Abstract
Recent studies indicate that molecules released by glia can induce synapse formation. However, what induces glia to produce such signals, their identity, and their in vivo relevance remain poorly understood. Here we demonstrate that supporting cells of the vestibular organ—cells that have many characteristics of glia—promote synapse formation only when induced by neuron-derived signals. Furthermore, we identify BDNF as the synaptogenic signal produced by these nonneuronal cells. Mice in which erbB signaling has been eliminated in supporting cells have vestibular dysfunction caused by failure of synapse formation between hair cells and sensory neurons. This phenotype correlates with reduced BDNF expression in supporting cells and is rescued by reexpression of BDNF in these cells. Furthermore, knockdown of BDNF expression in supporting cells postnatally phenocopies the loss of erbB signaling. These results indicate that vestibular supporting cells contribute in vivo to vestibular synapse formation and that this is mediated by reciprocal signals between sensory neurons and supporting cells involving erbB receptors and BDNF.
Keywords: erbB receptor signaling, glia, vestibular organ, glutamatergic synapse, hair cell
Synaptogenesis is typically viewed as a process involving only the pre- and postsynaptic neurons, but recent studies suggest that astrocytes are also involved. For example, studies show that astrocytes release thrombospondins, which interact with neuronal gabapentin receptors that, upon activation, induce the formation of excitatory synapses in the central nervous system (1–3). Other studies have shown that complexes of cholesterol and apolipoprotein E-containing lipoproteins released by astrocytes are required for synaptogenesis by purified retinal ganglion cells (4). Although these studies suggest that glia may regulate synapse formation, a number of key questions remain unanswered: (i) Are astrocytes the only glia with synaptogenic activity? (ii) Are thombospondin and cholesterol/ApoE complexes the only glial-derived synaptogenic factors? (iii) Do glia produce synaptogenic factors on their own or in response to other signals? And (iv) what are the functional consequences of disruption of glial-induced synaptogenesis? Our studies on neuron–glia interactions in the vestibular system answer these questions.
The vestibular organs provide information about head position and thus contribute to the maintenance of balance and posture. Linear acceleration is transformed into neural signals by mechanotransducing hair cells in sensory epithelia, called maculae, in the orthogonally oriented saccule and utricle. These hair cells synapse on primary sensory neurons that send the information to the brainstem. Vestibular hair cells and their associated sensory nerve terminals are surrounded by nonneuronal cells, called supporting cells (SCs). These SCs express glial markers such as vimentin (5), S100 (6), glutamate–aspartate transporter (7), low-affinity neurotrophin receptor p75 (8), glial fibrillary acidic protein (GFAP) (9), and proteolipid protein (PLP) (10). They surround synaptic contacts, as astrocytes and perisynaptic Schwann cells do at central and neuromuscular synapses, respectively. Moreover, during development, these nonneuronal cells are precursors for hair cells (11, 12), as radial glia are to neurons. Based on these similarities, we hypothesized that interactions of SCs with vestibular neurons and hair cells could be important for the vestibular system, and that the communication between neuronal and nonneuronal cells of the vestibular sensory epithelia could be mediated by some of the same molecules that mediate neuron–glia interactions elsewhere in the nervous system.
The growth factor neuregulin 1 (NRG1) and its receptors, the erbB family of tyrosine kinase receptors, have emerged as key mediators of neuron–glia interactions throughout the nervous system (13, 14). NRGs are members of the EGF family of growth factors and signal through erbB2, erbB3, and erbB4 (14, 15). NRG1 is expressed primarily by neurons, including many peripheral sensory neurons (16), whereas glial cells express erbB receptors (14, 17–19). The NRG1/erbB signaling pathway is important for axon–Schwann cell interactions that regulate myelination (20–23), and the function of unmyelinated sensory neurons (24). NRG1/erbB signaling also regulates radial glia morphology and neuronal migration (25, 26), oligodendrocyte maturation (27–29), the function of hypothalamic astrocytes (30), as well as the timing of astrogenesis (31).
Expression patterns of the NRG1/erbB signaling components in inner ear sensory epithelia suggested that this pathway is important for interactions between inner ear sensory neurons and their associated SCs; i.e., NRG1 is expressed solely by the sensory neurons (32, 33), whereas erbB2 and erbB3 expression is restricted to SCs (33, 34). To determine the roles of erbB receptor signaling in the interaction among SCs and nerve terminals in the postnatal vestibular organs, we used a transgenic (Tg) mouse line in which erbB receptor signaling in SC is blocked by expression of a dominant-negative erbB receptor under the control of the GFAP promoter (GFAP-DN-erbB4) (24, 33). GFAP-DN-erbB4 mice show severe vestibular dysfunction caused by a failure of synapse formation and/or maintenance. Analysis of several genetically modified mouse lines shows that SCs contribute to the formation of synapses in vestibular epithelia and that this contribution is mediated by reciprocal signals between sensory neurons and SCs involving NRG1-erbB and BDNF-TrkB signaling pathways.
Results
Expression of a DN-erbB4 Receptor Under the Control of the GFAP Promoter.
To study the roles of erbB receptor signaling in SC of the murine inner ear, we used a Tg approach based on expression of a dominant-negative erbB4 receptor (DN-erbB4). Expression of DN-erbB4, a truncated human erbB4 lacking the intracellular domain, blocks ligand-induced activation of erbB2, erbB3, and erbB4 without affecting signaling through other receptors, including erbB1 or Notch1 (30, 35). We previously showed that expression of DN-erbB4 in myelinating Schwann cells (20) phenocopies the loss of erbB2 expression (22, 36) and that DN-erbB4 expression in oligodendrocytes (27) leads to phenotypes consistent with those found in mice heterozygous for type III NRG1 (29), indicating that DN-erbB4 is a powerful tool to block erbB2, erbB3, and erbB4 signaling in vivo. To drive DN-erbB4 expression to inner-ear SCs, we used the human GFAP promoter (37), which we showed is active in these cells (9). We studied one Tg line, GFAP-DN-erbB4 Line-D, in which the transgene is expressed only by GFAP-expressing cells in the peripheral nervous system (24), including inner ear SCs (33), thus avoiding potential confounding effects of alterations in the central nervous system. As expected from the pattern of expression of the GFAP promoter (9), in situ hybridization showed that in vestibular organs of Line-D GFAP-DN-erbB4 mice, the transgene is expressed only by SCs of the sensory epithelium, but not by hair cells, vestibular sensory neurons, or any cell in WT tissues (Fig. S1).
Homozygous Tg mice displayed ataxia, circling behavior, and diminished orientation reflexes (Movie S1), signs consistent with vestibular dysfunction. To more directly test peripheral vestibular function, we recorded vestibular evoked potentials (VsEPs). These potentials, which are elicited by rapid head jerks, can provide a measure of the neural output from the utricle and saccule, the gravitational sensors of the inner ear (38). At postnatal day 21 (P21; the earliest time at which mature VsEPs can be recorded), Tg mice showed a complete absence of these evoked neural responses, even at stimulus amplitudes of 15 dB (i.e., factor of 6) above normal threshold (Fig. 1). To determine if the physiological deficits reflected a disruption in the structure of the vestibular system, we examined the histology of the inner ear. Vestibular organs of Tg mice were normal in size and general appearance at all ages examined (P6–P30). Light microscopy analysis showed no evidence of hair cell loss or alterations in the size of the saccular and utricular maculae at all ages examined (P6–P30; Fig. S2 A and B). Similarly, no alterations were found in the number of neuronal cell bodies in the vestibular ganglia, or of myelinated fibers projecting to the sensory epithelia (Fig. S2C). Neurofilament immunostaining (which labels the sensory nerve fibers and terminals) of the Tg sensory epithelium and electron microscopy showed that the contacts between primary sensory neurons and hair cells had normal morphology (Fig. S2 D and E). Phalloidin staining of the utricular maculae showed that the density and orientation of hair cell bundles appeared normal in Tg mice (Fig. S2 F and G). Finally, to determine if defects in hair cell function could be involved in the pathophysiology of Tg mice, we tested hair cell mechanotransduction by using FM1-43, a fluorescent dye that passes through hair cell transduction channels (39). FM1-43 uptake in sensory epithelia of Tg mice was the same as in WT, indicating that hair cell mechanotransduction was not altered. Importantly, FM1-43 uptake was blocked in both genotypes by La3+, a mechanotransduction channel blocker, indicating that it is specific (Fig. S2). Together, these results indicated that the vestibular dysfunction of GFAP-DN-erbB4 mice is not the consequence of a major disruption in the structure of the vestibular organ or in hair cell mechanotransduction.
Fig. 1.
Loss of erbB signaling in SCs causes vestibular dysfunction. (A) VsEPs from WT and GFAP-DN-erbB4 Tg mice demonstrate the severity of vestibular dysfunction at P21. Magnitude of the evoking head jerk is expressed in dB re: 1.0 g/ms. (B) Box plot analysis of VsEP thresholds: arrow illustrates the threshold shift in GFAP-DN-erbB4 mice, which had no response at the highest levels tested (ND, not detectable). (C) Box plot analysis of peak 1 amplitudes for VsEPs at 5 dB for WT (n = 4) and GFAP-DN-erbB4 mice (n = 4); ***P < 0.0001.
Vestibular Dysfunction Correlates with a Defect in Hair Cell Synapse Formation.
Based on the findings described earlier, we hypothesized that the vestibular dysfunction might reflect a defect in the functional connectivity between hair cells and primary vestibular neurons. To test this, we analyzed synaptic density in the utricular epithelium by determining the incidence of colocalization of hair cell presynaptic ribbons and neuronal postsynaptic receptors by using antibodies for RIBEYE/CtBP2 and GluR2/3, respectively (40). We observed a dramatic reduction in the density of putative synapses at P21 in Tg mice (Fig. 2A). Quantitative analysis of these tissues showed that presumptive synaptic sites in the utricle were reduced by 95%. This was caused by the presence of fewer pre- and postsynaptic specializations (RIBEYE/CtBP2 puncta by 67% and GluR2/3 puncta by 82%; Fig. 2B) and by the frequent misalignment of pre- and postsynaptic components. These results support the notion that the functional phenotype is a consequence of synaptic defects in the vestibular sensory epithelium.
Fig. 2.
Loss of erbB signaling in SCs causes defects in synapse formation. (A) Immunostaining for synapses [seen as colocalization of the pre- and postsynaptic markers RIBEYE (red) and GluR2/3 (green)] reveals a reduction in afferent synapses on utricular hair cells in P21 GFAP-DN-erbB4 mice (hair cell region delineated by fine white line). [Scale bars: 50 μm, 10 μm (Inset).] (B) Mean synaptic density (colocalized RIBEYE and GluR2/3), presynaptic ribbons (RIBEYE puncta), and postsynaptic receptor plaques (GluR2/3 puncta) from three to six utricles at each postnatal age. Error bars represent SEMs.
To understand the mechanisms that lead to the reduced synaptic numbers in the mature sensory organ, we examined the time course of synapse formation. As shown in Fig. 2B, in WT, the density of presynaptic ribbons, postsynaptic receptors, and putative synapses increases steadily between P0 and P21, indicating that significant synaptogenesis occurs postnatally in the vestibular epithelium. In contrast, Tg mice did not form many new synapses after birth. Although the density of postsynaptic receptor patches and presynaptic ribbons in Tg mice both increased significantly between P0 and P5, they did not align with one another, and therefore synaptic density remained unchanged. Furthermore, all three parameters decreased significantly between P5 and P21 (Fig. 2B). These data suggest that the vestibular phenotype of Tg mice reflects an initial failure in synapse formation and a subsequent disappearance of previously formed structures, possibly caused by a defect in synapse maintenance.
Loss of erbB Signaling in SC Results in Reduced BDNF Expression.
Earlier studies suggested that erbB signaling contributes to neuron–glia interactions by being part of reciprocal signaling loops, i.e., that activation of glial erbB receptors in response to neuronally derived erbB ligands induces glia to produce factors such as GDNF (24), NT3 (33, 41), or PGE2 (30), that then act on the neuron. Therefore, we tested if the expression of three trophic factors important for inner ear development (BDNF, GDNF, and NT3) is altered in the vestibular organs of GFAP-DN-erbB4 mice. Real-time quantitative RT-PCR showed that, at P26, there is a specific and significant reduction in BDNF mRNA levels in Tg mice (Fig. 3A). Semiquantitative immunofluorescence showed a similar reduction in BDNF protein in the sensory epithelium (Fig. 3 B and C) at P10, an age at which this neurotrophin is restricted to SCs (42) (Fig. 3B, Top). Together, these results suggest that BDNF expression in vestibular SCs depends on erbB receptor signaling and that alterations in BDNF produced by SCs could underlie the vestibular phenotype of GFAP-DN-erbB4 mice.
Fig. 3.
BDNF expression is reduced in GFAP-DN-erbB4 utricles. (A) Real-time quantitative RT-PCR shows BDNF mRNA is reduced at P26 in GFAP-DN-erbB4 (n = 6) compared with WT (n = 9) mice; ***P = 0.0002. In contrast, expression of two other trophic factors, GDNF and NT3, is unaffected (P = 0.63 and P = 0.34 for GDNF and NT3, respectively). (B) Immunostaining of utricular maculae shows reduced BDNF expression in GFAP-DN-erbB4 mice at P10 (Inset: high-magnification image). [Scale bars: 50 μm, 10 μm (Inset).] (C) Quantification of BDNF fluorescence (arbitrary units) in utricular maculae of P10 WT (n = 6) and GFAP-DN-erbB4 (n = 4) mice; *P = 0.0155. Error bars represent SEMs.
Loss of BDNF Expression in SC During the Perinatal Period Phenocopies the Vestibular Defects in GFAP-DN-erbB4 Mice.
To test the idea that synaptogenesis in vestibular epithelia depends on BDNF produced by SCs during early postnatal life, we eliminated BDNF expression in these nonneuronal cells using inducible, cell-specific Cre-dependent recombination. We achieved this by crossing mice with loxP sites flanking the bdnf coding exon (BDNFf mice) (43) with mice carrying a tamoxifen-inducible Cre recombinase (CreERT) under control of the proteolipid protein promoter (PLP/CreERT) (44), which can drive transgene expression in SCs of the embryonic inner ear (10). We recently demonstrated that, in BDNFf/f::PLP/CreERT mice, tamoxifen induces gene recombination of the bdnf locus and produces effective knockdown in BDNF mRNA expression in vestibular SCs but not in the hair cells or sensory neurons (45). Vestibular Schwann cells do not express BDNF (42), and therefore elimination of the BDNF gene from these cells should not impact the pattern or levels of BDNF expression in the inner ear.
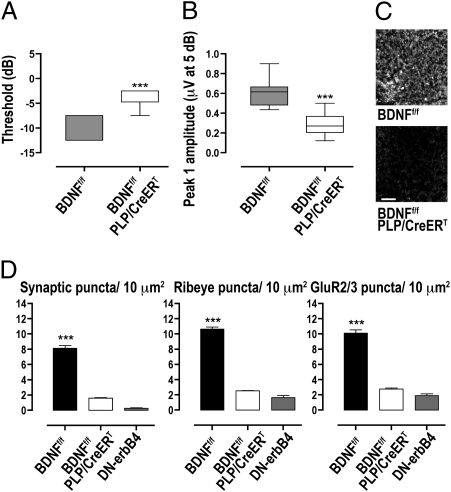
Pregnant dams were treated with tamoxifen from E14.5 to E17.5 and the resulting BDNFf/f and BDNFf/f::PLP/CreERT progeny were analyzed at P26. Tamoxifen-treated BDNFf/f::PLP/CreERT mice appeared uncoordinated and ataxic. VsEP recordings showed an elevation of thresholds and a decrease in response amplitude (Fig. 4 A and B), indicative of vestibular dysfunction similar to that observed in GFAP-DN-erbB4 mice. In contrast, response latency was normal (1,040 ± 23.1 μs vs. 1,010 ± 19.1 μs for peak1 in BDNFf/f and BDNFf/f::PLP/CreERT mice, respectively; P = 0.40), indicating that conduction velocity in vestibular nerve fibers was unchanged.
Fig. 4.
Knockdown of BDNF expression in SCs results in vestibular dysfunction and loss of synapses. Box plot analysis of VsEP thresholds (A) and peak 1 amplitudes at 5 dB (B) from control (BDNFf/f; n = 12) and BDNF conditional KO (BDNFf/f::PLP/CreERT; n = 9) mice injected with tamoxifen from embryonic day (E) 14.5 to E17.5 show that BDNF knockdown leads to vestibular dysfunction at P26. Magnitude of the evoking head jerk is expressed in dB re: 1.0 g/ms; ***P < 0.0001. (C) BDNF immunostaining of P26 utricular maculae from tamoxifen treated control and conditional KO mice illustrates the successful knockout of BDNF. (Scale bar, 20 μm.) (D) Quantification of synapses (colocalized RIBEYE and GluR2/3), presynaptic ribbons (RIBEYE puncta), and postsynaptic receptor plaques (GluR2/3 puncta) from BDNFf/f (n = 7), BDNFf/f::PLP/CreERT (n = 6), and GFAP-DN-erbB4 (n = 4) utricles show that reduced BDNF expression in SCs reduces synaptic density; ***P < 0.0001. Error bars in D represent SEM.
After the physiological tests, tissues were harvested, and one ear of each animal was used for analysis of BDNF expression and the other was used for synapse quantification. Immunostaining confirmed that tamoxifen reduced BDNF protein expression by SCs in all BDNFf/f::PLP/CreERT mice, but not in BDNFf/f mice (Fig. 4C). As in the GFAP-DN-erbB4 Tg mice, mice with BDNF KO in SCs did not show loss of sensory nerve terminals in the maculae (Fig. S3). This is in stark contrast to what occurs in BDNF-null mice (46) or if BDNF is eliminated globally and early in embryogenesis in the BDNFf/f line using a Cre line under the control of the GATA-1 promoter (47) (Fig. S3). These results indicate that BDNF provided by late embryonic/early postnatal SCs is not necessary for the formation and/or maintenance of vestibular sensory nerve terminals. Analysis of synaptic density in the vestibular epithelium showed that supporting cell-specific BDNF KO produces a synaptic phenotype similar to that seen in GFAP-DN-erbB4 mice, i.e., reduction in synapses, including both presynaptic ribbons and postsynaptic puncta (Fig. 4D). Furthermore, we found a correlation between synaptic density and VsEP amplitude in BDNFf/f::PLP/CreERT and in BDNFf/f mice (Fig. S4), supporting the notion that the synaptic loss is indeed the functionally important structural change. These results suggest that the phenotype observed by the elimination of erbB signaling in SCs could be the result of decreased BDNF expression.
Restoration of BDNF Expression in SC Rescues the Vestibular Defects in GFAP-DN-erbB4 Mice.
To further explore the link between erbB signaling, BDNF expression and vestibular synaptogenesis, we tested if the GFAP-DN-erbB4 phenotype could be rescued by restoring BDNF expression in SCs. For this, we used a conditional BDNF over-expression Tg line (BDNFstop) (48), which we have shown previously can be used in combination with the PLP/CreERT line to increase postnatal BDNF expression in vestibular SCs (45). Mice of the three Tg lines (BDNFstop, GFAP-DN-erbB4, and PLP/CreERT) were crossed, and progeny mice carrying different transgene combinations were treated with tamoxifen between P5 and P11 and analyzed at P26. Immunostaining confirmed that activation of the BDNF transgene in GFAP-DN-erbB4 mice restored BDNF protein to levels similar to those observed in WT vestibular epithelia (Fig. 5A). BDNF reexpression in SCs rescued vestibular function in GFAP-DN-erbB4 mice, as documented by restoration of normal VsEP thresholds and response amplitudes (Fig. 5 B and C), without affecting the latency for peak 1 (1,113 ± 140 μs and 1,202 ± 60.4 μs for BDNFstop and BDNFstop::GFAP-DN-erbB4::PLP/CreERT mice, respectively; P = 0.58). Immunostaining for synaptic markers showed that BDNF reexpression in SCs restored the density of both pre- and postsynaptic markers, as well as synaptic puncta (Fig. 5D).
Fig. 5.
BDNF reexpression in SCs rescues vestibular function and synapse formation in GFAP-DN-erbB4 mice. (A) BDNF immunostaining of P26 utricular, maculae from control (BDNFstop), GFAP-DN-erbB4 mice carrying the BDNFstop transgene, and mice carrying all three transgenes (GFAP-DN-erbB4, BDNFstop and PLP/CreERT) show that tamoxifen increases BDNF expression in SCs of the utricular macula of BDNFstop::GFAP-DN-erbB4::PLP/CreERT mice to levels similar to those of control mice. (Scale bar, 20 μm.) Box plot analysis of VsEP thresholds: arrow illustrates the threshold shift in GFAP-DN-erbB4 mice, which had no response at the highest levels tested (ND, not detectable) (B) and peak 1 amplitudes at 5 dB (C) from control mice (n = 6), GFAP-DN-erbB4 mice carrying the BDNFstop transgene (n = 6), and mice carrying all three transgenes (n = 4) show that conditional overexpression of BDNF rescues the vestibular function of GFAP-DN-erbB4 mice at P26. Magnitude of the evoking head jerk is expressed in dB re: 1.0 g/ms; ***P < 0.0001. (D) Quantification of synapses (colocalized RIBEYE and GluR2/3), presynaptic ribbons (RIBEYE puncta), and postsynaptic receptor plaques (GluR2/3 puncta) in utricular maculae of the same mice tested in A–C shows that BDNF overexpression in SCs rescues the synaptic loss in GFAP-DN-erbB4 mice; ***P < 0.0001. Error bars in D represent SEMs.
Discussion
The present data demonstrate that SCs of the vestibular system regulate synapse formation in the context of reciprocal interactions among these nonneuronal cells, the sensory neurons, and the hair cells, highlighting the tripartite relationship in the process of synaptogenesis. They also show that activation of SC erbB receptors, most likely by NRG1 provided by the sensory neurons, is required to evoke a synaptogenic signal from the SCs back to the other components and that BDNF is the requisite SC-derived synaptogenic factor. Together, these observations suggest that the release of synapse-inducing molecules by glia could depend on the target neurons.
Our findings also illustrate the stage-dependent nature of BDNF's biological roles in the vestibular system. It had been shown previously that early in development (E13–E16), BDNF is essential for the survival of vestibular neurons (46, 49), whereas at later embryonic stages it is necessary for the maintenance of the nerve terminals at the sensory epithelia (50). We now show that, in the early postnatal vestibular epithelium, BDNF expressed by SCs is essential for synapse formation but not for neuronal survival or axonal maintenance. These changes in BDNF function are paralleled by changes in its pattern of expression. Whereas hair cells and SCs are the source of BDNF in the embryonic vestibular epithelia (51), by P3 SCs become the sole source, as hair cells stop producing this factor (42). As Schwann cells in the postnatal vestibular organ do not express BDNF (42), SCs are probably the sole source of this key neurotrophin regulating vestibular synaptogenesis. This conclusion is strongly supported by the present results showing a dramatic reduction in synaptic density when BDNF expression is reduced by blocking erbB signaling in SCs, or by direct cell-specific knockdown of BDNF itself in SCs and by the complementary observation that the synaptic deficits induced by blocking BDNF production in SCs can be rescued by overexpression of BDNF in these nonneuronal cells. Importantly, although the phenotypes produced by loss of SC erbB signaling and BDNF knockdown are very similar, the one in GFAP-DN-erbB4 mice is more pronounced. As BDNF effectively rescues the phenotype produced by DN-erbB4 expression, it is unlikely that there is an additional erbB-induced factor that works together with BDNF. Rather, it is possible that the time course or extent of BDNF knockdown by CreERT is of a lesser magnitude than that produced by loss of erbB signaling.
The biological roles of NRG1/erbB signaling in neuron–glia interactions are also context dependent. The loss of erbB signaling in vestibular SC results in the failure of synapse formation and a reduction in synapse maintenance without any neuronal loss and that this is caused by alterations in BDNF expression. In contrast, loss of erbB signaling in nonmyelinating Schwann cells or cochlear SC results in degeneration of sensory neurons, which correlates with decreases in GDNF or NT3 expression, respectively (24, 33). Thus, erbB signaling seems to contribute to neuron–glia interactions in the peripheral nervous system by inducing the expression of specific trophic factors in the nonneuronal cells, which then act on the NRG1-expressing cells promoting different biological responses. The basis for the different responses to erbB signaling in the glial cells in vivo remains unknown, but the presence of different cofactors or differences in expression of intracellular signaling molecules in the different glia are possible scenarios.
The present data also provide insights into the molecular and cellular mechanisms of inner ear development. First, they highlight important differences between the roles of cochlear and vestibular SCs, i.e., cochlear SCs regulate spiral ganglion neuronal survival, possibly through erbB-dependent NT3 expression (33), whereas vestibular SCs regulate synapse formation through erbB-dependent BDNF expression. Second, they shed light on the role of NRG1-erbB signaling in the vestibular system, i.e., NRG1 had previously been shown to act as a SC mitogen during embryonic development and after injury (34, 52), whereas here we show that, at postnatal stages, erbB signaling in SCs promotes synapse formation.
Neurotrophins are important modulators of synaptic development and function in the central and peripheral nervous systems (53–55). For example, they have been shown to regulate transmitter release, synaptic size and stability, and to modulate short- and long-term synaptic plasticity (56–59). Although neurons have long been viewed as the primary source of BDNF in the central nervous system, recent studies have reported BDNF expression in both oligodendrocytes and astrocytes (60–62). As these central glia also express erbB receptors, it is important to consider the possibility that erbB signaling regulates neurotrophin expression in these cells as well. If this were the case, processes similar to the ones we describe here for the vestibular system could be at work in synapse formation and function throughout the nervous system. Furthermore, this could be of relevance for neurological and/or neuropsychiatric disorders, as NRG1-erbB and BDNF-TrkB signaling have been implicated in multiple sclerosis, traumatic brain and spinal cord injury, autism, depression, and schizophrenia (63–67).
Materials and Methods
Animals.
Methods for creating the GFAP-DN-erbB4 mice were described in ref. 24. PLP/CreRT (44) and BDNFf/f (43) mice were obtained from Jackson Laboratory. The BDNFstop mouse line (48) was provided by Rudolf Jaenisch (Whitehead Institute for Biomedical Research, Cambridge, MA). The GATA-1/Cre (47) mouse line was provided by Stuart Orkin (Children's Hospital Boston, Boston, MA).
In Situ Hybridization.
In situ hybridization was performed in whole-mount temporal bones of Tg mice. Detailed methods are provided in SI Materials and Methods.
Histological Analysis.
Histopathological analyses were performed as described in ref. 33. Details and EM methods are provided in SI Materials and Methods.
FM1–43 Dye Uptake.
Freshly dissected utricular maculae from P21 WT and Tg mice were incubated with FM1-43 dye for 2 min at room temperature and immediately visualized using confocal microscopy. Lanthanum (100 μM), a blocker of mechanotransduction channels, was used to verify the specificity of the FM1-43 uptake.
Tamoxifen Injection.
Tamoxifen (Sigma) was dissolved in corn oil at a concentration of 10 mg/mL at 55 °C. For prenatal administration, tamoxifen was injected i.p. to embryonic day 14.5 pregnant dams for 4 consecutive days at a dose of 50 mg/kg body weight. For postnatal administration, pups were injected i.p. beginning at P5 for 7 consecutive days with tamoxifen at a dose of 33 mg/kg body weight.
VsEP Recordings.
VsEPs were recorded as described in ref. 39. Detailed methods are provided in SI Materials and Methods.
Immunostaining.
Immunohistochemical detection of BDNF and RIBEYE-GluR2/3 was performed as described in refs. 43 and 41, respectively. Details are provided in SI Materials and Methods.
Real-Time Quantitative RT-PCR.
Real-time quantitative RT-PCR was performed as in ref. 33. Detailed information is provided in SI Materials and Methods.
Data Analysis.
Statistical analyses were performed using ANOVA followed by a Tukey test for multiple comparisons or by a Student two-tailed t test. P < 0.05 was selected as the criterion for statistical significance. To determine if the difference in normalized gene expression of BDNF, GDNF, and NT3 was statistically significant, we used the bootstrap method using Matlab 5.3 software (MathWorks). Mean values are quoted as means ± SEM.
Image Acquisition and Analysis.
A laser scanning confocal microscope (LSM 510 META; Zeiss) was used to acquire images of whole utricular maculae. The area of the maculae and the number of synaptic, RIBEYE, and GluR2/3 puncta per macula were measured using Metamorph advanced image analysis software.
Supplementary Material
Acknowledgments
We thank Dr. Rudolph Jaenisch for the BDNFstop Tg line (MIT, Cambridge, MA); Dr. Stuart Orkin for the GATA1-Cre mouse line (Children's Hospital, Boston, MA); Drs. Sherri and Timothy Jones for their advice on VsEP recordings; Pieter Dikkes, Bethany Taylor, Ishmael J. Stefanov-Wagner, and Dr. Lihong Bu for technical help; Dr. Samir Koirala for advice with microscopy; Dr. Martin Hemberg for advice on statistical analysis; and Drs. Thomas Schwarz, Xi He, and Kenneth Rosen for their helpful comments on the manuscript. This research was supported in part by National Institute on Deafness and Other Communication Disorders Grants R01 DC 004820 (to G.C.) and P30 DC 005209 (to M.C.L.), Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant P30-HD 18655 (Mental Retardation Research Center) (to G.C.), and by the Pew Latin American Fellowship Program (M.E.G.-C.).
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008938107/-/DCSupplemental.
References
- 1.Eroglu C, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christopherson KS, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Barres BA. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Mauch DH, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 5.Anniko M, Thornell LE, Gustavsson H, Virtanen I. Intermediate filaments in the newborn inner ear of the mouse. ORL J Otorhinolaryngol Relat Spec. 1986;48:98–106. doi: 10.1159/000275854. [DOI] [PubMed] [Google Scholar]
- 6.Pack AK, Slepecky NB. Cytoskeletal and calcium-binding proteins in the mammalian organ of Corti: Cell type-specific proteins displaying longitudinal and radial gradients. Hear Res. 1995;91:119–135. doi: 10.1016/0378-5955(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 7.Furness DN, Lehre KP. Immunocytochemical localization of a high-affinity glutamate-aspartate transporter, GLAST, in the rat and guinea-pig cochlea. Eur J Neurosci. 1997;9:1961–1969. doi: 10.1111/j.1460-9568.1997.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 8.Vega JA, San José I, Cabo R, Rodriguez S, Represa J. Trks and p75 genes are differentially expressed in the inner ear of human embryos. What may Trks and p75 null mutant mice suggest on human development? Neurosci Lett. 1999;272:103–106. doi: 10.1016/s0304-3940(99)00577-7. [DOI] [PubMed] [Google Scholar]
- 9.Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J Comp Neurol. 2002;442:156–162. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- 10.Morris JK, et al. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091:186–199. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morest DK, Cotanche DA. Regeneration of the inner ear as a model of neural plasticity. J Neurosci Res. 2004;78:455–460. doi: 10.1002/jnr.20283. [DOI] [PubMed] [Google Scholar]
- 12.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 13.Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- 15.Falls DL. Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 16.Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JA, Yachnis AT, Arai M, Davis JG, Scherer SS. Expression of the neu proto-oncogene by Schwann cells during peripheral nerve development and Wallerian degeneration. J Neurosci Res. 1992;31:622–634. doi: 10.1002/jnr.490310406. [DOI] [PubMed] [Google Scholar]
- 18.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 19.Abe Y, Namba H, Zheng Y, Nawa H. In situ hybridization reveals developmental regulation of ErbB1-4 mRNA expression in mouse midbrain: Implication of ErbB receptors for dopaminergic neurons. Neuroscience. 2009;161:95–110. doi: 10.1016/j.neuroscience.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, et al. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 23.Woldeyesus MT, et al. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, et al. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 25.Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 26.Schmid RS, et al. Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci USA. 2003;100:4251–4256. doi: 10.1073/pnas.0630496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy K, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Taveggia C, et al. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008;56:284–293. doi: 10.1002/glia.20612. [DOI] [PubMed] [Google Scholar]
- 30.Prevot V, et al. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003;23:230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Morley BJ. ARIA is heavily expressed in rat peripheral auditory and vestibular ganglia. Brain Res Mol Brain Res. 1998;54:170–174. doi: 10.1016/s0169-328x(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 33.Stankovic K, et al. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hume CR, Kirkegaard M, Oesterle EC. ErbB expression: The mouse inner ear and maturation of the mitogenic response to heregulin. J Assoc Res Otolaryngol. 2003;4:422–443. doi: 10.1007/s10162-002-3008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patten BA, Sardi SP, Koirala S, Nakafuku M, Corfas G. Notch1 signaling regulates radial glia differentiation through multiple transcriptional mechanisms. J Neurosci. 2006;26:3102–3108. doi: 10.1523/JNEUROSCI.4829-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J Cell Biol. 2000;148:1035–1046. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones TA, Jones SM. Short latency compound action potentials from mammalian gravity receptor organs. Hear Res. 1999;136:75–85. doi: 10.1016/s0378-5955(99)00110-0. [DOI] [PubMed] [Google Scholar]
- 39.Farris HE, LeBlanc CL, Goswami J, Ricci AJ. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol. 2004;558:769–792. doi: 10.1113/jphysiol.2004.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khimich D, et al. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- 41.Verdi JM, et al. A reciprocal cell-cell interaction mediated by NT-3 and neuregulins controls the early survival and development of sympathetic neuroblasts. Neuron. 1996;16:515–527. doi: 10.1016/s0896-6273(00)80071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montcouquiol M, Valat J, Travo C, Sans A. A role for BDNF in early postnatal rat vestibular epithelia maturation: Implication of supporting cells. Eur J Neurosci. 1998;10:598–606. doi: 10.1046/j.1460-9568.1998.00070.x. [DOI] [PubMed] [Google Scholar]
- 43.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 44.Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Casati ME, Murtie J, Taylor B, Corfas G. Cell-specific inducible gene recombination in postnatal inner ear supporting cells and glia. J Assoc Res Otolaryngol. 2010;11:19–26. doi: 10.1007/s10162-009-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 47.Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 49.Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 50.Schimmang T, et al. Developing inner ear sensory neurons require TrkB and TrkC receptors for innervation of their peripheral targets. Development. 1995;121:3381–3391. doi: 10.1242/dev.121.10.3381. [DOI] [PubMed] [Google Scholar]
- 51.Pirvola U, et al. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci USA. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng JL, Frantz G, Lewis AK, Sliwkowski M, Gao WQ. Heregulin enhances regenerative proliferation in postnatal rat utricular sensory epithelium after ototoxic damage. J Neurocytol. 1999;28:901–912. doi: 10.1023/a:1007078307638. [DOI] [PubMed] [Google Scholar]
- 53.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 55.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 57.Causing CG, et al. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- 58.Martínez A, et al. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J Neurosci. 1998;18:7336–7350. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flores-Otero J, Xue HZ, Davis RL. Reciprocal regulation of presynaptic and postsynaptic proteins in bipolar spiral ganglion neurons by neurotrophins. J Neurosci. 2007;27:14023–14034. doi: 10.1523/JNEUROSCI.3219-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai X, et al. The trophic role of oligodendrocytes in the basal forebrain. J Neurosci. 2003;23:5846–5853. doi: 10.1523/JNEUROSCI.23-13-05846.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagayogo IP, Dreyfus CF. Regulated release of BDNF by cortical oligodendrocytes is mediated through metabotropic glutamate receptors and the PLC pathway. ASN Neuro. 2009;1:e00001. doi: 10.1042/AN20090006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jean YY, Lercher LD, Dreyfus CF. Glutamate elicits release of BDNF from basal forebrain astrocytes in a process dependent on metabotropic receptors and the PLC pathway. Neuron Glia Biol. 2008;4:35–42. doi: 10.1017/S1740925X09000052. [DOI] [PubMed] [Google Scholar]
- 63.Esper RM, Pankonin MS, Loeb JA. Neuregulins: Versatile growth and differentiation factors in nervous system development and human disease. Brain Res Brain Res Rev. 2006;51:161–175. doi: 10.1016/j.brainresrev.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 65.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 67.Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.