Abstract
In most mammals, placentation is critical for fetal development and pregnancy success. Exposure to marijuana during pregnancy has adverse effects, but whether the placenta is a target of cannabinoid/endocannabinoid signaling is not known. Using mice as a model system, we found that the endocannabinoid system is present in the ectoplacental cone and spongiotrophoblast cells. We also observed that aberrant endocannabinoid signaling confers premature trophoblast stem cell differentiation, and defective trophoblast development and invasion. These defects are reflected in retarded fetal development and compromised pregnancy outcome. Because the endocannabinoid system is conserved in mice and humans, our study suggests that endocannabinoid signaling is critical to placentation and pregnancy success in humans and implicates its potential significance in stem cell biology.
Keywords: CNR1, fatty acid amide hydrolase, placenta, stem cell
In eutherians, the placenta is the sole bridge between the mother and fetus. Although maternal resources filtered across the selective barrier of the placenta nourish and protect the conceptus, the placenta also reshapes maternal physiology to facilitate fetal survival. Most placental functions are attributed to various trophoblast lineages: giant cells, spongiotrophoblast, syncytiotrophoblast, and trophoblast cells (1). The fact that all trophoblast cell types are derived from trophoblast stem (TS) cells suggests that TS cell differentiation is a tightly regulated process (2). An appropriate ratio of different trophoblast cell types is a prerequisite for normal placentation; any aberration in trophoblast differentiation compromises normal placentation.
In mice, immediately after implantation, the mural trophectoderm penetrates the uterine stroma, forming primary trophoblast giant cells (TGCs). The polar trophectoderm, adjacent to the inner cell mass (ICM), forms the extraembryonic ectoderm and ectoplacental cone (EPC), in which progenitor cells that differentiate into secondary TGCs reside. Both primary and secondary giant cells undergo multiple rounds of endoreduplication, resulting in polyploid cells (3). EPC and extraembryonic ectoderm cells give rise to the spongiotrophoblast (SPT) and labyrinth layers of the placenta (2). In vivo, progenitor cells in the extraembryonic ectoderm and EPC generate chorionic trophoblasts or SPT. However, TS cells in culture primarily differentiate into TGCs, leading to the concept that whereas differentiation to the giant cell lineage is a default pathway, differentiation to other lineages requires distinct signaling cues (4).
Endocannabinoid signaling is mediated by two G-protein–coupled receptors, CNR1 (5) and CNR2 (6), commonly known as CB1 and CB2, respectively. Endocannabinoids are bioactive lipid mediators that activate these receptors. The two most studied endocannabinoids are N-arachidonoylethanolamine, or anandamide (AEA) and 2-arachidonoylglycerol (2-AG) (7, 8). AEA levels are regulated by a balance between the rate of its synthesis and degradation. Several AEA synthetic pathways have been identified (9–11), providing evidence that biosynthesis of AEA is complex. AEA is degraded to ethanolamine and arachidonic acid by a membrane-bound fatty acid amide hydrolase (FAAH) (12). Although FAAH can hydrolyze other endocannabinoids including 2-AG, studies in Faah−/− mice show that FAAH is the major player in regulating the magnitude and duration of AEA signaling (10, 13).
The evolutionarily conserved nature of the endocannabinoid system across species from invertebrates to vertebrates suggests its important biological functions (14). Certain aspects of endocannabinoid signaling in pregnancy have similarities between mice and humans. For instance, pregnancy success in both mice and humans requires regulated AEA signaling (15–17). Whereas Cnr1−/− mice show oviductal retention of embryos (18), women with ectopic pregnancy have reduced CNR1 expression in the Fallopian tubes (19).
In humans and rats, the endocannabinoid system is associated with recurrent miscarriages (20), parturition (21), as well as placental production of PGE2 (22) and nitric oxide (23). However, the role of endocannabinoid signaling in TS cell differentiation and growth that occur early in placentation remains undefined. In this study, we used a genetic approach to address this question. We found midgestational fetal loss in Cnr1−/− females. Further in vivo and in vitro studies show that TS cells missing Cnr1 fail to undergo appropriate proliferation and differentiation required for normal placentation, leading to midgestational fetal demise and compromised pregnancy outcome.
Results
Placentation Is Compromised in Cnr1−/− Mice.
Endocannabinoid signaling plays a key role in regulating female fertility. We previously showed that mice missing Faah or Cnr1 have impaired preimplantation embryo development and oviductal embryo transport (18, 24). Nearly 30% of embryos are trapped in the oviducts of these mutant mice. In addition, breeding data of Cnr1−/− females mated with Cnr1−/− males, and Faah−/− females mated with Faah−/− males show a 50% reduction in litter sizes compared with crosses between WT females and males. In contrast, the average litter size of Cnr2−/− females mated with Cnr2−/− males is normal (Fig. 1A). This dramatic reduction in Cnr1−/− and Faah−/− litter sizes suggests that nearly 20% of embryos are lost during gestation, considering the 30% of embryos retained in the oviduct. Moreover, 40% of plug-positive Cnr1−/− females did not produce any litters (18), underscoring the severity of embryo loss during gestation.
Fig. 1.
Cnr1−/− mice have compromised placentation, embryo development, and pregnancy outcome. (A) Cnr1−/− and Faah−/− mice have smaller litter sizes (unpaired t test, *P < 0.05), whereas litter size in Cnr2−/− mice is comparable to that of WT mice. (B) Cnr1−/− mice show higher resorption rates from day 12 of pregnancy, whereas increased resorption rates in Faah−/− females are evident on day 14 (F test, *P < 0.05). (C) Cnr1−/− and Faah−/− implantation sites (IS) weigh less than those of WT at midgestation (unpaired t test, *P < 0.05). (D) Weights of Cnr1−/−, but not Faah−/−, placentas are lower than WT placentas on days 12 and 14 of pregnancy (unpaired t test, *P < 0.05). Numbers within bars indicate numbers of dams examined.
To determine the stage when pregnancy fails, we examined the pregnancy status on days 10–14 in WT, Cnr1−/−, and Faah−/− females. Compared with WT females, resorption rates in Cnr1−/− females were higher on days 12 and 14 of pregnancy. In Faah−/− females, the resorption rate was similar to WT females on day 12, but the rate was higher on day 14 (Fig. 1B). These results suggest that augmented and diminished endocannabinoid signalings regulate midgestational pregnancy events differently. Although weights of implantation sites in Cnr1−/− and Faah−/− mice were lower on days 10 through 14 (Fig. 1C), a careful examination of placental weights revealed that only Cnr1−/−, but not Faah−/−, females had reduced placental weights compared with weights of WT placentas (Fig. 1D). These results suggest that both high and low endocannabinoid signaling compromises normal pregnancy events. Muted signaling in Cnr1−/− females resulted in placentation defects and subsequent pregnancy loss starting from day 12 of pregnancy, whereas the augmented cannabinoid signaling was not reflected in altered placental weight and did not initiate pregnancy loss until late midgestation.
Endocannabinoid System Is Present in Midgestational Placentas.
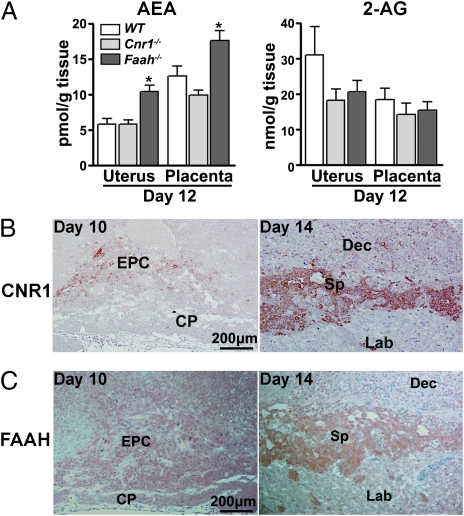
To study the role of the endocannabinoid system, we first measured the levels of AEA and 2-AG. We found that whereas uterine and placental AEA and 2-AG levels are comparable between WT and Cnr1−/− females on day 12 (Fig. 2A), AEA levels were elevated in these tissues in Faah−/− females on the same day. Consistent with our previous observation (13), the results indicate that FAAH is a major regulator of tissue AEA status. CNR1-positive cells are present in the EPC on day 10 of pregnancy as examined by immunohistochemistry. On day 14, CNR1 is expressed in the SPT layer which is derived from the EPC (Fig. 2B). FAAH showed an expression pattern similar to CNR1 (Fig. 2C). We also found that other endocannabinoid metabolic enzymes and Trpv1, which can be activated by AEA, are all present in the mouse placenta (Fig. S1), but without much variation in their expression levels between WT and Cnr1−/− placentas. In midgestation placentas, colocalization of FAAH with CNR1 and the presence of other players in the endocannabinoid system led us to speculate that generation, differentiation, and/or maintenance of SPT cells is regulated by endocannabinoid signaling, and that aberration of this signaling leads to abnormal placentation.
Fig. 2.
Endocannabinoid system is present in midgestational placentas. (A) In midgestational uteri and placentas, AEA levels are higher in Faah−/− mice, whereas in WT and Cnr1−/− mice, levels are comparable. Uterine and placental samples were pooled separately from five pregnant mice in each group (n = 4–5). (B) CNR1 is present in trophoblast cells in EPC on day 10 and SPT layer on day 14 of pregnancy. (C) FAAH expression pattern is similar to CNR1 in midgestational placentas. Results are representative of at least two to three independent samples. CP, chorionic plate; Dec, decidua basalis; EPC, ectoplacental cone; Lab, labyrinth; Sp, spongiotrophoblast.
Cnr1−/− Mice Have Compromised Spongiotrophoblast Development.
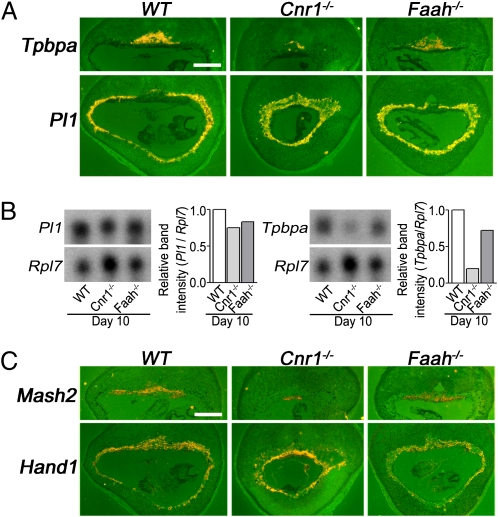
To better understand the cause of midgestational pregnancy defects resulting from aberrant endocannabinoid signaling, we assessed placental development in WT, Cnr1−/−, and Faah−/− placentas. Placental lactogen I (Pl1), encoding a prolactin-like hormone, is specifically expressed in TGCs (25), whereas trophoblast-specific protein α (Tpbpa) is explicitly expressed in SPT cells (26). In situ hybridization results showed that Pl1 expression in WT, Cnr1−/−, and Faah−/− placentas on day 10 of pregnancy is comparable (Fig. 3A). However, Tpbpa expression is appreciably reduced in Cnr1−/− placentas, providing evidence for compromised development of the Cnr1−/− SPT layer. These results are consistent with Northern hybridization results showing higher Tpbpa expression in WT placentas than in Cnr1−/− placentas on day 10 (Fig. 3B). In Faah−/− placentas, the overall expression intensity of Tpbpa was lower than WT cells, and this change was reflected in a modest reduction in Tpbpa-positive cell population (Fig. 3 A and B). We also examined the expression of these marker genes in Cnr2−/− placentas and found them to be normal (Fig. S2).
Fig. 3.
Cnr1−/− spongy layer is developmentally compromised. (A) Although the number of Tpbpa positive cells is considerably lower in Cnr1−/− placentas compared with WT and Faah−/− placentas, Pl1 expression is similar in placentas of all three genotypes on day 10. (Scale bar, 1,000 μm.) (B) Northern blot analyses of WT, Cnr1−/−, and Faah−/− implantation sites on day 10 of pregnancy show that Tpbpa, but not Pl1, levels in Cnr1−/− mice are reduced. Levels of Tpbpa and Pl1 mRNAs were normalized and quantified against Rpl7 (a house-keeping gene) using the same membrane. (C) Levels of mash2 transcripts were reduced in Cnr1−/− placentas, but Hand1 levels were comparable in WT, Cnr1−/−, and Faah−/− placentas. (Scale bar, 1,000 μm.)
Secondary TGCs are differentiated from precursor cells present in the EPC and later in the SPT layer. Mash2 maintains trophoblast precursor cells in an undifferentiated state (27), whereas Hand1 promotes differentiation into TGCs (28). Perhaps the compromised SPT layer in Cnr1−/− mice resulted from misregulated expression of Hand1 and Mash2. Our in situ hybridization results show that Hand1 expression in TGCs and SPT cells is similar in WT, Cnr1−/−, and Faah−/− mice on day 10 (Fig. 3C), but the number of Mash2-positive cells is reduced in Cnr1−/− placentas. This observation suggests that the absence of endocannabinoid signaling via CNR1 compromises Mash2-positive Tr precursor cells, whereas the elevated signaling does not affect the precursor cell pool. Collectively, the findings raise two possibilities: (i) Cnr1 deficiency in the EPC suppresses spongiotrophoblast cell proliferation, thus reducing the population of Tpbpa positive Tr cells; or (ii) the loss of Cnr1 in Tr precursor cells promotes their differentiation to TGCs but not SPT cells.
Trophoblast Cell Proliferation Is Stifled by Aberrant Endocannabinoid Signaling.
To test whether CNR1 deficiency inhibits trophoblast cell proliferation in the EPC, BrdU incorporation at the implantation sites on day 9 was assessed in WT, Cnr1−/− and Faah−/− mice. SPT cells are derived from Tr cells in the EPC (29), which show rapid proliferation on day 9 of pregnancy. In WT mice most cells in the EPC were BrdU positive (Fig. 4A). However, the number of BrdU-positive cells was markedly reduced in the Cnr1−/− EPC. Although many Cnr1−/− embryos showed retarded development, some Cnr1−/− embryos showing development comparable to WT embryos (Fig. S3) also had fewer BrdU-positive cells in the EPC and TGC. Reduced proliferation of Cnr1−/− Tr progenitor cells in the EPC, which later differentiate into SPT cells, most likely resulted in compromised SPT development. Faah−/− TGCs proliferate normally, but the number of the BrdU-positive Tr progenitor cells within the EPC was somewhat lower compared with those in WT EPC (Fig. 4A).
Fig. 4.
Compromised proliferation of trophoblast cells missing Cnr1. (A) BrdU incorporation results show that Cnr1−/− TS cells in the EPC proliferate at a much slower rate compared with WT TS cells, whereas Faah−/− TS cells show less reduction in proliferation. Em, embryo; EPC, ectoplacental cone. (Scale bar, 200 μm.) (B) RT-PCR analysis coupled with Southern blotting shows that Cnr1, but not Cnr2, is expressed in WT TS cells. RNA samples from brain (Br) and spleen (Sp) were used as positive controls for Cnr1 and Cnr2, respectively. TS, trophoblast stem cells. (C) Proliferation of Cnr1−/−/Cnr2−/− and Faah−/− TS cells is much slower than WT TS cells in vitro (n = 3). (D) Treatment with mAEA (7 nM) increases WT TS cell proliferation in vitro, and this effect is suppressed by a CNR1-specific inhibitor, SR141716 (n = 3; unpaired t test, *P < 0.05).
In WT blastocysts, Cnr1 is expressed in the Tr, whereas Cnr2 is expressed in the ICM (30). We confirmed the absence of Cnr2 in WT TS cells by RT-PCR analysis followed by Southern hybridization (Fig. 4B). To complement our findings in vivo, we generated WT, Cnr1−/−/Cnr2−/− and Faah−/− TS cells for in vitro experiments. We first assessed TS cell proliferation by MTS assays (31). The same numbers of TS cells in each genotype were seeded in multiple wells, and cell numbers were counted using standard curves on given time points shown in Fig. 4C. Cnr1−/−/Cnr2−/− and Faah−/− TS cell proliferation was remarkably slower than that in WT TS cells. To confirm that the effect is mediated by CNR1, we further tested WT TS cell proliferation in the presence of methanandamide (mAEA), a stable analogue of AEA, or vehicle (control). Compared with the control group, WT TS cells treated with mAEA (7 nM) proliferated at a faster rate (Fig. 4D), and the effect was blocked by SR141716 (SR1), a CNR1 selective antagonist, suggesting CNR1 mediated endocannabinoid signaling regulates TS proliferation.
SPT cell proliferation is tightly regulated by the PI3K-pAKT signaling pathway (32). Cannabinoid signaling via CNR1 can regulate AKT activation by the Gi/o/PI3K pathway (33, 34). We speculated that endocannabinoid signaling via CNR1 would regulate trophoblast cell proliferation by altering AKT activation. Our immunofluorescence results show that pAKT is localized in TS cells undergoing division (Fig. S4), suggesting that pAKT plays a role in TS cell proliferation. To test whether CNR1-mediated signaling involves AKT signaling in placentation, pAKT was localized by immunohistochemistry in day 10 placentas. Most Tr cells in the WT EPC were positive for pAKT, whereas the number of pAKT-positive cells was much lower in the Cnr1−/− and Faah−/− EPC (Fig. 5A). These results provide evidence that either higher or lower endocannabinoid signaling suppresses AKT activation, thereby attenuating cell proliferation in the EPC and SPT. To determine whether TS cell proliferation is indeed regulated by the PI3K/AKT pathway, WT TS cells were treated with the PI3K inhibitor LY294002. The inhibitor was effective in downregulating the pAKT level and proliferation of WT TS cells in a concentration-dependent manner (Fig. 5 B and C). Collectively, the results show that CNR1-mediated signaling via the PI3K–AKT pathway is important for appropriate TS cell proliferation.
Fig. 5.
Endocannabinoid signaling regulates TS cell proliferation via PI3K/AKT signaling. (A) Cnr1−/− placentas have reduced numbers of pAKT-positive cells in the EPC compared with Faah−/− and WT EPCs, although the numbers of pAKT-positive cells in Faah−/− EPCs were somewhat lower than WT. CP, chorionic plate; Dec, decidua basalis; EPC, ectoplacental cone. Results are representative of at least two to three independent samples. (Scale bar, 200 μm.) (B) Levels of pAKT in TS cells were reduced by PI3K inhibitor LY294002. Total AKT served as a loading control. (C) LY294002 attenuated WT TS cell proliferation in a concentration-dependent manner. TS cells were treated with 0, 2, 20, and 40 μM LY294002 for 72 h (n = 3).
Cnr1−/− TS Cells Are Biased to Differentiate into TGCs.
Because the SPT in the Cnr1−/− placenta is compromised, we examined whether the TS cell fate is biased under aberrant endocannabinoid signaling in addition to its role in cell proliferation. To circumvent the effects of maternal genotype on the development of Cnr1−/− placentas, we performed embryo transfer experiments. WT or Cnr1−/− embryos were transferred to WT recipients, and TS cell differentiation markers were examined on day 10 of pregnancy. We found that the population of Tpbpa-positive TS cells was sparse in placentas derived from Cnr1−/− embryos, but that Pl1 expression was comparable in both groups (Fig. S5). To further study the acute role of CNR1 during midgestation, WT females were treated with SR1 on days 8 and 9 of pregnancy when placentation is initiated. In situ hybridization experiments showed that SR1 treatment greatly reduced the number of Tpbpa-positive TS cells (Fig. S6) when examined on day 10. These results suggest that even an acute deficiency of CNR1 has some effects on placental differentiation.
In vitro studies also revealed that Cnr1−/−/Cnr2−/− TS cells cultured in the presence of FGF4 and mouse embryonic fibroblast (MEF) condition medium displayed dramatic morphological changes. Cnr1−/−/Cnr2−/− TS cells were larger with irregularly shaped large nuclei and numerous stress fibers (Fig. 6A). Cytoskeleton reorganization and larger cell size signify that TS cells are prone to differentiate into TGCs (35). In this respect, Cnr1−/−/Cnr2−/− TS cells resembled those in a differentiated state, forming loose and chaotic cell colonies (Fig. 6A). We also induced spontaneous differentiation in WT, Cnr1−/−/Cnr2−/−, and Faah−/− TS cells by culturing them in the absence of FGF4 and MEF condition medium. The trophoblast giant cell and spongy cell marker genes were examined by Northern blotting. Although all TS cells differentiated into trophoblast giant cells as evident by the expression of the Pl1 gene, the SPT marker Tpbpa was much reduced in Cnr1−/−/Cnr2−/− TS cells, confirming that TS cells are prone to differentiate into TGCs in the absence of CNR1 (Fig. 6B). To our surprise, we found that levels of Tpbpa transcripts are lower in Faah−/− TS cells, although Faah−/− placentas showed normal Tpbpa expression, suggesting that in vitro-derived results do not always necessarily correlate with in vivo findings. Nonetheless, these results indicate that regulated endocannabinoid signaling is required for maintaining appropriate TS cell state; the absence of CNR1 signaling suppresses differentiation of TS cells into SPT cells, but promotes differentiation to TGCs.
Fig. 6.
Cnr1−/−/Cnr2−/− TS cells are more prone to differentiate into TGC. (A) Under similar culture conditions, Cnr1−/−/Cnr2−/− TS cells have more stress fibers, as evident from increased f-actin immunofluorescence staining. Results are representative of at least two to three independent samples. (Scale bar, 50 μm.) (B) Northern blotting results from spontaneous differentiation experiments show that Tpbpa expression in WT TS cells on day 6 after withdrawal of the FGF4/condition medium were much higher than those in Cnr1−/−/Cnr2−/− and Faah−/− TS cells, suggesting that most Cnr1−/−/Cnr2−/− and Faah−/− TS cells had differentiated into TGCs in vitro. Levels of Tpbpa and Pl1 mRNAs were normalized and quantified against Rpl7 using the same membrane. 1, WT; 2, Cnr1−/−/Cnr2−/−; 3, Faah−/−.
Cnr1−/− Trophoblast Cells Show Shallow Invasion.
The maternal and fetal vascular systems are brought into close proximity through placentation and trophoblast cells directly tap into the maternal vascular system (36). Thus, trophoblast cell invasiveness is critical for successful placentation. COX2 is expressed in invading trophoblast cells with the initiation of placentation. Immunolocalization shows that the Cnr1−/− EPC has reduced COX2 expression compared with WT EPC on day 10 of pregnancy (Fig. 7B), suggesting that Cnr1−/− EPC cells are less invasive. This is consistent with previous findings that AEA increases PGE2 levels via CNR1 (22). Western blotting results are consistent with immunohistochemistry signals (Fig. 7C). The invasion of glycogen trophoblast cells (GTCs) into the decidua basalis is another indicator of trophoblast invasiveness. These cells, derived from the SPT on day 12, continue to express caudal type homeobox 2 (CDX2) (1). We examined CDX2 expression in the placenta on day 14. In WT females, an abundant number of CDX2-positive glycogen trophoblast cells invade into the decidual basalis, whereas only a sporadic number of them with shallow invasion was found beyond the TGC border in Cnr1−/− mice (Fig. 7A). The results suggest that trophoblast invasive ability is compromised in the absence of CNR1.
Fig. 7.
Cnr1−/− trophoblast cells are less invasive than WT cells. (A) Glycogen trophoblast cell invasion into decidua basalis on day 14 of pregnancy in Cnr1−/− mice is shallow as shown by CDX2 immunostaining. (Scale bar, 100 μm.) (B) In Cnr1−/− mice, the number of COX2-positive cells invading the maternal decidua around the ectoplacental cone is low. (Scale bar, 200 μm.) (C) Western blotting of COX2 levels in extracts of isolated EPC from day 10 WT and Cnr1−/− implantation sites. (D) In vitro invasion assays show that numbers of Cnr1−/−/Cnr2−/− TS cells successfully penetrating through the matrigel are much lower than those of Faah−/− and WT TS cells. Invasion of WT TS cells was attenuated by a CNR1 antagonist SR141716 (SR1, 7 nM), indicating that this receptor is critical for normal TS cell invasion. Results are representative of at least two to three independent samples.
Matrigel invasion assays are used to study the invasive ability of TGCs (37). Using this assay, we examined the effects of endocannabinoid signaling on TS cell invasive behavior in vitro. Only a limited number of Cnr1−/−/Cnr2−/− TS cells were able to penetrate through the matrigel membrane after 44 h in culture (Fig. 7D), suggesting that deficient endocannabinoid signaling suppresses the invasiveness of trophoblast cells. However, invasion of Faah−/− TS cells in culture was not significantly altered compared with WT TS cells; this was consistent with localization pattern of COX2 in day 10 Faah−/− and WT EPC (Fig. S7). In addition, WT TS cell invasiveness was blocked by the CNR1-selective antagonist SR1, but not SR144528 (SR2), a CNR2 antagonist. The results show that the silencing of CNR1 dampens trophoblast invasion.
Discussion
The functional placenta requires an appropriate and balanced repertoire of different Tr cell types to support fetal growth and survival. Using different murine models with aberrant endocannabinoid signaling, we show here that ablation of CNR1-mediated endocannabinoid signaling inhibits TS cell proliferation, differentiation into SPT cells, and Tr invasiveness, leading to defective placentation and fetal development with increased resorptions in Cnr1−/− females. However, TS cell proliferation and differentiation were modestly affected in Faah−/− females with higher AEA levels.
Interestingly, whereas placental weights in Cnr1−/− mice are lower, placental weights in Faah−/− mice are comparable to weights of WT placentas; this is consistent with the modest reduction of TS proliferation in Faah−/−placentas. These results suggest that the absence of endocannabinoid signaling causes abnormal placentation, whereas amplified endocannabinoid signaling adversely affects embryonic growth, reflected in reduced weights of implantation sites. Although Faah−/− placentas appear structurally normal, we cannot rule out the possibility of a functional abnormality at the molecular level. However, Faah−/− TS cells generated and cultured in vitro show inferior growth and differentiation, suggesting participation or deficiency of some other factors under in vitro conditions. These results also raise caution that in vivo and in vitro results cannot always be correlated.
The spongy layer is located between the outer secondary TGCs and inner labyrinth layer. The function of the SPT layer is still not clearly understood; it is thought to function as structural support to the developing labyrinth which is populated by maternal and fetal capillaries (36). Studies have shown that the labyrinth, SPT and TGC layers are all affected in Mash2−/− mice (38), although it is specifically expressed in the SPT layer. This suggests that a well-developed SPT layer is required for normal placentation. Our results show that CNR1 is expressed in EPC and SPT layers and Cnr1−/− placentas have reduced Tpbpa-positive SPT layers with unaffected TGCs. Thus, compromised SPT development leads to abnormal placentation and consequently resorption of the feto-placental unit.
The molecular mechanism by which endocannabinoid signaling regulates TS differentiation is not clearly understood. To better understand the role of this signaling in TS cell biology, we started generating TS cells from WT, Cnr1−/−, Cnr1−/−/Cnr2−/−, and Faah−/− blastocysts. Although we successfully generated TS cells from WT, Faah−/−, and Cnr1−/−/Cnr2−/− blastocysts, repeated attempts in parallel to generate Cnr1−/− TS cells were unsuccessful in our hands, the reason for which is not understood. Whether CNR2, which is expressed in the ICM (30), alters the behavior of Cnr1−/− Tr in vitro would require in-depth investigation. The finding that Cnr1−/−/Cnr2−/− TS cells are more prone to differentiate even in the presence of FGF4 and MEF condition medium suggests that TS cells missing endocannabinoid signaling have a different gene expression signature to maintain the stem cell property. In this respect, it would be interesting to see, in future studies, whether genes important for TS cell self-renewal and differentiation are differentially expressed in the blastocyst trophectoderm of different phenotypes.
Expression of Hand1 and Mash2 overlaps in the EPC and SPT in which Tpbpa-positive trophoblast precursor cells reside. Coordinated antagonistic effects of Hand1 and Mash2 regulate the differentiation of the precursor cells into secondary TGCs (39). However, a recent study shows that not all secondary TGCs are derived from Tpbpa-positive cells, indicating that the cell fate of some TGCs is decided before Tpbpa expression (40). In vitro spontaneous differentiation experiments with Cnr1−/−/Cnr2−/− TS cells show that Tpbpa is barely detected, suggesting that most, if not all, TS cells differentiated into TGCs. It is possible that deficient endocannabinoid signaling directly promotes TS cell differentiation to TGCs in vitro without going through the transition phase of Tpbpa-positive precursor cells. Alternatively, the reduced Tpbpa-positive cells in Cnr1−/− mice were the consequence of reduced TS cell proliferation by decreased pAKT levels. These results are consistent with studies in other systems that CNR1 is coupled with Gi/o and activates AKT signaling by turning on 1-phosphatidylinositol 3-kinase (33, 34, 41).
Endocannabinoid signaling via CNR1 is operative in both human and rodent placentas (20, 23, 42, 43) and is associated with recurrent miscarriages (20), initiation of labor (21), and placental production of vasoactive mediators (22). However, it is not yet known how endocannabinoid signaling regulates TS cell proliferation and differentiation in the initial stage of placentation. Our present findings that endocannabinoid signaling via CNR1 directs the fate of TS cell differentiation and that silencing of endocannabinoid signaling causes defective placental development in mice could be clinically relevant to placentation in humans.
Materials and Methods
Reagents.
Antibodies to anti-CNR1 (N-15), anticytokeratin, and anti-phospho-AKT (Ser-473 or 308) were purchased from Santa Cruz Biotechnology, DAKO Diagnostics, and Cell Signaling, respectively. Anti-FAAH antibody is a gift from Benjamin F. Cravatt (The Scripps Research Institute, La Jolla, CA). SR141716 and SR144528 (National Institute on Drug Abuse, National Institutes of Health) were dissolved in ethanol and diluted to a final concentration containing less than 0.1% of ethanol.
Animals.
Adult WT Cnr1 and Faah mutant mice on C57BL/6J genetic background were generated as described elsewhere (13, 44) and housed in the animal care facility of Cincinnati Children's Hospital Medical Center according to National Institutes of Health and institutional guidelines for laboratory animals. Experimental procedures to analyze implantation sites and placenta are provided in SI Materials and Methods.
In Situ and Northern Hybridization.
In situ hybridization and Northern blotting were performed as previously described (45). Northern bands were quantified using Scion Image software.
Analysis of Anandamide and 2-AG.
Anandamide and 2-AG were assayed as previously described (46).
Trophoblast Stem Cell Culture.
WT, Cnr1−/−Cnr2−/−, and Faah−/− TS cells were generated as previously reported (47) and described in SI Materials and Methods.
Proliferation Assay.
Proliferation assays of WT, Cnr1−/−Cnr2−/−, or Faah−/− TS cells were performed using a Cell Proliferation Assay kit (Promega) and detailed in SI Materials and Methods.
Invasion Assay.
Invasion assays were performed in 24-well BD Biocoat Matrigel invasion chambers following the manufacturer's instructions and as detailed in SI Materials and Methods.
Statistical Analysis.
Comparison of means was performed by using the Student t test. Data are shown as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Erin L. Adams for editing the manuscript and Bliss Magella for technical assistance with several experiments. This work was supported by National Institutes of Health Grants DA06668 and HD12304 (to S.K.D.). and DA018224 (to H.B.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010892107/-/DCSupplemental.
References
- 1.Adamson SL, et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- 2.Cross JC, et al. Genes, development and evolution of the placenta. Placenta. 2003;24:123–130. doi: 10.1053/plac.2002.0887. [DOI] [PubMed] [Google Scholar]
- 3.Varmuza S, Prideaux V, Kothary R, Rossant J. Polytene chromosomes in mouse trophoblast giant cells. Development. 1988;102:127–134. doi: 10.1242/dev.102.1.127. [DOI] [PubMed] [Google Scholar]
- 4.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: Key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 5.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 6.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 7.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 8.Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- 12.Cravatt BF, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 13.Cravatt BF, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onaivi ES, et al. Endocannabinoids and cannabinoid receptor genetics. Prog Neurobiol. 2002;66:307–344. doi: 10.1016/s0301-0082(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 15.Paria BC, et al. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. J Biol Chem. 2001;276:20523–20528. doi: 10.1074/jbc.M100679200. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Dey SK, Maccarrone M. Jekyll and Hyde: Two faces of cannabinoid signaling in male and female fertility. Endocr Rev. 2006;27:427–448. doi: 10.1210/er.2006-0006. [DOI] [PubMed] [Google Scholar]
- 17.Habayeb OM, Bell SC, Konje JC. Endogenous cannabinoids: Metabolism and their role in reproduction. Life Sci. 2002;70:1963–1977. doi: 10.1016/s0024-3205(01)01539-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, et al. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat Med. 2004;10:1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- 19.Horne AW, et al. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS ONE. 2008;3:e3969. doi: 10.1371/journal.pone.0003969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamley LW, et al. Nuclear localisation of the endocannabinoid metabolizing enzyme fatty acid amide hydrolase (FAAH) in invasive trophoblasts and an association with recurrent miscarriage. Placenta. 2008;29:970–975. doi: 10.1016/j.placenta.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Acone G, et al. Low type I cannabinoid receptor levels characterize placental villous in labouring delivery. Placenta. 2009;30:203–205. doi: 10.1016/j.placenta.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell MD, et al. Cannabinoids stimulate prostaglandin production by human gestational tissues through a tissue- and CB1-receptor-specific mechanism. Am J Physiol Endocrinol Metab. 2008;294:E352–E356. doi: 10.1152/ajpendo.00495.2007. [DOI] [PubMed] [Google Scholar]
- 23.Cella M, et al. Dual effect of anandamide on rat placenta nitric oxide synthesis. Placenta. 2008;29:699–707. doi: 10.1016/j.placenta.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, et al. Fatty acid amide hydrolase deficiency limits early pregnancy events. J Clin Invest. 2006;116:2122–2131. doi: 10.1172/JCI28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faria TN, Ogren L, Talamantes F, Linzer DI, Soares MJ. Localization of placental lactogen-I in trophoblast giant cells of the mouse placenta. Biol Reprod. 1991;44:327–331. doi: 10.1095/biolreprod44.2.327. [DOI] [PubMed] [Google Scholar]
- 26.Lescisin KR, Varmuza S, Rossant J. Isolation and characterization of a novel trophoblast-specific cDNA in the mouse. Genes Dev. 1988;2:1639–1646. doi: 10.1101/gad.2.12a.1639. [DOI] [PubMed] [Google Scholar]
- 27.Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner AL. Essential role of Mash-2 in extraembryonic development. Nature. 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- 28.Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- 29.Cross JC. How to make a placenta: Mechanisms of trophoblast cell differentiation in mice—a review. Placenta. 2005;26(Suppl A):S3–S9. doi: 10.1016/j.placenta.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc Natl Acad Sci USA. 1995;92:9460–9464. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 32.Yang ZZ, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 33.Gómez del Pulgar T, Velasco G, Guzmán M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J. 2000;347:369–373. doi: 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina-Holgado E, et al. Cannabinoids promote oligodendrocyte progenitor survival: Involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parast MM, Aeder S, Sutherland AE. Trophoblast giant-cell differentiation involves changes in cytoskeleton and cell motility. Dev Biol. 2001;230:43–60. doi: 10.1006/dbio.2000.0102. [DOI] [PubMed] [Google Scholar]
- 36.Cross JC, et al. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol. 2002;187:207–212. doi: 10.1016/s0303-7207(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 37.Hemberger M, Hughes M, Cross JC. Trophoblast stem cells differentiate in vitro into invasive trophoblast giant cells. Dev Biol. 2004;271:362–371. doi: 10.1016/j.ydbio.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Gertsenstein M, Rossant J, Nagy A. Mash2 acts cell autonomously in mouse spongiotrophoblast development. Dev Biol. 1997;190:55–65. doi: 10.1006/dbio.1997.8685. [DOI] [PubMed] [Google Scholar]
- 39.Scott IC, Anson-Cartwright L, Riley P, Reda D, Cross JC. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Mol Cell Biol. 2000;20:530–541. doi: 10.1128/mcb.20.2.530-541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304:567–578. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102:1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 42.Park B, Gibbons HM, Mitchell MD, Glass M. Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta. 2003;24:990–995. doi: 10.1016/s0143-4004(03)00165-6. [DOI] [PubMed] [Google Scholar]
- 43.Habayeb OM, Taylor AH, Bell SC, Taylor DJ, Konje JC. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149:5052–5060. doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- 44.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das SK, et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: A possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 46.Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol. 2006;291:R349–R358. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.