Abstract
Ecological cues during prenatal and postnatal development may allow organisms to adjust reproductive strategy. The hypothalamic-pituitary-gonadal (HPG) axis is a prime candidate for adaptive plasticity as a result of its critical period of birth to 6 mo (B6M) in humans and the role of testosterone in the development and maintenance of costly sexually dimorphic somatic and behavioral traits. We hypothesized that weight velocity specific to B6M would predict male life history characteristics, including maturational timing, reproductive hormones, adult size, strength, and sexual activity. Data come from 770 Filipino men (age 20.5–22.5 y) followed since birth, with predictor variables including birth weight and weight velocities calculated at 6-mo intervals during the first 2 y of life. As expected, infants who were breastfed experienced less diarrhea, lived in wealthier households with better hygiene, and grew faster from B6M. Males with rapid B6M growth reached puberty earlier and, as young adults, had higher testosterone levels, were taller, more muscular, and had higher grip strength. They also had sex earlier and were more likely to report having had sex in the past month, resulting in more lifetime sex partners. Relationships between B6M weight gain and physical outcomes were generally not present or weaker in female subjects. We conclude that rapid weight gain specific to the brief postnatal hypothalamic-pituitary-gonadal critical period predicts early maturation and sexual activity, elevated hormone production, and more costly adult somatic characteristics among the male subjects in this sample. These findings provide evidence for early life developmental plasticity in male life history and reproductive strategy in humans.
Keywords: adaptation, developmental plasticity, testosterone, endocrinology, growth and development
Developmental plasticity in response to nutrients and hormones during fetal and infancy development can modify growth patterns, adult metabolism, and hormone regulation (1, 2). These effects are hypothesized to have evolved to allow modification of nutritional requirements and reproductive strategy as ecological conditions change (3–5). Maternally derived ecological cues transferred via the placenta or in breast milk could convey information about typical energetic or social experiences in the past (5, 6), and thus allow the developing organism to modify biological settings in anticipation of conditions likely to be experienced in the future (3, 4, 7).
Although widely cited, this hypothesis has been subjected to minimal empirical testing in humans. A small number of human studies provide evidence for early life plasticity in reproductive biology, which has clear implications for genetic fitness and thus could be under selection. Jasienska and colleagues (8) reported that the threshold of energetic stress that suppresses ovarian steroidogenesis varies according to a woman's own birth weight, and they speculated that this indicates a capacity to reset energetic thresholds regulating initiation of pregnancy in response to early life energetic cues. Studies in other species similarly point to evidence for long-term effects of fetal or early postnatal nutritional stress on sex steroids or ovulation rate in female offspring (9, 10). Whether such responses reflect an adaptive capacity to adjust reproductive strategy, a nonadaptive, vestigial feature of ontogeny (i.e., constraint), or imperfect buffering of developmental biology from impairment is a matter of ongoing debate (11).
In males the hypothalamic-pituitary-gonadal (HPG) axis is a good candidate for adaptive developmental plasticity (12). The axis influences energy needs by regulating resource allocation in support of growth and maintenance of sexually dimorphic, costly traits like muscle (13, 14), while also promoting reproductive behaviors (15, 16). In many mammals, including humans and other primates (17–20), the HPG axis generates two early-life surges in testosterone that have durable, organizational effects on multiple target tissues (21–24). The first begins in utero when testosterone production “masculinizes” the genitalia, sexually dimorphic regions of the CNS, and somatic traits (20, 21, 25–28). There is a second increase in luteinizing hormone (LH) during the first 6 mo of life, leading to circulating testosterone comparable to adult levels, followed by a decline in testosterone until puberty (18, 29). Although the function of this second testosterone surge in humans is poorly understood (30), there is evidence from animal models that early postnatal testosterone further masculinizes the brain and can lead to a higher growth trajectory and accretion of greater muscle mass (31–34).
Animal models show that exogenous stressors, hormones, or nutrients during early HPG critical periods can modify the organizational effects of testosterone, with downstream effects on growth and behavior. For instance, gestational stress imposed on rodents suppresses fetal testosterone (35) and has lasting effects on reproductive behavior (21, 26). In male rats and sheep, restricting prenatal or early postnatal nutrition can delay puberty and reduce adult testosterone (36, 37). Although comparable human data are limited, a clinical study found reduced testicular testosterone in adolescent males born small for gestational age (38). Collectively, these studies suggest that nutrition during the early HPG critical periods influences how the male phenotype is organized, and can have long-term effects on hormone regulation and physical and behavioral development.
Here we test the hypothesis that the rate of weight gain from birth to 6 mo—a proxy for nutritional and growth conditions during the early postnatal HPG critical period in humans—will predict characteristics of male life history and reproductive strategy. We test this hypothesis using data from 770 young adult male participants of a representative birth cohort study in urban and rural areas of metropolitan Cebu, Philippines. The study began in 1983, when pregnant mothers and later their newborns were enrolled, and has since followed offspring into adulthood. Here we relate maturational tempo, adult body size and composition, strength, reproductive hormones, and sexual activity to birth weight and to weight velocities calculated at 6-mo intervals between birth and 2 y of age. We recently showed that testosterone in early adulthood predicts lean mass, arm muscle, and strength in these men (39), and that, consistent with prior work in humans and other species (40, 41), testosterone and LH are both reduced in fathers (42). Here we add a developmental perspective to this work by testing whether weight velocity specific to the postnatal HPG critical period predicts variation in male life history characteristics among these men.
Results
Table 1 summarizes early life characteristics of the full sample and also stratifies the sample on tertiles of weight velocity corresponding with the postnatal HPG critical period in humans [birth to 6 mo (B6M)]. As expected (43), newborns who gained weight rapidly had slightly lighter birth weights. Although 0.1 kg lighter at birth, by 6 mo, fast growers were approximately 1.5 kg heavier than male subjects in the slowest growth tertile. Fast growth during the B6M interval was strongly related to favorable nutritional and growth conditions. Early fast growers lived in wealthier households with better hygiene scores and were born to more highly educated and better nourished mothers. They were more likely to have been breastfed late into the B6M interval and had fewer concurrent diarrheal episodes by maternal report. In bivariate tests, infants who grew fast after birth were taller, heavier, and had more lean mass and daily energy intake as adults (Table 2). They also matured earlier, and had had more lifetime sex partners by the time of the adult interview.
Table 1.
Early life (1983–1986) household, nutritional, and growth characteristics of Cebu male subjects stratified on tertiles of B6M weight velocity
| Weight velocity tertile |
|||||
| Variable | All (N = 706) | Slow (n = 237) | Middle (n = 233) | Fast (n = 236) | P value* |
| Maternal nutritional status and household characteristics | |||||
| Household assets, 0–10 | 2.6 (2.0) | 2.4 (1.9) | 2.7 (1.9) | 2.8 (2.1) | 0.1023 |
| Household income, pesos | 235 (349) | 217 (238) | 231 (387) | 257 (475) | 0.4831 |
| Mother's highest completed grade, 0–17 | 7.4 (3.7) | 6.9 (3.6) | 7.2 (3.6) | 8.1 (3.8) | 0.0009 |
| Hygiene index, 0–9 | 5.4 (1.9) | 5.1 (1.9) | 5.2 (1.9) | 5.8 (1.8) | 0.0001 |
| Mother's height, cm | 150.8 (5.2) | 149.7 (5.2) | 150.8 (5.0) | 151.9 (5.2) | 0.0001 |
| Mother's triceps skinfold, mm | 12.2 (4.5) | 11.7 (4.3) | 12.1 (4.4) | 12.9 (4.7) | 0.0121 |
| Mother's BMI, kg/m2 | 20.7 (2.8) | 20.2 (2.8) | 20.7 (2.9) | 21.2 (2.8) | 0.0009 |
| Infancy nutrition and growth | |||||
| Not breastfed at 4 mo of age | 17.6% | 23.6% | 14.2% | 14.8% | 0.011 |
| Birth–6 mo diarrhea, 0–3 maternal reports† | 0.26 (0.53) | 0.33 (0.60) | 0.25 (0.51) | 0.19 (0.47) | 0.044 |
| Weight at birth, kg | 3.03 (0.43) | 3.09 (0.44) | 3.03 (0.43) | 2.98 (0.43) | 0.022 |
| Weight 6 mo, kg | 7.14 (0.86) | 6.37 (0.60) | 7.12 (0.45) | 7.94 (0.63) | 0.0001 |
| Weight 24 mo, kg | 10.09 (1.14) | 9.39 (1.03) | 10.02 (0.90) | 10.87 (0.97) | 0.0001 |
| Weight velocity, birth to 6 mo, kg/wk | 0.16 (0.03) | 0.12 (0.02) | 0.16 (0.01) | 0.19 (0.02) | 0.0001 |
| Weight velocity, 6–24 mo, kg/wk | 0.04 (0.01) | 0.04 (0.01) | 0.04 (0.01) | 0.04 (0.01) | 0.180 |
Values are presented as mean (SD) where appropriate. BMI, body mass index.
*One-way ANOVA or χ2 testing for significant differences across B6M weight velocity tertiles.
†Mothers asked at 2, 4, and 6 mo if child had had diarrhea in past 7 d (0–3 possible).
Table 2.
Adult characteristics (2005) of Cebu men stratified on tertiles of B6M weight velocity
| Weight velocity tertile |
|||||
| Variable | All (N = 770) | Slow (n = 257) | Middle (n = 257) | Fast (n = 256) | P value* |
| Physical development | |||||
| Age, y | 21.48 (0.3) | 21.46 (0.3) | 21.46 (0.3) | 21.51 (0.3) | 0.087 |
| Height, cm | 163.0 (5.9) | 160.8 (5.9) | 162.7 (5.5) | 165.4 (5.5) | 0.0001 |
| Weight, kg | 55.9 (9.2) | 52.6 (7.4) | 55.4 (9.3) | 59.6 (9.5) | 0.0001 |
| BMI, kg/m2 | 21.0 (3.0) | 20.3 (2.4) | 20.9 (3.2) | 21.8 (3.2) | 0.0001 |
| Lean mass, kg† | 54.6 (8.8) | 51.6 (7.2) | 54.1 (8.8) | 58.1 (9.2) | 0.0001 |
| Arm muscle area, cm2 | 35.0 (7.7) | 34.1 (7.4) | 34.9 (7.8) | 36.1 (7.9) | 0.017 |
| Grip strength, kg | 73.1 (22.7) | 72.9 (21.5) | 71.3 (22.6) | 75.2 (24.0) | 0.141 |
| Adult energy intake, kcal/d | 2,188 (925) | 2,119 (813) | 2,136 (1026) | 2,309 (916) | 0.009 |
| Matured early‡ | 40.8% | 31.7% | 39.6% | 50.8% | 0.0001 |
| Reproductive status and behaviors | |||||
| Adult household income, pesos | 552 (805) | 496 (467) | 606 (1172) | 553 (594) | 0.939 |
| Currently pairbonded§ | 19.4% | 19.5% | 19.5% | 19.1% | 0.995 |
| Has ever had sex | 67.7% | 67.3% | 66.5% | 69.1% | 0.811 |
| In past month | 45.3% | 38.7% | 46.8% | 50.3% | 0.085 |
| No. of lifetime sex partners | 2.5 (7.4) | 1.7 (2.5) | 2.7 (7.2) | 3.2 (10.4) | 0.0001 |
| Has ever gotten woman pregnant | 21.4% | 21.0% | 18.7% | 24.6% | 0.257 |
| Does not live with child | 9.7% | 8.2% | 7.8% | 13.3% | 0.064 |
| Testicular function | |||||
| Morning testosterone, pg/mL | 190.4 (75.7) | 184.5 (68.8) | 190.6 (76.5) | 196.1 (81.1) | 0.210 |
| Evening testosterone, pg/mL† | 117.7 (53.1) | 117.6 (53.3) | 116.6 (55.1) | 119.0 (51.1) | 0.662 |
| Plasma LH, mIU/mL | 10.3 (4.6) | 10.6 (5.0) | 10.1 (4.4) | 10.0 (4.5) | 0.284 |
| Plasma FSH, mIU/mL | 3.0 (2.3) | 3.2 (3.0) | 2.9 (1.7) | 2.9 (2.0) | 0.430 |
Values are presented as mean (SD) where appropriate. BMI, body mass index.
*One-way ANOVA, χ2 or negative binomial regression (count data) testing for significant differences across B6M weight velocity tertiles.
†n = 762.
‡n = 748.
§Legally married or cohabiting with partner.
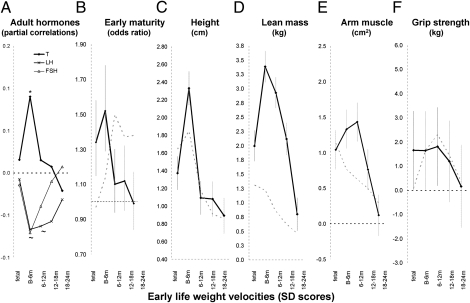
We next ran a series of multivariate models that evaluated whether early-life weight velocities predicted adult hormonal, physical, and reproductive outcomes, adjusting for potential confounding influences. To evaluate the specificity of timing of any associations with early weight gain, we included birth weight and postnatal weight velocities calculated at 6-mo intervals as predictors in all models (Materials and Methods provides details of the modeling approach and confounding factors included in each set of models). Fig. 1 shows partial correlations relating birth weight and 6-mo weight velocities with testosterone, LH, and follicle-stimulating hormone (FSH; Table S1). No weight velocity predicted evening testosterone (all P > 0.3; Table S1). B6M weight velocity was a significant predictor of waking testosterone (positive) and a borderline-significant predictor (inverse) of waking LH and FSH. Maternal stress results in lower birth weight and may program metabolism expressed later in life. Because rapid weight gain after birth may be a marker of fetal growth restriction (43), we tested for interactions between birth size and B6M weight velocity in models predicting each adult hormone. In these models, there were no significant or borderline significant birth weight-by-B6M interactions (all P > 0.6).
Fig. 1.
Early weight velocities (SD scores) as predictors of maturational tempo and adult hormones and physical outcomes: (A) partial correlations relating early life weight velocities and salivary waking testosterone and plasma LH and FSH adjusted for time of saliva or blood collection, wake time, usual wake time, fatherhood status, and age; (B) odds ratio for being an early maturer adjusting for income in adolescence; (C–F) regression coefficients reflecting change in adult physical outcomes predicted by a 1-SD change in early life weight velocities (gray bars, ±95% CI); y axis range set to 0.4 SD for visual comparability; all adjusted for age and household income, and D–F adjusted for demanding work, basketball playing, and weightlifting (male subjects) and physically demanding work and household activities (female subjects). Coefficients for female subjects are shown in dotted gray (SI Results). *P < 0.02, ∼P < 0.1.
We tested relationships between early growth and physical development, beginning with maturational tempo when participants were 15 to 16 y old (1998–1999). Logistic regression was used to predict which male subjects were relatively early maturers, as indicated by being in the two most advanced pubic hair stages (PH4 or PH5). Only birth weight and B6M weight velocity were significant predictors of early maturity, with B6M the strongest predictor (Fig. 1 and Table S2). In multiple regression models predicting adult height, lean mass, arm muscle area, and grip strength (Fig. 1), all four outcomes showed relationships with weight velocity during the first 6 to 12 mo of life, with weight velocities after 12 mo comparably weak predictors (Table S3). When models were rerun with birth weight-by-B6M weight velocity interaction terms, there was a significant interaction only in the model predicting adult height (interaction, P < 0.034), suggesting that rapid B6M weight velocity facilitated some degree of catch-up growth among men born small: a 1-SD increase in B6M weight velocity predicted a 2.1 ± 0.3 cm increase in adult height among men born in the upper half of the birth weight distribution, but this coefficient was increased by 0.48 ± 0.4 cm among men in the lower half of the birth weight distribution.
We next tested relationships with maturational tempo and adult physical outcomes in same-aged, nonpregnant female cohort members (n = 690; age, 21.5 ± 0.3 y). In contrast to findings in male subjects, B6M weight velocity was not a strong predictor of menarcheal age, lean mass, or arm muscle area among females (Fig. 1 and Table S4). The relationship between B6M and height was similar, although weaker in females, whereas relationships with grip strength were similar for both sexes and generally weaker than relationships with other outcomes. There were no significant birth weight-by-B6M interactions in models predicting any female outcome (interaction, P > 0.4 for all models).
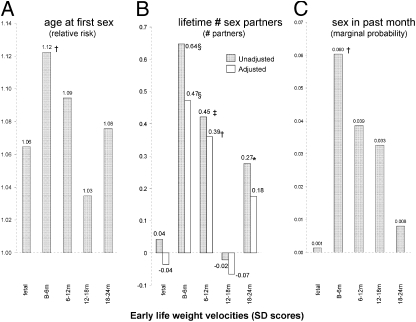
We next modeled the relationship between early weight velocities and reproductive behaviors in male subjects (SI Results). In a survival time regression (Weibull), B6M weight velocity was the only significant predictor of an earlier age at first sex (Fig. 2A). Fig. 2B shows the lifetime number of sex partners predicted by a 1-SD change in each predictor before (gray bars) and after (white bars) adjustment for maturational tempo. B6M weight velocity was the strongest predictor of lifetime partners, followed by 6 to 12 mo and 18 to 24 mo weight velocities. Adjusting for maturational tempo reduced all coefficients, but those for B6M weight velocity remained strongest and most significant. Because B6M remained a significant positive predictor of sex partners independent of the earlier maturity of fast early growers, we next investigated associations between early weight velocities and frequency of sexual activity. Adjusting for pair-bond status and other key potential confounders, B6M weight velocity was the only significant predictor of having had sex in the previous 1 month (Fig. 2C).
Fig. 2.
Early weight velocities (SD scores) as predictors of adult behaviors: (A) hazard of having first sex adjusted for income, education, and urbanicity; (B) lifetime number of sex partners adjusted for age, pair-bond status, income, education, and urbanicity with (white bars) and without (gray bars) adjustment for maturational tempo (SI Results; *P < 0.05, †P < 0.01, ‡P < 0.001, and §P < 0.0001); and (C) probability of having had sex in the previous 1 month among sexually active men and adjusting for age, pair-bond status, income, education, and urbanicity.
Associations between B6M weight velocity and adult outcomes could be confounded by differences in household resources, which could influence growth and development across the life course. Although all models described adjusted for household income (Materials and Methods), we also calculated correlations between each weight velocity and two robust measures of wealth (household income and assets) when the participants were 2, 8.5, 11, 15, and 22 y old. Men raised in wealthier homes grew faster between 6 and 12 mo and between 12 and 18 mo, but B6M weight velocity was a comparably weak correlate of these potential confounding influences (Fig. S1).
Discussion
In this large, representative sample of young adult Filipino men, weight velocity specific to the timing of the postnatal critical period of HPG axis development is a strong, and often the strongest, predictor of male life history traits measured two decades later. Men who grew rapidly from B6M had higher testosterone levels; were taller; had more lean mass, arm muscle, and grip strength; and had higher energy intake at 22 y of age. They also reached sexual maturity at an earlier age and reported a younger age of first sex, which in turn contributed to a greater lifetime number of sex partners. Although rapid postnatal growth may be a response to fetal growth restriction (43), the only significant birth weight-by-B6M interaction was found in models predicting adult height in male subjects, suggesting that rapid postnatal weight gain may facilitate some degree of catch-up growth among male, but not female, subjects when born small. All other relationships with B6M were independent of birth weight. Collectively, these findings link developmental, somatic, endocrine, and behavioral features of human male life history strategy to weight gain during a brief, early life period when the reproductive axis is highly active and is known to have organizational effects on sexually dimorphic regions of the CNS, peripheral organs, and somatic traits.
Mechanistically, several explanations for these associations are possible. Genes with pleiotropic effects could lead to genetic correlations between infancy growth and adult outcomes, as shown for other traits (44). Although there is evidence for moderate heritability of early growth in well nourished populations (45, 46), no heritability estimates have been calculated under conditions of nutritional stress and growth faltering, which were common in the Cebu cohort during infancy. Future studies should strive to incorporate analysis of growth-influencing genotypes (47), which could contribute to, or moderate, the associations we document.
Environmental factors are important determinants of infancy weight gain in this population (48, 49) and in other populations similarly faced with poverty and infancy undernutrition (50, 51). As expected, men who grew rapidly after birth experienced favorable growth conditions as indexed by household wealth and hygiene. This, combined with their higher incidence of breastfeeding, likely helps explain the less frequent diarrhea that their mothers reported for them. The environmental correlates of infancy weight gain documented here are consistent with prior analyses in the full cohort (48, 49), and suggest an important nutritional influence on B6M weight gain. Although we find little evidence that birth weight predicts adult outcomes in this sample, serial ultrasound measurements were not collected when the study began in 1983, which would be needed to evaluate whether fetal growth or nutrition specific to the fetal HPG critical period also predicts life history outcomes.
There was evidence for male-specific associations between early postnatal growth and adult phenotypes for most outcomes. This was particularly true for lean mass, arm muscle, and early maturity, which were strongly predicted by B6M weight velocity in male but not female subjects. Little is known about sex biases and their possible effects on development in this sample. However, differential treatment based on gender could contribute to the apparent sex differences in relationships between B6M weight velocity and adult outcomes that we document here (52). Although we are unable to rule out such biases, this is unlikely to explain why male-only traits like testicular hormone production show evidence for stronger associations with B6M weight gain than with weight gain that the same individual experienced at other ages in infancy and early childhood. In a rat model, maternal dietary restriction during the age of suckling has been shown to result in lower testosterone in adult male offspring, pointing to a long-term impact of early postnatal nutrition on HPG axis function (36).
Girls born light or thin, or who grow rapidly in infancy and childhood, experience earlier menarche (53, 54). The lack of an easily observed marker of male puberty has hampered similar investigations in male subjects. One recent exception is a study of German male subjects who reached peak height velocity (a marker of pubertal growth) earlier if they were born light or gained weight rapidly from birth to 24 mo (55). We find strong evidence for similar effects in the male subjects at Cebu, among whom B6M weight velocity was the strongest predictor of early maturation indicated by pubic hair stage. The absence of similar associations with early maturity among female subjects in Cebu is additional evidence for sex-specific developmental processes immediately after birth.
Although the earlier maturity and age at first sex of early fast growers at Cebu helps explain their increased lifetime number of sex partners, B6M remained a positive predictor of sex partners even after adjusting for early maturity. In follow-up analyses, we found that B6M weight velocity was also the only significant positive predictor of the frequency of recent sex in our sample, hinting at heightened sexual activity among these men. We can only speculate on possible explanations for the latter association, which could involve some combination of socially mediated behavioral changes facilitated by, for example, increased body size or earlier maturity (56), or direct organizational effects of infancy nutrition or androgens on the CNS (57).
Whether the developmental responses documented here reflect adaptive plasticity, a vestigial or nonadaptive form of plasticity, or developmental impairment is uncertain (11, 58, 59). Many species adjust developmental trajectory and adult phenotype based on early experience (60–62). In some birds, mothers modify yolk androgen deposition in response to social or nutritional cues, which leads to faster posthatching growth and adult size (63). Similar observations have been made in reptiles (64) and mammals (65). In wild ungulates, preweaning undernutrition disproportionately impacts male growth, attenuates adult size dimorphism, and diminishes testosterone-dependent adult traits (e.g., ornamentation) and reproductive success (66–69).
The relationships documented here are similar to these findings in wild mammal populations, and suggest that reproductive expenditure in human males is modified developmentally in response to early nutritional experiences. Unlike females, who invest metabolic resources directly in offspring production during pregnancy and lactation, males shunt a component of reproductive effort into building and maintaining a larger, more muscular body (12, 13, 70, 71). It has been postulated that this somatic component of reproductive effort should track longer-term energetic trends (70). Our findings are in agreement with this model and provide human evidence that nutritional and growth conditions specific to the HPG critical period influence adult male reproductive energetics (12). This is reflected in the taller stature and greater lean mass, strength, and caloric requirements of men with fast B6M growth. As adults, fast early growers also have higher testosterone levels, which has anabolic effects on muscle (72) and which we have recently shown positively relates to arm muscle area and strength among the men in our sample (39). Thus, our findings provide evidence for an important developmental influence on adult reproductive expenditure in human males. In contrast, among females, the intensive commitment of energetic resources required of each gestation and lactation may require acute sensitivity of ovulation and ovarian function to a woman's current energetic status and fat reserves (70).
The changes in maturational tempo that we document in relation to B6M weight velocity may also have fitness implications (73, 74). A related research tradition in developmental psychology has noted early maturity among females facing unstable household environments or psychosocial stress, which has been hypothesized to reflect an adaptive response to cues signaling high extrinsic mortality risk (75–80). Although early reproduction may be an appropriate response to high unavoidable mortality (76), the earlier maturity we document in response to favorable nutrition is also consistent with expectations of life history theory (5). Because extrinsic mortality is always greater than zero, all else equal, early reproduction should be favored by natural selection if faster growth allows adult size to be attained at an earlier age (5, 81). Our findings emphasize the importance of nutrition, in addition to mortality cues, as an important source of developmental information (82).
This study has several limitations. Our hormonal measures were based upon single saliva or plasma samples, and averaging measures across multiple days would reduce measurement error (83). We compensated by carefully standardizing sample collection times in a large sample of men. Because neither the field-based methods nor scientific rationale to collect infant steroids existed when this study began in 1983, we are not able to directly test the role of testosterone itself as a programming influence during the early infancy HPG critical period. The associations we document linking growth rate at this age with multiple aspects of life history and reproductive phenotype provide a strong rationale to incorporate measures of infancy HPG function in future longitudinal birth cohorts.
Methods
Study Population.
Data are from the Cebu Longitudinal Health and Nutrition Survey, a population-based study that has followed a birth cohort born in 1983–1984 (49, 84). This research was conducted with informed consent and human subjects clearance from the institutional review boards of Northwestern University and the University of North Carolina.
Anthropometry and Questionnaire Data.
Weight was measured at birth and at bimonthly intervals until 2 y of age (1983–1986). Birth weight was adjusted for gestational age at birth. Mothers answered questions about infant health and feeding and socioeconomic conditions (SI Methods). Adult outcomes were measured by trained interviewers (2005–2006). Anthropometrics were measured using standard techniques (SI Methods). Energy intake (from two 24-h recalls) was calculated using Food Composition Tables for use in the Philippines (85). Men answered questions about their households, families, and reproductive behaviors. In 1998 (age 15–16 y), participants reported pubic hair development by comparing themselves with drawings of pubic hair stages physician-validated among Filipino high school students. As possible, we tested comparable models in female cohort members limited to women who had early life and adult somatic outcomes (height, lean mass, arm muscle area, grip strength, n = 655 with 55 pregnant women excluded). Early weight velocities were also used to predict female maturational tempo (n = 744).
Salivary Testosterone and Plasma LH and FSH.
Salivary testosterone was measured in saliva collected before bed and immediately after waking. Testosterone was determined in duplicate using an enzyme immunoassay protocol for use with saliva samples (1–2402; Salimetrics). The between-assay coefficients of variation were 5.6% and 6.7% for high and low controls, respectively. Plasma LH and FSH were measured in morning plasma samples collected after an overnight fast in EDTA tubes and using a commercially available enzyme immunoassays (for LH, IB19104; Immuno-Biological Laboratories; for FSH; 40–056-205005; Genway Biotech). Between-assay coefficients of variation were 5.7% and 8.0% for low and high controls for LH and 7.2% and 12.6% for low and high controls for FSH.
Sample Selection.
In the 2005 survey, 1,008 male subjects among the original cohort of 1,633 liveborn male subjects were located and interviewed. Of these, 770 individuals agreed to participate and had sufficient sample for biomarker analysis and complete infancy weight characteristics. There were no significant differences in birth order or mother's height, age, education, or household income among the subset compared with the full birth sample of 1,633 (all P > 0.25). Participants in the subsample were 54 g heavier at birth (P < 0.003) than those included at birth but not in the final sample, although they had similar B6M weight velocities (P < 0.31).
Statistical Analyses.
All analyses used Stata software, version 10. Continuous and dichotomous outcomes were modeled using multiple regression and multiple logistic regression, with birth weight and all weight velocities included in the same model (first converted to sample-specific SD scores to allow comparison of coefficients). All models were adjusted for age and household income. Hormonal outcomes were adjusted for fatherhood status (42). Models predicting lean muscle and strength also adjusted for demanding work, basketball playing, and weightlifting (for male subjects) and demanding work and household activities (for female subjects). Behavioral outcomes in addition adjusted for education (highest grade) and urbanicity scale, and when modeling sexual activity, pair-bond status (SI Results). All count data were overdispersed and thus were modeled with negative binomial regression.
Supplementary Material
Acknowledgments
Yarrow Axford, Jay Belsky, Jared Bragg, Robert Denver, Dan Eisenberg, Peter Ellison, Lee Gettler, Pathik Wadhwa, Carol Worthman, and Zane Thayer gave helpful feedback. Elizabeth Quinn, Katy Sharrock, Jeffrey Huang, Iram Azam, Divya Mallampati, Brian Dubin, Amy Desantis, and Laura Rogers helped with laboratory work. We thank researchers at the Office of Population Studies, University of San Carlos, for their role in study design and data collection, and the Filipino participants who generously provided their time. This work was funded by Wenner Gren Foundation Grant 7356 and National Science Foundation Grant BCS-0542182. Fieldwork and sample collection were supported by pilot funds from the Interdisciplinary Obesity Center (RR20649) and the Center for Environmental Health and Susceptibility (ES10126; project 7-2004-E).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006008107/-/DCSupplemental.
References
- 1.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman PD, Hanson MA. Developmental Origins of Health and Disease. New York: Cambridge Univ Press; 2006. [Google Scholar]
- 3.Bateson P. Fetal experience and good adult design. Int J Epidemiol. 2001;30:928–934. doi: 10.1093/ije/30.5.928. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 5.Kuzawa CW. Fetal origins of developmental plasticity: Are fetal cues reliable predictors of future nutritional environments? Am J Hum Biol. 2005;17:5–21. doi: 10.1002/ajhb.20091. [DOI] [PubMed] [Google Scholar]
- 6.Wells JC, Stock JT. The biology of the colonizing ape. Am J Phys Anthropol. 2007;45(suppl):191–222. doi: 10.1002/ajpa.20735. [DOI] [PubMed] [Google Scholar]
- 7.Kuzawa CW, Quinn EA. Developmental origins of adult function and health: Evolutionary hypotheses. Annu Rev Anthropol. 2009;38:131–147. [Google Scholar]
- 8.Jasienska G, Thune I, Ellison PT. Fatness at birth predicts adult susceptibility to ovarian suppression: an empirical test of the Predictive Adaptive Response hypothesis. Proc Natl Acad Sci USA. 2006;103:12759–12762. doi: 10.1073/pnas.0605488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rae MT, et al. The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim Reprod Sci. 2002;72:63–71. doi: 10.1016/s0378-4320(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 10.Rhind SM. Effects of maternal nutrition on fetal and neonatal reproductive development and function. Anim Reprod Sci. 2004;82-83:169–181. doi: 10.1016/j.anireprosci.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Ellison PT, Jasienska G. Constraint, pathology, and adaptation: How can we tell them apart? Am J Hum Biol. 2007;19:622–630. doi: 10.1002/ajhb.20662. [DOI] [PubMed] [Google Scholar]
- 12.Kuzawa CW. Developmental origins of life history: Growth, productivity, and reproduction. Am J Hum Biol. 2007;19:654–661. doi: 10.1002/ajhb.20659. [DOI] [PubMed] [Google Scholar]
- 13.Bribiescas RG. Reproductive ecology and life history of the human male. Am J Phys Anthropol. 2001;33(suppl):148–176. doi: 10.1002/ajpa.10025.abs. [DOI] [PubMed] [Google Scholar]
- 14.Ellison PT. Energetics and reproductive effort. Am J Hum Biol. 2003;15:342–351. doi: 10.1002/ajhb.10152. [DOI] [PubMed] [Google Scholar]
- 15.Isidori AM, et al. Effects of testosterone on sexual function in men: Results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- 16.Archer J. Testosterone and human aggression: An evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Forest MG, Cathiard AM, Bertrand JA. Evidence of testicular activity in early infancy. J Clin Endocrinol Metab. 1973;37:148–151. doi: 10.1210/jcem-37-1-148. [DOI] [PubMed] [Google Scholar]
- 18.Winter JS, Faiman C, Hobson WC, Prasad AV, Reyes FI. Pituitary-gonadal relations in infancy. I. Patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J Clin Endocrinol Metab. 1975;40:545–551. doi: 10.1210/jcem-40-4-545. [DOI] [PubMed] [Google Scholar]
- 19.Mann DR, Fraser HM. The neonatal period: A critical interval in male primate development. J Endocrinol. 1996;149:191–197. doi: 10.1677/joe.0.1490191. [DOI] [PubMed] [Google Scholar]
- 20.Herman RA, Jones B, Mann DR, Wallen K. Timing of prenatal androgen exposure: Anatomical and endocrine effects on juvenile male and female rhesus monkeys. Horm Behav. 2000;38:52–66. doi: 10.1006/hbeh.2000.1608. [DOI] [PubMed] [Google Scholar]
- 21.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 22.Worthman C. Epidemiology of human development. In: Panter-Brick C, Worthman C, editors. Hormones, Health and Behavior: A Socio-Ecological and Lifespan Perspective. Cambridge, UK: Cambridge Univ Press; 1999. pp. 47–104. [Google Scholar]
- 23.Davies MJ, Norman RJ. Programming and reproductive functioning. Trends Endocrinol Metab. 2002;13:386–392. doi: 10.1016/s1043-2760(02)00691-4. [DOI] [PubMed] [Google Scholar]
- 24.Rhind SM, Rae MT, Brooks AN. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction. 2001;122:205–214. doi: 10.1530/rep.0.1220205. [DOI] [PubMed] [Google Scholar]
- 25.Jost A. The role of fetal hormones in prenatal development. Harvey Lect. 1961;55:201–226. [PubMed] [Google Scholar]
- 26.Dörner G. Hormones and Brain Differentiation. New York: Elsevier; 1976. [Google Scholar]
- 27.Shimada M, Murayama N, Yamazoe Y, Kamataki T, Kato R. Further studies on the persistence of neonatal androgen imprinting on sex-specific cytochrome P-450, testosterone and drug oxidations. Jpn J Pharmacol. 1987;45:467–478. doi: 10.1254/jjp.45.467. [DOI] [PubMed] [Google Scholar]
- 28.Gorski RA. Hypothalamic imprinting by gonadal steroid hormones. Adv Exp Med Biol. 2002;511:57–70. doi: 10.1007/978-1-4615-0621-8_5. [DOI] [PubMed] [Google Scholar]
- 29.Andersson AM, et al. Longitudinal reproductive hormone profiles in infants: Peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab. 1998;83:675–681. doi: 10.1210/jcem.83.2.4603. [DOI] [PubMed] [Google Scholar]
- 30.Quigley CA. Editorial: The postnatal gonadotropin and sex steroid surge-insights from the androgen insensitivity syndrome. J Clin Endocrinol Metab. 2002;87:24–28. doi: 10.1210/jcem.87.1.8265. [DOI] [PubMed] [Google Scholar]
- 31.Tarttelin MF, Shryne JE, Gorski RA. Patterns of body weight change in rats following neonatal hormone manipulation: a “critical period” for androgen-induced growth increases. Acta Endocrinol (Copenh) 1975;79:177–191. doi: 10.1530/acta.0.0790177. [DOI] [PubMed] [Google Scholar]
- 32.Jansson JO, Ekberg S, Isaksson O, Mode A, Gustafsson JA. Imprinting of growth hormone secretion, body growth, and hepatic steroid metabolism by neonatal testosterone. Endocrinology. 1985;117:1881–1889. doi: 10.1210/endo-117-5-1881. [DOI] [PubMed] [Google Scholar]
- 33.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 34.Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- 35.Ward IL, Weisz J. Maternal stress alters plasma testosterone in fetal males. Science. 1980;207:328–329. doi: 10.1126/science.7188648. [DOI] [PubMed] [Google Scholar]
- 36.Zambrano E, et al. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Silva P, Aitken RP, Rhind SM, Racey PA, Wallace JM. Influence of placentally mediated fetal growth restriction on the onset of puberty in male and female lambs. Reproduction. 2001;122:375–383. doi: 10.1530/rep.0.1220375. [DOI] [PubMed] [Google Scholar]
- 38.Cicognani A, et al. Low birth weight for gestational age and subsequent male gonadal function. J Pediatr. 2002;141:376–379. doi: 10.1067/mpd.2002.126300. [DOI] [PubMed] [Google Scholar]
- 39.Gettler LT, Agustin SS, Kuzawa CW. Testosterone, physical activity, and somatic outcomes among Filipino males. Am J Phys Anthropol. 2010;142:590–599. doi: 10.1002/ajpa.21282. [DOI] [PubMed] [Google Scholar]
- 40.Wingfield JC, Hegner RE, Dufty AM, Ball GF. The “challenge hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- 41.Gray PB, Campbell BC. Human male testosterone, pair bonding and fatherhood. In: Ellison PT, Gray PB, editors. Endocrinology of Social Relationships. Cambridge, MA: Harvard Univ Press; 2009. pp. 270–293. [Google Scholar]
- 42.Kuzawa CW, Gettler LT, Muller MN, McDade TW, Feranil AB. Fatherhood, pairbonding and testosterone in the Philippines. Horm Behav. 2009;56:429–435. doi: 10.1016/j.yhbeh.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prader A, Tanner JM, von Harnack G. Catch-up growth following illness or starvation. An example of developmental canalization in man. J Pediatr. 1963;62:646–659. doi: 10.1016/s0022-3476(63)80035-9. [DOI] [PubMed] [Google Scholar]
- 44.Suarez F, Zeghoud F, Rossignol C, Walrant O, Garabédian M. Association between vitamin D receptor gene polymorphism and sex-dependent growth during the first two years of life. J Clin Endocrinol Metab. 1997;82:2966–2970. doi: 10.1210/jcem.82.9.4232. [DOI] [PubMed] [Google Scholar]
- 45.van Dommelen P, de Gunst MC, van der Vaart AW, Boomsma DI. Genetic study of the height and weight process during infancy. Twin Res. 2004;7:607–616. doi: 10.1375/1369052042663805. [DOI] [PubMed] [Google Scholar]
- 46.Demerath EW, et al. Genetic and environmental influences on infant weight and weight change: the Fels Longitudinal Study. Am J Hum Biol. 2007;19:692–702. doi: 10.1002/ajhb.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dennison EM, et al. Southampton Genetic Epidemiology Research Group. Polymorphism in the growth hormone gene, weight in infancy, and adult bone mass. J Clin Endocrinol Metab. 2004;89:4898–4903. doi: 10.1210/jc.2004-0151. [DOI] [PubMed] [Google Scholar]
- 48.Popkin BM, et al. Breast-feeding and diarrheal morbidity. Pediatrics. 1990;86:874–882. [PubMed] [Google Scholar]
- 49.Adair L, et al. Growth dynamics during the first two years of life: A prospective study in the Philippines. Eur J Clin Nutr. 1993;47:42–51. [PubMed] [Google Scholar]
- 50.Rowland MG, Cole TJ, Whitehead RG. A quantitative study into the role of infection in determining nutritional status in Gambian village children. Br J Nutr. 1977;37:441–450. doi: 10.1079/bjn19770047. [DOI] [PubMed] [Google Scholar]
- 51.Schroeder DG, Martorell R, Rivera JA, Ruel MT, Habicht JP. Age differences in the impact of nutritional supplementation on growth. J Nutr. 1995;125(4, Suppl):1051S–1059S. doi: 10.1093/jn/125.suppl_4.1051S. [DOI] [PubMed] [Google Scholar]
- 52.Stinson S. Sex differences in environmental sensitivity during growth and development. Yearbook Phys Anthropol. 1985;28(suppl 6):123–147. [Google Scholar]
- 53.Adair LS. Size at birth predicts age at menarche. Pediatrics. 2001;107:E59. doi: 10.1542/peds.107.4.e59. [DOI] [PubMed] [Google Scholar]
- 54.van Weissenbruch MM, Delemarre-van de Waal HA. Early influences on the tempo of puberty. Horm Res. 2006;65(suppl 3):105–111. doi: 10.1159/000091514. [DOI] [PubMed] [Google Scholar]
- 55.Karaolis-Danckert N, Buyken AE, Sonntag A, Kroke A. Birth and early life influences on the timing of puberty onset: Results from the DONALD (DOrtmund Nutritional and Anthropometric Longitudinally Designed) Study. Am J Clin Nutr. 2009;90:1559–1565. doi: 10.3945/ajcn.2009.28259. [DOI] [PubMed] [Google Scholar]
- 56.Magnusson D, Stattin H, Allen VL. Biological maturation and social development: A longitudinal study of some adjustment processes from mid-adolescence to adulthood. J Youth Adolesc. 1985;14:267–283. doi: 10.1007/BF02089234. [DOI] [PubMed] [Google Scholar]
- 57.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 58.Schell LM, Magnus PD. Is there an elephant in the room? Addressing rival approaches to the interpretation of growth perturbations and small size. Am J Hum Biol. 2007;19:606–614. doi: 10.1002/ajhb.20669. [DOI] [PubMed] [Google Scholar]
- 59.Jones JH. Fetal programming: Adaptive life-history tactics or making the best of a bad start? Am J Hum Biol. 2005;17:22–33. doi: 10.1002/ajhb.20099. [DOI] [PubMed] [Google Scholar]
- 60.West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford Univ Press; 2003. [Google Scholar]
- 61.Denver RJ. Environmental stress as a developmental cue: Corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm Behav. 1997;31:169–179. doi: 10.1006/hbeh.1997.1383. [DOI] [PubMed] [Google Scholar]
- 62.Adkins-Regan E, Ottinger MA, Park J. Maternal transfer of estradiol to egg yolks alters sexual differentiation of avian offspring. J Exp Zool. 1995;271:466–470. [Google Scholar]
- 63.Schwabl H, Palacios MG, Martin TE. Selection for rapid embryo development correlates with embryo exposure to maternal androgens among passerine birds. Am Nat. 2007;170:196–206. doi: 10.1086/519397. [DOI] [PubMed] [Google Scholar]
- 64.Hews DK, Knapp R, Moore MC. Early exposure to androgens affects adult expression of alternative male types in tree lizards. Horm Behav. 1994;28:96–115. doi: 10.1006/hbeh.1994.1008. [DOI] [PubMed] [Google Scholar]
- 65.Dloniak SM, French JA, Holekamp KE. Rank-related maternal effects of androgens on behaviour in wild spotted hyaenas. Nature. 2006;440:1190–1193. doi: 10.1038/nature04540. [DOI] [PubMed] [Google Scholar]
- 66.Clutton-Brock TH, Guinness FE, Albon SD. Red Deer: Behavior and Ecology of Two Sexes. Chicago: Univ Chicago Press; 1982. [Google Scholar]
- 67.Post E, Langvatn R, Forchhammer MC, Stenseth NC. Environmental variation shapes sexual dimorphism in red deer. Proc Natl Acad Sci USA. 1999;96:4467–4471. doi: 10.1073/pnas.96.8.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Festa-Bianchet M, Jorgenson JT, Reale D. Early development, adult mass, and reproductive success in bighorn sheep. Behav Ecol. 2000;11:633–639. [Google Scholar]
- 69.Lummaa V. Early developmental conditions and reproductive success in humans: Downstream effects of prenatal famine, birthweight, and timing of birth. Am J Hum Biol. 2003;15:370–379. doi: 10.1002/ajhb.10155. [DOI] [PubMed] [Google Scholar]
- 70.Ellison PT. On Fertile Ground. A Natural History of Human Reproduction. Cambridge, MA: Harvard Univ Press; 2001. [Google Scholar]
- 71.Lancaster J, Kaplan H. The endocrinology of the human adaptive complex. In: Ellison PT, Gray PB, editors. Endocrinology of Social Relationships. Cambridge, MA: Harvard Univ Press; 2009. pp. 95–119. [Google Scholar]
- 72.Bhasin S, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 73.Stearns S, Koella J. The evolution of phenotypic plasticity in life-history traits: Predictions of reaction norms for age and size at maturity. Q Rev Biol. 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 74.Hill K, Hurtado A. Ache Life History: The Ecology and Demography of a Foraging People. New York: Aldine De Gruyter; 1996. [Google Scholar]
- 75.Belsky J, Steinberg L, Houts RM, Halpern-Felsher BL, NICHD Early Child Care Research Network The development of reproductive strategy in females: early maternal harshness —> earlier menarche —> increased sexual risk taking. Dev Psychol. 2010;46:120–128. doi: 10.1037/a0015549. [DOI] [PubMed] [Google Scholar]
- 76.Chisholm JS. Death, hope, and sex: Life-history theory and the development of reproductive strategies. Curr Anthropol. 1993;34:1–24. [Google Scholar]
- 77.Nettle D, Coall DA, Dickins TE. Birthweight and paternal involvement predict early reproduction in British women: Evidence from the National Child Development Study. Am J Hum Biol. 2010;22:172–179. doi: 10.1002/ajhb.20970. [DOI] [PubMed] [Google Scholar]
- 78.Coall DA, Chisholm JS. Reproductive development and parental investment during pregnancy: Moderating influence of mother's early environment. Am J Hum Biol. 2010;22:143–153. doi: 10.1002/ajhb.20965. [DOI] [PubMed] [Google Scholar]
- 79.Quinlan RJ. Father absence, parental care, and female reproductive development. Evol Hum Behav. 2003;24:376–390. [Google Scholar]
- 80.Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Hum Nat. 2009;20:204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- 81.Hill K, Kaplan H. Life history traits in humans: Theory and empirical studies. Annu Rev Anthropol. 1999;28:397–430. doi: 10.1146/annurev.anthro.28.1.397. [DOI] [PubMed] [Google Scholar]
- 82.Dabbs JM., Jr Salivary testosterone measurements: Reliability across hours, days, and weeks. Physiol Behav. 1990;48:83–86. doi: 10.1016/0031-9384(90)90265-6. [DOI] [PubMed] [Google Scholar]
- 83.Walker R, et al. Growth rates and life histories in twenty-two small-scale societies. Am J Hum Biol. 2006;18:295–311. doi: 10.1002/ajhb.20510. [DOI] [PubMed] [Google Scholar]
- 84.Adair LS, Kuzawa CW, Borja J. Maternal energy stores and diet composition during pregnancy program adolescent blood pressure. Circulation. 2001;104:1034–1039. doi: 10.1161/hc3401.095037. [DOI] [PubMed] [Google Scholar]
- 85.Food and Nutrition Research Institute of the Philippines . Food Composition Tables Recommended for Use in the Philippines. Manila, Philippines: Food and Nutrition Research Institute; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.