic heterogeneity and pleiotropy of a repeat polymorphism in tyrosine hydroxylase: prediction of catecholamines and response to stress in twins. Physiol Genomics. 2004;19:277–291. doi: 10.1152/physiolgenomics.00151.2004. [DOI] [PubMed] [Google Scholar]
- 13.Stridsberg M, Eriksson B, Oberg K, Janson ET. A panel of 13 region-specific radioimmunoassays for measurements of human chromogranin B. Regul Pept. 2005;125:193–199. doi: 10.1016/j.regpep.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Stridsberg M, Eriksson B, Oberg K, Janson ET. A panel of 11 region-specific radioimmunoassays for measurements of human chromogranin A. Regul Pept. 2004;117:219–227. doi: 10.1016/j.regpep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–2153. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 17.Palmer GJ, Ziegler MG, Lake CR. Response of norepinephrine and blood pressure to stress increases with age. J Gerontol. 1978;33:482–487. doi: 10.1093/geronj/33.4.482. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, Fung MM, Stridsberg M, et al. Naturally occurring human genetic variation in the 3′-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sex-dependent fashion. J Am Coll Cardiol. 2008;52:1468–1481. doi: 10.1016/j.jacc.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert EA, Schlaich MP. Reduced sympathoneural responses to the cold pressor test in individuals with essential hypertension and in those genetically predisposed to hypertension. No support for the “pressor reactor” hypothesis of hypertension development. Am J Hypertens. 2004;17:863–868. doi: 10.1016/j.amjhyper.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 21.Huh YH, Jeon SH, Yoo SH. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem. 2003;278:40581–40589. doi: 10.1074/jbc.M304942200. [DOI] [PubMed] [Google Scholar]
- 22.Videen JS, Mezger MS, Chang YM, O'Connor DT. Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J Biol Chem. 1992;267:3066–3073. [PubMed] [Google Scholar]
- 23.Montesinos MS, Machado JD, Camacho M, Diaz J, Morales YG, Alvarez de la Rosa D, et al. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci. 2008;28:3350–3358. doi: 10.1523/JNEUROSCI.5292-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connor DT, Klein RL, Thureson-Klein AK, Barbosa JA. Chromogranin A: localization and stoichiometry in large dense core catecholamine storage vesicles from sympathetic nerve. Brain Res. 1991;567:188–196. doi: 10.1016/0006-8993(91)90795-w. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor DT, Insel PA, Ziegler MG, Hook VY, Smith DW, Hamilton BA, et al. Heredity and the autonomic nervous system in human hypertension. Curr Hypertens Rep. 2000;2:16–22. doi: 10.1007/s11906-000-0053-8. [DOI] [PubMed] [Google Scholar]
- 26.Lillie EO, O'Connor DT. Early phenotypic changes in hypertension: a role for the autonomic nervous system and heredity. Hypertension. 2006;47:331–333. doi: 10.1161/01.HYP.0000203980.44717.aa. [DOI] [PubMed] [Google Scholar]
- 27.Shih PA, O'Connor DT. Hereditary determinants of human hypertension: strategies in the setting of genetic complexity. Hypertension. 2008;51:1456–1464. doi: 10.1161/HYPERTENSIONAHA.107.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 29.Kasagi F, Akahoshi M, Shimaoka K. Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension. 1995;25:71–76. doi: 10.1161/01.hyp.25.1.71. [DOI] [PubMed] [Google Scholar]
- 30.Menkes MS, Matthews KA, Krantz DS, Lundberg U, Mead LA, Qaqish B, et al. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension. 1989;14:524–530. doi: 10.1161/01.hyp.14.5.524. [DOI] [PubMed] [Google Scholar]
- 31.Schneider GM, Jacobs DW, Gevirtz RN, O'Connor DT. Cardiovascular haemodynamic response to repeated mental stress in normotensive subjects at genetic risk of hypertension: evidence of enhanced reactivity, blunted adaptation, and delayed recovery. J Hum Hypertens. 2003;17:829–840. doi: 10.1038/sj.jhh.1001624. [DOI] [PubMed] [Google Scholar]
- 32.Biswas N, Vaingankar SM, Mahata M, Das M, Gayen JR, Taupenot L, et al. Proteolytic cleavage of human chromogranin a containing naturally occurring catestatin variants: differential processing at catestatin region by plasmin. Endocrinology. 2008;149:749–757. doi: 10.1210/en.2007-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimsdale JE, O'Connor DT, Ziegler M, Mills P. Chromogranin A correlates with norepinephrine release rate. Life Sci. 1992;51:519–525. doi: 10.1016/0024-3205(92)90029-o. [DOI] [PubMed] [Google Scholar]
- 34.Takiyyuddin MA, Parmer RJ, Kailasam MT, Cervenka JH, Kennedy B, Ziegler MG, et al. Chromogranin A in human hypertension. Influence of heredity. Hypertension. 1995;26:213–220. doi: 10.1161/01.hyp.26.1.213. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor DT, Takiyyuddin MA, Printz MP, Dinh TQ, Barbosa JA, Rozansky DJ, et al. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press. 1999;8:285–295. doi: 10.1080/080370599439508. [DOI] [PubMed] [Google Scholar]
- 36.Mahata SK, Mahata M, Parmer RJ, O'Connor DT. Desensitization of catecholamine release. The novel catecholamine release-inhibitory peptide catestatin (chromogranin a344-364) acts at the receptor to prevent nicotinic cholinergic tolerance. J Biol Chem. 1999;274:2920–2928. doi: 10.1074/jbc.274.5.2920. [DOI] [PubMed] [Google Scholar]
- 37.Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, et al. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352-372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 38.Wing LM, Reid JL, Davies DS, Dargie HJ, Dollery CT. Apparent resistance to hypotensive effect of clonidine. Br Med J. 1977;1:136–138. doi: 10.1136/bmj.1.6054.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celik I, Surucu O, Dietz C, Heymach JV, Force J, Hoschele I, et al. Therapeutic efficacy of endostatin exhibits a biphasic dose-response curve. Cancer Res. 2005;65:11044–11050. doi: 10.1158/0008-5472.CAN-05-2617. [DOI] [PubMed] [Google Scholar]
- 40.Tjin Tham Sjin RM, Naspinski J, Birsner AE, Li C, Chan R, Lo KM, et al. Endostatin therapy reveals a U-shaped curve for antitumor activity. Cancer Gene Ther. 2006;13:619–627. doi: 10.1038/sj.cgt.7700938. [DOI] [PubMed] [Google Scholar]
- 41.Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ. Interferon-alpha-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res. 1999;5:2726–2734. [PubMed] [Google Scholar]