Abstract
BACKGROUND
Dopamine concentrations in the nucleus accumbens fluctuate on phasic (subsecond) and tonic (over minutes) timescales in awake rats. Acute ethanol increases tonic concentrations of dopamine, but its effect on subsecond dopamine transients has not been fully explored.
METHODS
We measured tonic and phasic dopamine fluctuations in the nucleus accumbens of rats in response to ethanol (within-subject cumulative dosing, 0.125 – 2 g/kg, i.v.).
RESULTS
Microdialysis samples yielded significant tonic increases in dopamine concentrations at 1 – 2 g/kg ethanol in each rat, while repeated saline infusions had no effect. When monitored with fast scan cyclic voltammetry, ethanol increased the frequency of dopamine transients in 6 of 16 recording sites, in contrast to the uniform effect of ethanol as measured with microdialysis. In the remaining 10 recording sites that were unresponsive to ethanol, dopamine transients either decreased in frequency or were unaffected by cumulative ethanol infusions, patterns also observed during repeated saline infusions. The responsiveness of particular recording sites to ethanol was not correlated with either core versus shell placement of the electrodes or the basal rate of dopamine transients. Importantly, the phasic response pattern to a single dose of ethanol at a particular site was qualitatively reproduced when a second dose of ethanol was administered, suggesting that the variable between-site effects reflected specific pharmacology at that recording site.
CONCLUSIONS
These data demonstrate that the relatively uniform dopamine concentrations obtained with microdialysis can mask a dramatic heterogeneity of phasic dopamine release within the accumbens.
Keywords: dopamine, ethanol, fast scan cyclic voltammetry, microdialysis, phasic
Introduction
Concentrations of dopamine (DA) in the striatum can fluctuate on a timescale of several minutes (tonic) or of seconds (phasic). These aspects of DA release are thought to reflect different firing patterns of DA neurons (Wightman and Robinson, 2002), which can range from single spike activity to bursts of spikes (Freeman et al., 1985; Freeman and Bunney, 1987; Hyland et al., 2002). For example, tonic DA concentrations measured with microdialysis increase when more DA neurons are active (i.e., increased population activity) but not when burst firing of DA neurons is induced (Floresco et al., 2003). In contrast, phasic DA fluctuations are measured with voltammetry (Kawagoe et al., 1992; Suaud-Chagny et al., 1992) when DA neurons are electrically stimulated in patterns that mimic the rapid firing rates achieved during bursts (Kiyatkin and Rebec, 1998; Hyland et al., 2002). Moreover, spontaneous fluctuations in DA (termed DA transients) that resemble the electrically evoked DA signals occur throughout the dorsal and ventral striatum (Robinson et al., 2002b), although these naturally-occurring transients have not been proven to result from burst-firing of DA neurons.
Tonic and phasic DA concentrations appear to serve different functions in animal behavior. For example, tonic DA levels dictate the amount of effort a rat will expend to obtain a reward (Salamone et al., 2007), while phasic DA firing is hypothesized to act as a neural signal that encodes prediction error (Schultz, 1998, 2007). Specifically, DA neurons briefly burst at the presentation of unexpected rewards (Mirenowicz and Schultz, 1996) or at unexpected stimuli that predict rewards (Mirenowicz and Schultz, 1994). Likewise, DA transients occur in response to salient sensory input (Rebec et al., 1997; Robinson et al., 2002b; Robinson and Wightman, 2004) and rewards (Roitman et al., 2004; Day et al., 2007) and can be conditioned to stimuli predicting reward (Phillips et al., 2003; Day et al., 2007). Addictive drugs are known to increase tonic levels of DA (e.g., Di Chiara and Imperato, 1986; Church et al., 1987), a trait which is thought to relate to their addictive properties, but less is known of their effects on phasic DA fluctuations. While recent studies have demonstrated that cocaine, nicotine and ethanol can increase the frequency of DA transients in rats (Stuber et al., 2005; Cheer et al., 2007), a dose-response curve has been generated only for cocaine (Heien et al., 2005).
Ethanol is a psychoactive drug that dose-dependently increases the firing rate of DA neurons in brain slices (Brodie et al., 1990; Okamoto et al., 2006), anesthetized rats (Inoue, 2000; Foddai et al., 2004) and paralyzed, awake rats (Mereu et al., 1984; Gessa et al., 1985). Furthermore, the in vivo studies described an increase in burst firing induced by ethanol (Mereu et al., 1984; Foddai et al., 2004). Consistent with electrophysiological findings, microdialysis measurements of DA in the nucleus accumbens (NAc) typically yield modest dose-dependent increases in extracellular DA at 0.5 – 2 g/kg ethanol administered intraperitoneally (e.g., Imperato and Di Chiara, 1986; Yoshimoto et al., 1992b; Yim et al., 2000). To further explore the pharmacological effects of ethanol on in vivo DA release, the present experiments assessed tonic DA concentrations by using microdialysis and phasic DA transients by using fast scan cyclic voltammetry after intravenous infusion of cumulative doses of ethanol (as used in Mereu et al., 1984; Gessa et al., 1985). Specifically, we predicted that intravenous ethanol would induce both tonic and phasic DA release in the NAc.
Experimental Procedures
Animals
Male Sprague-Dawley rats were purchased from Charles River Laboratories at 225 – 250 g with jugular catheters implanted. Rats were housed individually in a temperature (25°C) and light (12 hour light/12 hour dark) controlled room and had access to food and water ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committees at the University of North Carolina and the University of Texas, in accordance with the Public Health Service policy on Humane Care and Use of Laboratory Animals.
Surgical preparation
Rats were anesthetized with ketamine (80 – 100 mg/kg) and xylazine (12 – 20 mg/kg) or 2% isoflurane and positioned in a stereotaxic frame. For the microdialysis preparation, a single guide cannula (20 or 21 gauge TW, 8 mm length) was placed 1 or 4 mm below the skull surface above the NAc at 1.7 mm anterior and 1.0 – 1.4 mm lateral to bregma (Robinson et al., 2002a; Doyon et al., 2003). For the voltammetric preparation, a guide cannula (Bioanalytical Systems, West Lafayette, IN) was positioned above the NAc at 1.7 mm anterior and 1.7 mm lateral to bregma, and extended 2.5 mm below the skull surface. A reference electrode (Ag/AgCl, ~ 5 mm length) was placed in the contralateral cortex, extending to the dorsal caudate. Finally, a bipolar stimulating electrode (Plastics One, Roanoke, VA) was positioned ipsilateral to the guide cannula at the medial forebrain bundle (4.0 mm posterior and 1.4 mm lateral to bregma, ~ 8.5 mm ventral from the skull surface) in order to stimulate dopaminergic fibers. Rats were allowed at least 3 days to recover from surgery.
Microdialysis measurements
The microdialysis probes were constructed according to the methods described by Doyon et al. (2003). Briefly, fused silica tubing (i.d. = 40 μm; Polymicro Technologies, Phoenix, AZ) formed the inlets and outlets of the probes, and hollow cellulose fiber (o.d. = 250 μm; molecular weight cut-off = 13,000; Spectrum Laboratories, Inc., Rancho Dominguez, CA) formed the dialysis membrane. All the probes were constructed so that the active dialysis region spanned 2.2 mm (the distance between the end of the inlet and the epoxy that sealed the membrane) and the ventral tip would reach 8 mm below the skull surface.
On the day preceding the dialysis experiment, the microdialysis probes were perfused (flow rate = 2 μL/min) with artificial cerebral spinal fluid (149 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 0.25 mM ascorbic acid, and 5.4 mM D-glucose). The probes were inserted into the brain through the guide cannula while the rats were briefly anesthetized with 2% isoflurane or a minimally effective dose of propofol via the catheter. A syringe pump (CMA, Solna, Sweden) was used to pump the perfusate through a transfer line into a single-channel swivel that was suspended above the cage. The perfusion flow rate was decreased to 0.2 μL/min after the animal recovered from the implantation procedure. On the day of the dialysis experiment, the flow rate was returned to 2.0 μL/min two hours before baseline sampling began.
All of the dialysate samples were immediately frozen on dry ice and stored at −80°C until analyzed. The HPLC system used to separate and quantify DA was amperometric and based on reversed phase chromatography using an ion-pairing agent with electrochemical detection, as previously described in Doyon et al. (2003). Seven-μl samples were injected with an autosampler onto a Polaris 2 × 50 mm column (C18, 3-μm particle size; Varian, Palo Alto, CA, USA). The mobile phase was pumped at 0.3 mL/min, and the electrochemical flow cell (VT-03, 2 mm working electrode diameter, potential: +450 mV against a Ag/AgCl reference; Antec Leyden BV, Zoeterwoude, The Netherlands) was controlled with an Intro controller (GBC Separations Inc., Hubbardston, MA, USA). A second HPLC system was used for some samples in which the reference was an in situ Ag/AgCl (ISAAC). KCl was added to the mobile phase in appropriate concentrations in this case. The comparison of DA peak heights from dialysate samples to external standards was used to quantify data. The signal-to-noise rations were calculated and recorded for all samples. Only animals with ratios of 3 or higher for the 0.625 DA standard and 6 or higher for the first basal sample were included in the study.
Voltammetric measurements
Single carbon fibers (6-μm diameter) were prepared as previously described (Robinson et al., 2002b) with approximately 100 μm of fiber exposed (range 35 – 125 μm). The electrodes were secured in micromanipulators that fit in the guide cannula and allowed ventral placement of the electrode in 75-μm increments. Voltammetric recordings were made as previously described (Robinson and Wightman, 2004), with the modification of applying a triangle waveform potential of −0.4 to +1.3 V versus Ag/AgCl reference electrode at 400 V/s (Heien et al., 2003; Robinson et al., 2003). All recordings were made in NAc sites (6 – 8 mm below the skull surface) that supported electrically evoked DA release following stimulation of the bipolar electrode in the medial forebrain bundle (30 – 60 Hz, 12 – 24 pulses, biphasic, 2 ms per phase).
Electrochemical data were collected in 60-s files that were later two-dimensionally smoothed (two passes). Next, a high-throughput algorithm within the LabVIEW collection and analysis program was used to statistically target DA concentration transients by comparison to a template of a DA cyclic voltammogram generated at the same anatomical site by electrically stimulating the DA fibers, as previously described (Robinson et al., 2003; Robinson and Wightman, 2004). Current at the DA oxidation potential was converted to concentration using post-experiment in vitro calibration of the electrode (Logman et al., 2000). To calibrate, the carbon fiber tips of the electrodes were submerged in a flow of TRIS buffer (2.5 mM KCl, 2.4 mM CaCl2, 1.2 mM MgCl2, 2.0 mM Na2SO4, 1.2 mM NaH2PO4, 15 mM TRIS HCl, 126 mM NaCl, pH = 7.4), which was switched to buffer with known concentrations (up to 1 μM) of DA while current was recorded.
Ethanol dose-response study
In order to facilitate comparison of the present data with previous electrophysiology studies, a within-subject design was employed to measure the dopaminergic response to increasing doses of intravenous ethanol (Mereu et al., 1984). Measurements began with a baseline period (“basal”) that was 10 minutes for dialysis collection and 5 minutes for fast scan cyclic voltammetry. Next, 1 mL of saline was administered and collection continued for 5 min. Thereafter, incremental volumes of ethanol were administered (30% w/v ethanol in saline, plus NaCl to make the solution 0.9% NaCl) to obtain the following cumulative doses: 0.125, 0.25, 0.5, 1.0 and 2.0 g/kg; catheter lines were cleared in between doses with 0.4 mL saline. Doses were administered 5 minutes apart, and all solutions were warmed on a heated pad (37°C) prior to injection. Collection continued for 5 minutes after the 2 g/kg cumulative dose. The total recording time was 40 minutes for microdialysis and 35 minutes for voltammetry. Dialysate was collected in 5-minute samples, with the timing of the sample collection adjusted for the volume of the outlet of the probe. Voltammetric recordings were made continuously; however, the first minute of each dose (which included the infusion) was not analyzed because of electrical noise introduced by opening the Faraday cage for the infusion. Voltammetric and dialysis measurements were made in separate rats. To control for the effects of time and multiple infusions, a separate group of rats were given infusions of saline in equivalent volumes to the ethanol infusions.
Repeated 1 g/kg ethanol study
To test the reproducibility of ethanol effects on phasic DA release, additional rats underwent voltammetric measurements during two sequential infusions of a single dose of ethanol. Measurements began with a 10-minute baseline period (“basal”), followed by an initial infusion of 1 g/kg ethanol and recording continued for 20 minutes. A second infusion of 1 g/kg ethanol was made 60 minutes later, with identical recording periods.
Statistical analysis
Due to the variable response patterns produced by ethanol among the voltammetric recording sites, we classified the phasic DA response at each site by z-tests. Specifically, transients during the basal and saline phases were combined to indicate the baseline transient rates, which were then compared to rates following each dose of ethanol (target) by using the equation
When the calculated z score was > 2 or < −2, the target rate was judged significantly different than baseline (p < 0.05, two-tailed). Based on these comparisons, dose-response curves were categorized as “increase” (multiple doses of ethanol significantly increased the rate of DA transients from baseline), “decrease” (multiple doses significantly decreased transient rate) or “no change.” Similar calculations were made for the repeated 1 g/kg ethanol study, except that the baseline frequency rate was taken from multiple baseline samples (i.e., no saline infusion).
Dialysate concentrations of DA and frequency of DA transients after cumulative doses of ethanol or saline were analyzed with repeated-measures two-way ANOVA of group by infusion in either SPSS (manova command; SPSS Inc., Chicago, IL) or GraphPad Prism (GraphPad Software, Inc. San Diego California USA). Post-hoc comparisons were one-way ANOVAs or t-tests and were Bonferroni-corrected. There was one missing point from one rat in the microdialysis data due to a lost HPLC sample, and this was estimated by averaging the adjacent points; the degrees of freedom of the error term was decreased by one to account for this.
Histological analysis
After data collection, each rat was anesthetized with a lethal dose of urethane (> 1.5 g/kg) or pentobarbital (150 mg/kg) and perfused through the heart with saline followed by 10% formalin. The brain was removed, frozen and sectioned. The slices were Nissl stained and inspected to determine the location of the probe or electrode within the NAc.
Results
Placement of microdialysis probes and carbon-fiber microelectrodes
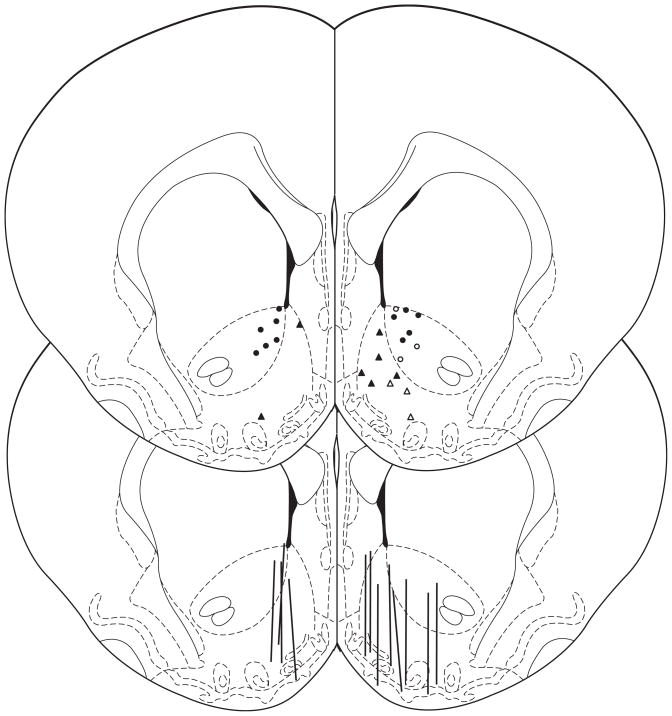
Reconstruction of the recording sites for the microdialysis and voltammetric experiments of cumulative ethanol doses are shown in Figure 1. Note that all voltammetric recordings were made in the right hemisphere and microdialysis recordings were made in the left hemisphere of separate rats. However, for ease of presentation, placements for the saline infusion group are depicted on the left while those for ethanol are on the right. Microdialysis probes were located primarily in the shell of the NAc (Figure 1) with most probes also entering the core. In contrast, the voltammetric electrodes sampled either the core (circles) or the shell (triangles).
Figure 1.
Anatomical sites for voltammetric recording (circles, triangles) and microdialysis sampling (lines) in the NAc. For clarity, the saline groups are drawn on the left while the ethanol groups are on the right. Circles indicate core and triangles indicate shell sites for recording DA transients; open symbols indicate sites that were responsive to ethanol (i.e., exhibited significant increases in DA transient frequency after multiple ethanol infusions). The anterior/posterior position of the placements ranged from +1.0 to +2.2 mm anterior to bregma (slice shown is 1.7 mm anterior, adapted from Paxinos and Watson, 1998).
Ethanol-induced changes in DA concentrations measured with microdialysis
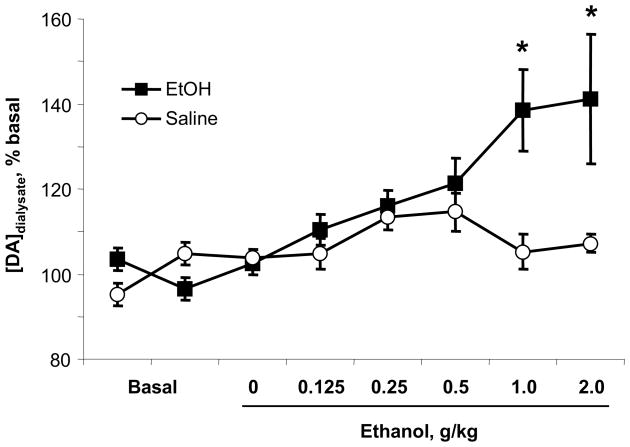
The baseline concentration of DA in dialysate was 1.6 ± 0.2 nM (n = 12 rats), uncorrected for probe recovery. A two-way, repeated measures ANOVA of the DA concentrations yielded a significant interaction of group (saline, ethanol) by infusion (F7,70 = 2.6, p < 0.02). While one-way ANOVA for repeated measures revealed that the saline infusions did not significantly alter tonic DA levels (F7,70 = 0.4, p > 0.05, n = 4), the cumulative ethanol dosing produced a gradual increase in DA concentration (F7,70 = 8.0, p < 0.01, n = 8), peaking at the 1 and 2 g/kg doses, as shown in Figure 2 (significantly higher then baseline, p < 0.05, Bonferroni corrected). Importantly, increases in DA concentration were observed in each rat that received ethanol, with peak DA concentrations in individual rats ranging from 115 – 200% of basal after 1 (n = 3 rats) or 2 g/kg ethanol (n = 5 rats).
Figure 2.
Tonic DA concentrations in the NAc across the cumulative ethanol dosing regimen measured with microdialysis. Ethanol infusion significantly increased DA concentrations at 1 and 2 g/kg (n = 8), while saline had no effect (n = 4). The concentrations are not corrected for probe recovery. * significant increase from baseline, p < 0.05.
Ethanol-induced changes in DA concentrations measured with fast scan cyclic voltammetry
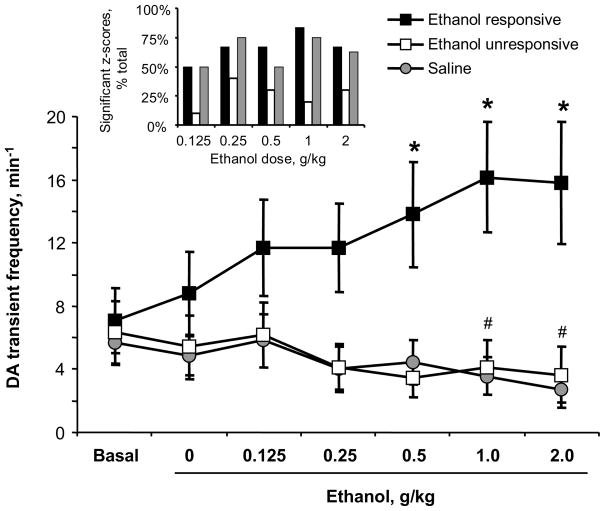
The basal rate of DA transients across all recordings (n = 24 rats) was 6.3 ± 1.0 transients per minute. Figure 3 depicts changes in DA transient frequency across the experiment. When individually analyzed via z scores, DA transient frequency in rats receiving repeated saline infusions (n = 8) either decreased or did not change over time. Cumulative ethanol dosing (n = 16 rats) had a heterogeneous effect on the frequency of DA transients as analyzed via z scores. In 10 recordings, DA transient frequencies were unresponsive to ethanol: they either decreased or did not change across the dosing regimen, identical to the response patterns seen in control rats receiving repeated saline infusions. However, in the remaining 6 recordings (shown as open symbols in Figure 1), ethanol significantly increased the frequency of DA transients at two or more ethanol doses versus the basal and saline frequencies. The proportion of significant z-scores by group is shown in Figure 3 inset. When all the data were analyzed with a two-way repeated measures ANOVA (Figure 3), there was a significant group (saline, ethanol unresponsive, ethanol responsive) by infusion interaction (F21,126 = 7.39, p < 0.0001). Next we compared whether each ethanol group was different from the saline control by calculating additional two-way ANOVAs (Bonferroni-corrected). The frequency of DA transients in the ethanol-responsive group significantly differed from the saline group (group by time interaction: F12,72 = 8.36, p < 0.0001). Importantly, the ethanol-unresponsive group did not differ from the saline group (group by time interaction: F16,96 = 0.29, p > 0.05; main effect of group: F1,96 = 0.13, p > 0.05), although there was a significant main effect of time (F6,96 = 4.62, p < 0.0001). Next we investigated significant changes in transient frequencies over time by calculating one-way ANOVAs for each group (Bonferroni-corrected). The ethanol-responsive group exhibited significant increases in transient frequency over time (F6,30 = 4.44, p < 0.003), and posthoc comparisons showed that transients were more frequent after 0.5, 1 and 2 g/kg ethanol versus baseline (Bonferroni-corrected, all p’s < 0.05). In contrast, the saline group exhibited significant decreases in transient frequency over time (F6,42 = 4.65, p < 0.001), and posthoc comparisons showed that transients were less frequent after 1 and 2 g/kg ethanol versus baseline (Bonferroni-corrected, all p’s < 0.05). Finally, DA transients in the ethanol-unresponsive group did not significantly change over time after Bonferroni correction (F6,54 = 2.53).
Figure 3.
Frequency of DA transients across the cumulative ethanol dosing regimen measured with fast scan cyclic voltammetry. Individual dose-response curves were statistically evaluated and separated into “ethanol responsive” (increased transient frequency in response to at least two doses of ethanol) or “ethanol unresponsive.” Phasic DA release increased at all doses of ethanol in responsive sites (n = 6), but not in unresponsive sites (n = 10) or in saline-treated rats (n = 8). * significant increase from baseline in ethanol-responsive group, p < 0.05; # significant decrease from baseline in saline group, p < 0.05. Inset: Histogram of significant z-scores, indicating > 2-standard-deviation changes in DA transient frequency after subsequent infusions of ethanol or saline versus baseline.
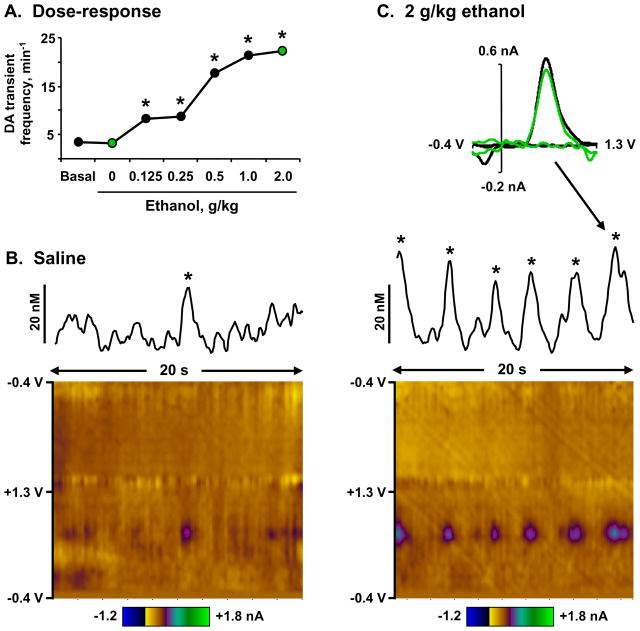
Figure 4 displays an example of a recording site that yielded increasing rates of DA transients in response to ethanol. During basal and saline episodes of the experiment, DA transients occurred at a rate of 3.3 – 3.5 per minute. However, this frequency significantly increased at each dose of ethanol, peaking at 22.3 transients per minute after 2 g/kg ethanol. Transients during the saline (panel B) and 2 g/kg ethanol (panel C) episodes are illustrated by concentration versus time traces as well as color plots of the current versus applied potential versus time. These figures clearly demonstrate the dramatic increase in transient frequency induced by ethanol at this site. Importantly, each transient was identified by a statistical match (r > 0.87) of its cyclic voltammogram to one of electrically-evoked DA release, as shown at the top of panel C.
Figure 4.
Ethanol-induced phasic DA release in the NAc core of an awake rat. Panel A. The rate of DA transients across the dosing regimen. DA transient frequency at each dose of ethanol was significantly higher than baseline and saline episodes (* all z scores < −2, p < 0.05). Frequencies during the saline and 2 g/kg episodes highlighted in panels B and C are depicted as green circles. Panel B. DA transient during a 20-s trace in the saline episode. The line graph shows current fluctuations at the oxidation potential of DA, and the confirmed DA transient is marked with an asterisk. The scale bar depicts DA concentration per unit current, as determined by in vitro calibration of the electrode. The color plot (Michael et al., 1998) shows all changes in current (color) at various applied potentials (y-axis) over time (x-axis). Fluctuations in current due to DA oxidation are best viewed at +0.65 V on the anodic scan. Panel C. Increased frequency of DA transients during a 20-s trace in the 2 g/kg episode. Data are depicted as in panel B, with the addition of a cyclic voltammogram (top) of a DA transient (green line) compared to that of electrically-stimulated DA release in the same site (black), confirming that the change in current is due to DA oxidation.
We divided all of the recordings from rats receiving ethanol infusions into NAc core (n = 8) and shell sites (n = 8) to investigate potential differences in ethanol-response patterns in the two regions.. There was no difference in the basal rates of transients between the two regions (core 7.4 ± 2.1 and shell 5.8 ± 2.0 transients per minute; t-test, p > 0.05). Furthermore, no difference in responsiveness to ethanol was observed between the regions; a two-way repeated measures ANOVA of region by infusion yielded no significant interaction or main effects. Of the six recordings that were ethanol-responsive, three were in the core and three in the shell (open symbols in Figure 1). Thus, we did not find core versus shell differences in the responsiveness to ethanol as measured by DA transient frequency.
As a recent study suggested that the response of phasic DA transients to cocaine may be related to basal rates of transients (Wightman et al., 2007), we also tested whether the basal rate of DA transients predicted the subsequent response to ethanol. First we separated all of the ethanol-infusion recordings into high (> 5 transients per minute; n = 9) and low (< 5 transients per minute; n = 7) basal rates. Next, a two-way repeated measures ANOVA of basal rate by infusion yielded no significant interaction or main effect of dose, but did reveal a significant main effect of basal rate (F1,84 = 31.75, p < 0.01), reflecting that recording sites that supported high basal rates of transients tended to maintain those rates relative to those with low basal rates. Of the six recordings that were ethanol responsive, two were in sites of low and four were in sites of high basal rates. Importantly, electrically stimulated DA release was similar across recording sites, suggesting no difference in DA innervation between the groups. Specifically, the amplitude of electrically stimulated DA signals was 190 ± 49 nM in unresponsive sites and 136 ± 32 nM in responsive sites (t-test, p > 0.05). Thus, we did not find that basal rates of DA transients were associated with the responsiveness to subsequent ethanol infusions.
Reproducibility of phasic DA response to acute ethanol
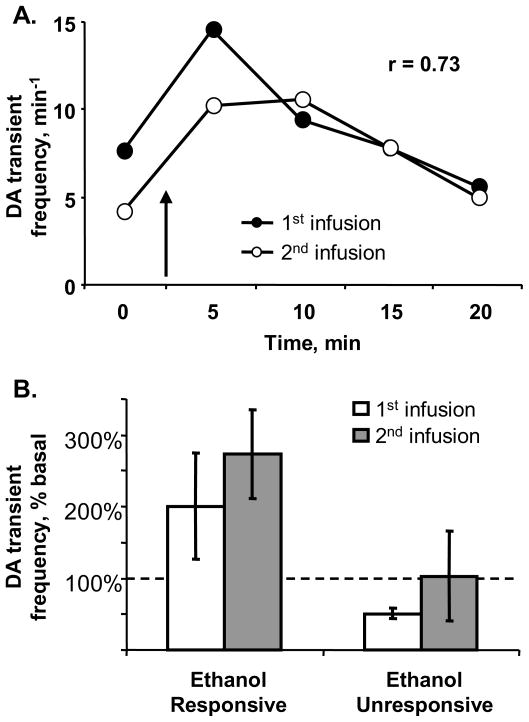
In light of the variable responsiveness of DA transient rates to ethanol, we tested whether the effect of 1 g/kg ethanol measured at a particular recording site was reproducible at that site (n = 7 rats). In five recordings, 1 g/kg ethanol significantly increased the rate of DA transients in the first (n = 4) or second (n = 1) 5-minute bin following infusion (mean z-scores: 7.4 ± 1.6). Importantly, in each of these responsive recording sites, a second infusion of 1 g/kg ethanol also significantly increased DA transient frequency in the same bins (mean z-scores: 5.6 ± 1.6; Figure 5). The remaining two sites were unresponsive to ethanol and showed no significant change in frequency of DA transients after either infusion. An example of an ethanol-responsive site is shown in Figure 5A. In this recording, the rate of DA transients doubled in the 5 min following each infusion of 1 g/kg ethanol. Thus, these data demonstrated that the responsiveness to ethanol was reproducible at a particular recording site.
Figure 5.
Reproducibility of ethanol-induced increases in DA transients at a single recording site. DA transients were measured before and after two infusions of 1 g/kg ethanol. Panel A. Representative data from one rat: Transient frequency was significantly higher than baseline in the 5 minutes following the initial infusion of ethanol (closed circles), an effect that was repeated 60 min later at a second infusion (open circles). Ethanol infusion is indicated by the arrow. Panel B. Group data as percent change from baseline: Sites that yielded increased rates of transients in the 5 min following the first ethanol infusion also yielded increased rates after the second infusion (“ethanol responsive,” n = 5). In contrast, sites that did not increase transient rate following the first ethanol infusion also did not increase rates after the second infusion (“ethanol unresponsive,” n = 2).
Discussion
This study is the first to compare the effect of ethanol on phasic versus tonic extracellular DA concentrations in the NAc, measured in 100-ms increments and 100-μm dimensions with fast scan cyclic voltammetry and in 5-minute increments and 2-mm dimensions with microdialysis, respectively. As predicted, microdialysis revealed dose-dependent increases in extracellular DA after intravenous ethanol, consistent with intraperitoneal administration (Imperato and Di Chiara, 1986; Yoshimoto et al., 1992b) and single doses of intravenous ethanol (Howard et al., 2008). In contrast, by using in vivo electrochemistry we observed increases in the frequency of DA transients in approximately 40% of recording sites within the NAc. The remaining recording sites were unresponsive to ethanol, exhibiting rates of DA transients identical to those during repeated saline infusions. Moreover, individual response patterns to a given dose of ethanol were reproducible at a particular recording site, suggesting that the responsiveness of phasic DA release to ethanol administration is site-specific within microdomains of the NAc core and shell. Thus, the major finding of this study is that while ethanol produces reliable increases in tonic DA concentrations, its effects on phasic DA release are not uniform, but rather spatially selective across the NAc.
The concentration profile of tonic DA after cumulative ethanol administration measured with microdialysis is consistent with the dose-dependent firing pattern of DA neurons described by Mereu et al. (1984) and Gessa et al. (1985). These studies found that intravenous ethanol increased firing in a large proportion of DA neurons, with higher concentrations affecting more DA neurons. The effect of ethanol was fast (within seconds) and persisted for more than 10 minutes in a dose-dependent manner, with peak effects at 0.5 g/kg in ventral tegmental area cells and 1 – 2 g/kg in substantia nigra pars compacta cells. Notably, the present study observed increases in DA concentrations that peaked at 1 or 2 g/kg ethanol in each rat, suggesting that the tonic dopaminergic response to ethanol is predictable when monitored across the vertical range of the NAc. In addition, these microdialysis data validate the intravenous dosing regimen used in the present study, as they are consistent with previous dose-response curves using intraperitoneal injections (Imperato and Di Chiara, 1986; Yoshimoto et al., 1992a, 1992b; Kiianmaa et al., 1995; Yim et al., 2000), although it can be noted that the brain concentrations would be higher with intravenous administration (Robinson et al., 2002a; Howard et al., 2008).
In contrast, when measured on the 100-μm dimension by fast scan cyclic voltammetry, only a subset of recordings yielded increases in phasic DA release to ethanol as predicted by the electrophysiological data (Mereu et al., 1984; Gessa et al., 1985). Specifically, increases in DA transient frequency to ethanol were observed in only 6 out of 16 of the recording sites. The remaining 10 sites were unresponsive to ethanol and exhibited transients rates that were identical to the those in rats receiving saline. Thus, these data are consistent with a report by Cheer et al. (2007), which also measured phasic DA release by using fast scan cyclic voltammetry and found “responsive” and “unresponsive” sites in the NAc after a single dose of 1 g/kg ethanol, with approximately half of the recording sites yielding significant increases in transient rates in the 5 min after ethanol infusion. One explanation for this heterogeneity may be individual differences among rats in overall sensitivity to ethanol; however, this interpretation is unlikely for two reasons. First, tonic DA responses to ethanol measured with microdialysis were consistent, in that each rat showed a comparable extracellular DA profile that peaked at the 1 or 2 g/kg dose. Second, each rat in the experiment became immobile and lost muscle tone after the 2 g/kg dose, indicating that all subjects were behaviorally responsive to ethanol. Rather, we interpret the heterogeneity of ethanol induced changes in the rate of DA transients as anatomically site-specific. This interpretation is strengthened by the apparent reproducibility of the response: both Cheer et al. (2007) and this study demonstrated that in those sites that were “responsive” (i.e., exhibited phasic DA release in response to ethanol), a subsequent dose of ethanol elicited a similar phasic DA response.
The present and previous studies (Robinson and Wightman, 2007; Wightman et al., 2007) have reported large variability in the baseline rates of DA transients within the NAc core and shell. Similarly, DA neurons have been shown to vary widely in their tendency to fire in bursts, and in electrophysiological experiments DA neurons have been identified as either “low” or “high” bursting, with bursts of 3 or more spikes occurring at an average of 0.05 and 0.48 Hz, respectively (Hyland et al., 2002). This variation in bursting is likely reflected in the basal rates of DA transients reported here (0.004 – 0.31 Hz) and in previous studies. Similarly, it is possible that DA neurons may differ in response to ethanol in the amount of bursting as opposed to simple increases in firing rate, and fast scan cyclic voltammetry may only detect release from the former. However, it is unknown whether such cell-specific variation in ethanol-induced bursting of DA neurons occurs in awake animals. While Mereu et al. (1984) stated that burst-firing was part of the ethanol-induced excitation of DA neurons in awake, paralyzed rats, that firing pattern was only quantified in anesthetized rats (Foddai et al., 2004). Nevertheless, DA neurons within the ventral tegmental area have heterogeneous electrophysiological properties and pharmacological responses (Ford et al., 2006; Margolis et al., 2006; Margolis et al., 2008). In addition, there are reports of regional differences in the effects of ethanol within the ventral tegmental area, suggesting differential sensitivity to the drug among DA cell body regions. For example, NAc increases in tonic DA induced by ethanol can be blocked by mecamylamine (nicotinic acetylcholine receptor antagonist) infusion to the anterior but not posterior ventral tegmental area (Ericson et al., 2008). In addition, rats will self-administer ethanol into the posterior but not the anterior ventral tegmental area (Rodd et al., 2004). As DA neurons have a general topographic projection to the ventral striatum (Haber et al., 2000; Ikemoto, 2007), it is possible that each voltammetric recording reveals the propensity of a subset of DA neurons to burst at baseline as well as at increasing concentrations of ethanol; in contrast, the temporal and spatial information of the microdialysis probe is insufficient to resolve this. Importantly, when comparing DA cell firing with DA release, note that it is unknown whether all bursts will result in DA transients. For instance, there may be a requirement that multiple DA neurons burst in synchrony (Robinson and Wightman, 2007), or local influences such as glutamate and acetylcholine might independently modulate DA release at the terminal (Wu et al., 2000; Avshalumov et al., 2003; Rice and Cragg, 2004; Zhang and Sulzer, 2004). Thus, the heterogeneous response of DA transients to ethanol observed in this and previous studies (Cheer et al., 2007) may be due to variable ethanol effects on DA burst firing, on synchronous activity of DA neurons, on regulation of DA release at the terminal, or some combination of these.
It is well known that tonic DA concentrations in the shell region of the NAc are particularly responsive to drugs of abuse, including ethanol (e.g., Di Chiara et al., 2004). While tonic measurements in the present study were primarily (but not exclusively) in the shell, Howard et al. (2008) recently demonstrated that tonic DA release in response to single intravenous doses of 1 or 1.5 g/kg ethanol is greater in the NAc shell than the core. Thus, we also investigated whether NAc subregion contributed to the heterogeneous response of phasic DA release to ethanol administration. However, we found no subregional differences either in baseline rates of transients or in the responsiveness to ethanol. In contrast, a recent study revealed that while cocaine increases the frequency of DA transients across the NAc, more DA transients are evoked in the shell (Aragona et al., 2008). This discrepancy may be related to the different mechanisms of action of the two drugs: while cocaine increases phasic DA release by blocking uptake to amplify ongoing events (Robinson and Wightman, 2004; Venton et al., 2006; Wightman et al., 2007), ethanol does not reliably alter DA uptake (Samson et al., 1997, Yim and Gonzales, 2000; Robinson et al., 2005; Jones et al., 2006; Mathews et al., 2006).
In summary, these data illustrate a disparity between the tonic and phasic response of DA in the NAc to ethanol administration when monitored with microdialysis versus fast scan cyclic voltammetry. Consistent with previous studies (Wightman and Robinson, 2002; Floresco et al., 2003), these results suggest that tonic and phasic aspects of DA transmission may be pharmacologically distinct. However, testing this hypothesis would require suppressing the phasic response and still observing the tonic response (or vice versa). A simpler interpretation is that the DA signal measured with microdialysis provides a composite view of all tonic and phasic DA activity in the broad area of the microdialysis probe, including some microregions that show increased DA release after ethanol and others that do not. In the case of ethanol-induced DA release, the heterogeneity observed on the 100-μm scale may explain the generally modest increases in DA concentration consistently measured with microdialysis (typically < 200%; e.g., Imperato and Di Chiara, 1986; Yoshimoto et al., 1992b; Kiianmaa et al., 1995; Moscary and Bradberry, 1996; Yan, 1999; Yim et al., 2000). In fact, it is unknown to what extent DA transients contribute to DA concentrations measured with microdialysis. While is it clear that a single transient is not detected by microdialysis due to damage around the electrode (Yang et al., 1998), it seems reasonable that a sustained increase in the frequency of DA transients may generate sufficient concentrations of DA to be detected.
Given that phasic DA transients may provide a learning signal about natural and drug reinforcers (Phillips et al., 2003; Day et al., 2007; Schultz, 2007), a pharmacological effect of ethanol on the frequency of DA transients may influence the reinforcing properties of ethanol. Moreover, heterogeneity in the phasic DA response to ethanol is ideologically consistent with the hypothesis that the NAc is functionally segregated into distinct microcircuits (Pennartz et al., 1994; Carelli and Wightman, 2004). In this context, the finding that ethanol enhances phasic DA release in some sites but not others may be one mechanism by which ethanol selectively modulates particular NAc microcircuits during ethanol self-administration (Janak et al., 1999; Robinson and Carelli, 2008). Thus, it will be important for future experiments to measure DA release events in the context of ethanol self-administration and reinforcement.
Acknowledgments
The authors thank Ms. Dawnya Bohager, Ms. Lisa Gurdin, Ms. Amber Kinard and Mr. Wilbur Williams for assistance with voltammetry, Dr. Sheneil Anders for assistance with microdialysis, and the NIH (AA014591 to D.L.R., DA10900 to R.M.W. and AA011852 to R.A.G.) and the Bowles Center for Alcohol Studies at the University of North Carolina for funding.
Abbreviations
- DA
dopamine
- NAc
nucleus accumbens
References
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Marshall SP, Pena DM, Rice ME. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J Neurosci. 2003;23:2744–2750. doi: 10.1523/JNEUROSCI.23-07-02744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opinion Neurobio. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church WH, Justice JB, Jr, Byrd LD. Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine and benztropine. Eur J Pharmacol. 1987;139:345–348. doi: 10.1016/0014-2999(87)90592-9. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Preferential stimulation of dopamine release in the nucleus accumbens by opiates, alcohol, and barbiturates: studies with transcerebral dialysis in freely moving rats. Ann N Y Acad Sci. 1986;473:367–381. doi: 10.1111/j.1749-6632.1986.tb23629.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacol. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Ericson M, Lof E, Stomberg R, Chau P, Soderpalm B. Nicotinic acetylcholine receptors in the anterior, but not posterior, ventral tegmental area mediate ethanol-induced elevation of accumbal dopamine levels. J Pharmacol Exp Ther. 2008;326:76–82. doi: 10.1124/jpet.108.137489. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacol. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AS, Bunney BS. Activity of A9 and A10 dopaminergic neurons in unrestrained rats: further characterization and effects of apomorphine and cholecystokinin. Brain Res. 1987;405:46–55. doi: 10.1016/0006-8993(87)90988-7. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Meltzer LT, Bunney BS. Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci. 1985;36:1983–1994. doi: 10.1016/0024-3205(85)90448-5. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst. 2003;128:1413–1419. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Nat Aca Sci USA. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neurosci. 2008;154:1042–1053. doi: 10.1016/j.neuroscience.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neurosci. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Inoue H. Effects of naltrexone on the accumulation of L-3, 4-dihydroxyphenylalanine and 5-hydroxy-L-tryptophan and on the firing rate induced by acute ethanol administration. Eur J Pharmacol. 2000;406:375–380. doi: 10.1016/s0014-2999(00)00703-2. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chang JY, Woodward DJ. Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Res. 1999;817:172–184. doi: 10.1016/s0006-8993(98)01245-1. [DOI] [PubMed] [Google Scholar]
- Jones SR, Mathews TA, Budygin EA. Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse. 2006;60:251–255. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM. Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neurosci. 1992;51:55–64. doi: 10.1016/0306-4522(92)90470-m. [DOI] [PubMed] [Google Scholar]
- Kiianmaa K, Nurmi M, Nykanen I, Sinclair JD. Effect of ethanol on extracellular dopamine in the nucleus accumbens of alcohol-preferring AA and alcohol-avoiding ANA rats. Pharmacol Biochem Behav. 1995;52:29–34. doi: 10.1016/0091-3057(95)00097-g. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Heterogeneity of ventral tegmental area neurons: single-unit recording and iontophoresis in awake, unrestrained rats. Neuroscience. 1998;85:1285–1309. doi: 10.1016/s0306-4522(98)00054-2. [DOI] [PubMed] [Google Scholar]
- Logman MJ, Budygin EA, Gainetdinov RR, Wightman RM. Quantitation of in vivo measurements with carbon fiber microelectrodes. J Neurosci Methods. 2000;95:95–102. doi: 10.1016/s0165-0270(99)00155-7. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse. 2006;60:288–294. doi: 10.1002/syn.20301. [DOI] [PubMed] [Google Scholar]
- Mereu G, Fadda F, Gessa GL. Ethanol stimulates the firing rate of nigral dopaminergic neurons in unanesthetized rats. Brain Res. 1984;292:63–69. doi: 10.1016/0006-8993(84)90890-4. [DOI] [PubMed] [Google Scholar]
- Michael D, Travis ER, Wightman RM. Color images for fast-scan CV measurements in biological systems. Anal Chem. 1998;70:586A–592A. doi: 10.1021/ac9819640. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol. 1994;72:1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Mocsary Z, Bradberry CW. Effect of ethanol on extracellular dopamine in nucleus accumbens: comparison between Lewis and Fischer 344 rat strains. Brain Res. 1996;706:194–198. doi: 10.1016/0006-8993(95)01200-1. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; San Diego, CA: 1998. CD-ROM. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. J Neurochem. 2004;90:894–903. doi: 10.1111/j.1471-4159.2004.02559.x. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. Rapid dopamine release in freely moving rats. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. CRC Press; Boca Raton: 2007. pp. 17–34. [PubMed] [Google Scholar]
- Robinson DL, Carelli RM. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. Eur J Neurosci. 2008;28:1887–1894. doi: 10.1111/j.1460-9568.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Brunner LJ, Gonzales RA. Effect of gender and estrous cycle on the pharmacokinetics of ethanol in the rat brain. Alcohol Clin Exp Res. 2002a;26:165–172. [PubMed] [Google Scholar]
- Robinson DL, Heien ML, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci. 2002b;22:10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Volz TJ, Schenk JO, Wightman RM. Acute ethanol decreases dopamine transporter velocity in rat striatum: in vivo and in vitro electrochemical measurements. Alcohol Clin Exp Res. 2005;29:746–755. doi: 10.1097/01.alc.0000164362.21484.14. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacol. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Erickson HL, Niehus JS, Gerhardt GA, Kalivas PW, Floyd EA. The effects of local application of ethanol in the n. accumbens on dopamine overflow and clearance. Alcohol. 1997;14:485–492. doi: 10.1016/s0741-8329(96)00216-9. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacol. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Chergui K, Chouvet G, Gonon F. Relationship between dopamine release in the rat nucleus accumbens and the discharge activity of dopaminergic neurons during local in vivo application of amino acids in the ventral tegmental area. Neurosci. 1992;49:63–72. doi: 10.1016/0306-4522(92)90076-e. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with ‘reward’. J Neurochem. 2002;82:721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Pearl SM, Zigmond MJ, Michael AC. Inhibitory glutamatergic regulation of evoked dopamine release in striatum. Neurosci. 2000;96:65–72. doi: 10.1016/s0306-4522(99)00539-4. [DOI] [PubMed] [Google Scholar]
- Yan QS. Extracellular dopamine and serotonin after ethanol monitored with 5-minute microdialysis. Alcohol. 1999;19:1–7. doi: 10.1016/s0741-8329(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Yang H, Peters JL, Michael AC. Coupled effects of mass transfer and uptake kinetics on in vivo microdialysis of dopamine. J Neurochem. 1998;71:684–692. doi: 10.1046/j.1471-4159.1998.71020684.x. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol. 2000;22:107–115. doi: 10.1016/s0741-8329(00)00121-x. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Robinson DL, White ML, Jaworski JN, Randall PK, Lancaster FE, Gonzales RA. Dissociation between the time course of ethanol and extracellular dopamine concentrations in the nucleus accumbens after a single intraperitoneal injection. Alcohol Clin Exp Res. 2000;24:781–788. [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O’Connor SJ, Morris ED. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res. 2007;31:965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Ethanol enhances the release of dopamine and serotonin in the nucleus accumbens of HAD and LAD lines of rats. Alcohol Clin Exp Res. 1992a;16:781–785. doi: 10.1111/j.1530-0277.1992.tb00678.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992b;9:17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]