Abstract
At the end of World War II, a severe 5-mo famine struck the cities in the western part of The Netherlands. At its peak, the rations dropped to as low as 400 calories per day. In 1972, cognitive performance in 19-y-old male conscripts was reported not to have been affected by exposure to the famine before birth. In the present study, we show that cognitive function in later life does seem affected by prenatal undernutrition. We found that at age 56 to 59, men and women exposed to famine during the early stage of gestation performed worse on a selective attention task, a cognitive ability that usually declines with increasing age. We hypothesize that this decline may be an early manifestation of an accelerated cognitive aging process.
Keywords: developmental origins, fetal, nutrition, cognition, aging
Chronic mild caloric restriction is one of the most effective ways of postponing aging and increase lifespan, as shown across species (1). Restricting caloric intake seems to beneficially affect several body systems, including the central nervous system, maintaining cognitive function in older age (2). In sharp contrast, it has been shown in animals as well as humans that caloric restriction occurring before birth has various negative effects on mental and physical health in later life (3). Evidence for a negative effect of prenatal caloric restriction on cognitive function has been shown in animals, and studies in humans have demonstrated effects of prenatal diet variation in micronutrients on childhood cognitive function (4–10). Little is known about the consequences of prenatal nutritional deprivation on cognitive health in later human life.
During the winter of 1944 to 1945, the western part of The Netherlands was struck by a period of severe food scarcity. The famine was a consequence of an embargo on the food transports imposed by the German occupying forces as retaliation for a strike of the Dutch railways, aimed at hampering transport of German troops. The previously and subsequently well-nourished Dutch population's daily rations dropped acutely to as little as 400 to 800 calories during the 5 to 6 mo of famine. The famine was a humanitarian disaster, but left the opportunity to study the effects of maternal malnutrition on the offspring's health in later life.
In their landmark study of long-term consequences of prenatal exposure to the Dutch famine for cognitive function among 19-y-old conscripts, Stein et al. found no effects on the ability for abstract reasoning rates or on rates of mental retardation (11). At later ages, between 50 and 58 y, we found convincing evidence that prenatal exposure to famine increases the risk of coronary heart disease and type 2 diabetes (12). As these are aging-associated diseases, we hypothesized that exposure to the famine in utero may lead to an age-associated decline in cognitive function in later life.
We set out to investigate this hypothesis in the Dutch Famine Birth Cohort, which consists of men and women born as term singletons in the Wilhelmina Gasthuis, a local teaching hospital in Amsterdam, The Netherlands. We measured several aspects of cognitive function in cohort members at the age of 56 to 59 y. The measures of cognitive function we used included a general intelligence test [the Alice Heim test, fourth version (AH4)], a memory task (paragraph recall), a perceptual motor-learning task (mirror drawing), and a selective attention task (a Stroop-like color-word incongruency task). We compared these measures between those exposed and those unexposed to famine during gestation, based on the criteria we have used in all of our previous studies of the Dutch Famine Birth Cohort. Additionally, to compare the results from the present study with those of the 1972 study of Stein et al. (11), we applied the criteria of exposure to famine as used in their study.
Results
Of the 737 participants, 297 (40%) had been exposed to famine in utero (Table 1). People exposed to famine in mid or late gestation were lighter and had smaller head circumferences at birth than those unexposed to famine during gestation (all P < 0.01). People exposed to famine during early gestation had a somewhat longer gestational period (P = 0.02). Interestingly, those exposed to famine during any stage of gestation had significantly smaller adult head circumference (all P < 0.05).
Table 1.
General, birth, and adult characteristics according to timing of prenatal exposure to the Dutch famine
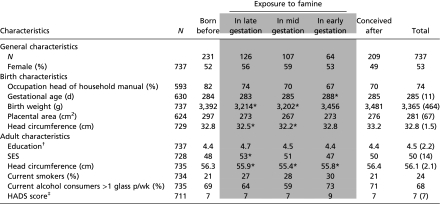 |
Data are given as frequencies, means (SD) or medians (interquartile range, IQR). HADS, Hospital Anxiety and Depression Scale;.SES, socioeconomic status. Shaded areas indicate the groups exposed to famine during gestation compared with the control groups unexposed to famine during gestation.
*Statistically significant difference (based on linear regression analysis, P < 0.05, adjusted for sex) compared with participants unexposed to famine during gestation.
†Educational level measured on a 10-point scale (1 = primary education not completed, 10 = university completed).
‡Data given as median (IQR).
As expected, education and adult socioeconomic status (SES) were highly positively correlated with cognitive performance (Table 2). Being male was strongly correlated with better performance on most of the cognitive measures, as was a larger adult head circumference. Smoking, alcohol consumption, and a higher score on the Hospital Anxiety and Depression Scale showed negative correlations with performance elements of all four cognitive tests. Head circumference at birth showed small positive correlations with performance on the AH4 and the Stroop-like task only. Birth weight was not associated with any of the four cognitive measures.
Table 2.
Correlations between cognitive measures and birth and adult characteristics
| Test or task | Sex† | B-SES† | Gestational age | Birth weight | Placental area | Head circumference at birth | Adult head circum-ference | Education‡ | C-SES | Smoking† | Alcohol† | HADS score |
| AH 4 test | ||||||||||||
| Response time | −0.06 | 0.08 | 0.05 | 0.03 | 0.00 | −0.02 | −0.10** | −0.29** | −0.20** | 0.03 | 0.07 | 0.01 |
| Score | 0.22** | −0.13** | −0.03 | 0.06 | 0.01 | 0.10** | 0.20** | 0.46** | 0.32** | −0.15** | −0.15** | −0.10** |
| Memory task | ||||||||||||
| Immediate recall | 0.01 | −0.09 | 0.05 | -0.02 | −0.02 | 0.00 | 0.05 | 0.32** | 0.21** | −0.13** | −0.08* | −0.16** |
| Retrieval | −0.01 | 0.08 | −0.07 | 0.01 | 0.02 | 0.04 | −0.05 | 0.12** | 0.04 | −0.07 | 0.01 | −0.05 |
| Mirror task | ||||||||||||
| Rounds | −0.21** | 0.01 | 0.04 | 0.06 | 0.06 | 0.04 | 0.20** | 0.14** | 0.12** | −0.09* | −0.06 | −0.09* |
| Errors | −0.32** | −0.03 | −0.04 | −0.07 | −0.07 | −0.06 | −0.24** | −0.15** | −0.09* | 0.03 | 0.09* | 0.07 |
| Errors per round | −0.29** | −0.08 | −0.05 | −0.08 | −0.06 | −0.03 | −0.26** | −0.17** | −0.13** | 0.04 | 0.08 | 0.12** |
| Stroop task | ||||||||||||
| Response time | −0.12** | 0.08 | 0.01 | −0.01 | −0.04 | −0.05 | −0.11** | −0.30** | −0.26** | 0.13** | 0.14** | 0.08* |
| Score | 0.23** | −0.07 | −0.02 | 0.03 | 0.10* | 0.11** | 0.24** | 0.38** | 0.31** | −0.06 | −0.11** | −0.14** |
Correlations are Spearman's rank correlations and point biserial correlations [sex (female = 0, male =1)]; B-SES (head of household has nonmanual occupation =0, head of household has manual occupation = 1); smoking (nonsmoking = 0, smoking = 1); alcohol consumption (less than 1 glass a week = 0, more than 1 glass a week = 1).
*P for correlation < 0.05. **P for correlation < 0.01.
†Correlations are point biserial.
‡Educational level measured on a 10-point scale (1 = primary education not completed, 10 = university completed).
Participants who had been prenatally exposed to famine performed worse on the Stroop-like task compared with unexposed participants (Table 3). The median score of those exposed was 33.0 compared with 43.5 in those unexposed to famine in utero [β = −30 (95% confidence interval [CI]: −60 to 0, P = 0.047)] derived from regression analysis on ranked scores, adjusted for sex. The effect was largest and statistically significant in those exposed to famine during early gestation [β = −85 (−139 to −32), P = 0.002]. Adjusting for potential confounders minimally changed the association [β = −75 (−131 to −19), P = 0.009]. For comparison, the effect of famine exposure during early gestation on performance on the Stroop-like task was almost as large as the effect of sex [β = 102 (73 to 132)], more than twice as large as the effect of smoking [β = −31 (−65 to 4), adjusted for sex] and comparable to the difference of 1 SD decrease in educational level [β = −73 (−87 to −59), adjusted for sex] and 1 SD decrease in SES level [β = −61 (−75 to −47), adjusted for sex].
Table 3.
Mean actual cognition test scores according to timing of prenatal exposure to the Dutch famine
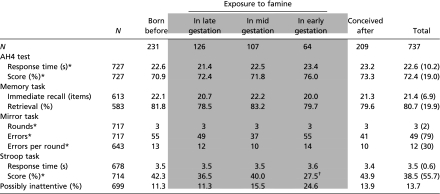 |
Data are given as means (SD) or *medians (IQR). Shaded areas indicate the groups exposed to famine during gestation compared with the control groups unexposed to famine during gestation.
†Statistically significant difference compared with participants unexposed to famine during gestation (based on linear regression analysis, P < 0.05, adjusted for sex).
Following the criteria of prenatal famine exposure as applied by Stein et al. (11), those exposed to famine in utero also performed worse on the Stroop-like task (Table 4). Not only did those prenatally exposed to famine score lower on the Stroop-like task [median scores 33.3 compared with 48.1 in those unexposed, β = −36 (−67 to −5), P = 0.022], they also responded significantly slower than those unexposed to famine during fetal life [mean scores 3.5 s vs. 3.4 s, β = −0.1 (−0.2 to 0.0), P = 0.044]. Adjusted for sex, the effect was largest and statistically significant in group D1, which consists of people exposed to famine during the first and second trimester of gestation [β = −66 (−117 to −15), P = 0.011 for score and β = −0.3 s (−0.4 to −0.1), P = 0.002 for response time].
Table 4.
Mean actual cognition test scores according to timing of prenatal exposure to the Dutch famine [definition as used before by Stein et al. (11)]
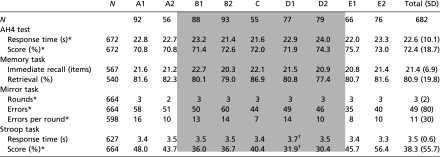 |
Cohort members born between January and July 1944 (A1); August and October 1944 (A2); November 1944 and January 1945 (B1); February and April 1945 (B2); May and June 1945 (C); July and September 1945 (D1); October 1945 and January 1946 (D2); February and May 1946 (E1); June and December 1946 (E2). Data are given as means (SD) or medians (IQR). Shaded areas indicate the groups exposed to famine during gestation compared with the control groups unexposed to famine during gestation.
*Data given as medians (IQR).
†Statistically significant difference compared with participants unexposed to famine during gestation (based on linear regression analyses, P < 0.05, adjusted for sex).
There were 96 participants who had a faster than average reaction time and also less than 40% correct responses, making it likely that they were inattentive or indifferent to the Stroop-like task. Individuals exposed to famine during early gestation were more likely to be among this group [odds ratio = 2.1 (95% CI: 1.1–4.1), P = 0.029]. However, after excluding inattentive and indifferent participants, the effect of early exposure to famine on performance on the Stroop-like task was sustained [β = −80 (−142 to −17), P = 0.012].
Prenatal famine exposure was not significantly associated with performance on the other cognitive measures of AH4, memory task, and mirror task (irrespective of the criteria used for famine exposure).
Discussion
Our results demonstrate that maternal malnutrition during fetal life may negatively influence aspects of cognitive function in later life, as suggested by lower performance of men and women in utero during the famine on a Stroop-like task. Stroop-like tasks require selective attention to inhibit an automatic reaction to the advantage of a nonautomatic one, a function situated in the prefrontal cortex generally declining with age (13–15). We suggest that the poorer selective attention performance in those exposed to famine in early gestation is caused by early aging. However, there is a chance that the deficit has always been there. The only way to establish this is by further testing at a later age.
In line with the results of Stein et al. (11), we found no effects of prenatal famine exposure on general intelligence, nor did we find effects on memory function and perceptual motor learning. However, if the decreased selective attention in those exposed to famine in early gestation is an early manifestation of an accelerated aging process, we may expect to find differences in other cognitive functions at a later age. Recently, error rate on a trial-by-trial computerized Stroop task was shown to be a strong predictor for conversion to Alzheimer's disease even before memory deficits were present (16).
We can only speculate on the pathophysiology of the association between prenatal famine exposure and poorer selective attention. Famine exposure in early gestation has been associated with increased schizophrenia, schizoid personality disorder, and white-matter hyperintensities (17–19). The lower Stroop scores achieved by people suffering from schizophrenia have been suggested to be caused by a dysfunction of the anterior cingulated gyrus, which may play an important role in selective attention (20, 21). White-matter lesions have also been associated with reduced Stroop task performance (22, 23) and increase with age, supporting the above-mentioned suggestion that the decreased Stroop performance in those exposed to famine in early gestation is because of an accelerated aging process.
Regardless of the criteria for famine exposure used, the data suggest that those exposed to malnutrition during the first part of pregnancy are most vulnerable to its effects on selective attention performance. Because the central nervous system is structurally formed in the beginning of gestation, this finding may not come as a surprise. Direct devastating effects of famine exposure during early gestation are illustrated by the increased prevalence of congenital abnormalities reported by Stein et al. in 1975 (24).
An alternative explanation for the association between prenatal famine exposure and poorer selective attention may be vascular-related rather than neurodegenerative. Evidence shows that age-related cognitive decline is associated with atherosclerosis and other vascular damage (25, 26). We have previously demonstrated that exposure to famine during early gestation is associated with a more atherogenic lipid profile and an increased risk of coronary heart disease (12). Prenatal undernutrition may possibly lead to poorer selective attention performance via increased vascular damage. The reported increase in white-matter lesions among those prenatally exposed to famine also fit this explanation, as white-matter lesions are strongly related to cardiovascular risk factors and disease (19, 27).
In addition to effects of prenatal famine exposure on selective attention, we found that exposure during any stage of gestation was associated with a smaller head circumference at the ages of 56 to 59 y. Head size is related to brain size and reduced size has been associated with decreased cognitive abilities in the elderly (28). Indeed, the present study results showed associations between small adult head circumference and reduced cognitive task results. Although we found no evidence that adult head circumference mediates the associations between prenatal famine exposure and cognitive task results, the link between exposure to famine in utero and adult head circumference is in itself an interesting observation. In a previous study performed on subjects at age 50 y, we did not find differences in head circumference (29). In search of an explanation, we found that head circumference decreased with age and this was more so among those prenatally exposed to famine (during all periods of gestation) (Table S1). We cannot explain why head circumference seemed to diminish more in the groups exposed to famine during fetal life. In the literature we found no studies reporting repeated measurements of head circumference with age. This correlation may be because of changes in the skull, increased skin atrophy, hair loss, or redistribution of facial fat. In future studies, we will further investigate this matter.
A number of methodological issues should be considered. Sample size was small. The number of participants exposed to famine during early gestation was only 64. Although the reported effect was strong, it could have been because of chance. About 60% of eligible cohort members participated in the study. Selective participation may therefore present a source of bias; however, birth weights of participants and nonparticipants did not differ. Participation rate in the group exposed to famine in early gestation was somewhat lower compared with most other groups, but if this is a reflection of a poorer (mental) health status, this would probably only have led to an underestimation of the effect. A further limitation may be that we did not use the classic Stroop task-card version with a control and a test trial. Instead, we used a computerized, single-trial Stroop-like task in which the interference effect may be lessened. However, if so, this again would have led to an underestimation of the reported effect rather than an overestimation (30). A final limitation is that we cannot be absolutely certain that the reported effects are because of prenatal undernutrition. Prenatal stress may provide an alternative explanation. On the other hand, we did not find differences in cognitive function between people who were born before the famine and those conceived after the famine, whereas we would expect these differences because mothers of individuals born before famine experienced stressful war circumstances during pregnancy and mothers of those conceived after famine did not.
In conclusion, the current work suggests that prenatal undernutrition negatively influences selective attention ability in later life. We suggest that this may be an early manifestation of an accelerated cognitive aging process, which we will further investigate by future examinations in the present cohort.
Materials and Methods
The Famine.
The Dutch famine was a consequence of a cascade of events that happened at the end of World War II. Although the southern part of The Netherlands was already liberated by the Allied forces, liberation of the northern part of The Netherlands came to a halt when Operation Market Garden, aimed at gaining control of the bridge across the Rhine at Arnhem, failed. To support the Allied offensive, the Dutch government in exile had arranged a strike of the national railways to hamper movement of German troops. In turn, as a reprisal, the German administration put an embargo on all food transports. In early November 1944 this embargo was partially lifted by allowing transport of food across water. At that time, however, an unusually early and severe winter had set in and all canals were frozen. Food stocks in the western cities of The Netherlands ran out rapidly and rations fell below 1,000 calories per person on November 26, 1944. The amount of protein, carbohydrate, and fat decreased more or less proportionately. The rations varied between about 400 and 800 calories from December 1944 to April 1945, and rose above 1,000 calories again after May 12, 1945, 1 wk after the liberation by the Allied forces (31, 32). In addition to the official rations, food also came from other sources (e.g., church organizations, central kitchens, and the black market). People may have had access up to double the rationed amount at the peak of the famine. The rations do, however, adequately reflect the fluctuation of food availability during the famine. Children younger than 1 y of age were relatively protected, as their rations never fell below 1,000 calories. Before the famine pregnant women received extra rations, but during the famine these extra supplies were no longer available. Because of a shortage in fuel, the shutting down of the production of gas and electricity, and in some places even a lack of water, most women gave birth in the hospital.
Participants.
All singleton babies born alive in the Wilhelmina Gasthuis (a teaching hospital in Amsterdam, The Netherlands) between November 1, 1943 and February 28, 1947 were candidates to be included in the Dutch Famine Birth Cohort. We excluded those whose birth records were not available (1%) or those who were born prematurely (8.9%, gestational age below 259 d). In all, 2,414 men and women were included. The population registry of Amsterdam traced 2,155 (89%) of the 2,414 included babies. Of these, 160 babies had not been registered in Amsterdam at birth, 328 people had died, 213 people had emigrated, 157 people refused permission to record their address, 125 people were not traceable to a current address, and 8 people requested their address be removed from the study's database. At the start of the study, 1,423 individuals (66%) were eligible for participation. We invited them by mail to participate in the study. A total of 860 (60%) took part in the study, of whom 120 were visited at home or interviewed by telephone because they were physically or mentally unable to visit the clinic. This group did not participate in the cognitive measurements. For 738 of the 740 remaining participants, a least one cognitive measure was available. Birth weights of the 738 participants did not differ from eligible individuals who did not participate [3,364 g (SD 464) vs. 3,341 g (SD 490), P = 0.36]. There was one person with a self-reported history of schizophrenia. Because of known negative associations between schizophrenia and performance on a Stroop-like task (20), we excluded this person from our analyses. Unfortunately, we had no data on whether male members of the Dutch Famine Birth Cohort were also participants in the study by Stein et al. (11), making any comparison impossible. The local Medical Ethics Committee of the Academic Medical Center of the University of Amsterdam approved the study, which was conducted according to the Helsinki declaration. All participants gave written informed consent. Data were collected between 2002 and 2004.
Exposure.
The official daily food-rations for the general population of 21 y and older were used to define exposure to famine (31, 32). In correspondence with all previous publications on the Dutch Famine Birth Cohort (12), we considered a person prenatally exposed to famine if the mother's average daily food-ration during any 13-wk period of gestation contained fewer than 1,000 calories. Therefore, we considered babies born between January 7, 1945 and December 8, 1945 as exposed to famine in utero. We delineated periods of 16 wk each to differentiate between those exposed in late gestation (born between January 7 and April 28, 1945), in mid-gestation (born between April 29 and August 18, 1945) and in early gestation (born between August 19 and December 8, 1945). People born before January 7, 1945 and after December 8, 1945 were considered unexposed to famine in utero. In the original article by Stein et al. (11), prenatal exposure to famine was based on the month of birth, with shorter than 13-wk periods of exposure to famine also included in the definition. Stein et al. considered those born between November 1944 and January 1946 as exposed to famine in utero. Cohort members born between January and July 1944 (A1) and between August and October 1944 (A2) were considered as conceived and born before the famine; those born between November 1944 and January 1945 (B1) were considered as exposed for the third trimester of gestation; those born between February and April 1945 (B2) as exposed for the second as well as the third trimesters; those born between May and June 1945 (C) as exposed during the middle 6 mo of gestation; those born between July and September 1945 (D1) as exposed during the first and second trimesters; those born between October 1945 and January 1946 (D2) as exposed for only the first trimester; and those born between February and May 1946 (E1) and between June and December 1946 (E2) as never exposed to famine. For a schematic overview of the definition of exposure status for the two study cohorts, see Table 5.
Table 5.
Schematic overview of definitions of prenatal exposure to the Dutch famine for the two study cohorts: Dutch Famine Birth Cohort from Amsterdam (12) and the Dutch Famine Cohort of Stein et al. (11)
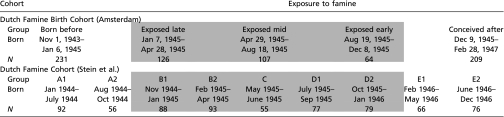 |
Shaded areas indicate the groups exposed to famine during gestation compared with the control groups unexposed to famine during gestation.
Study Parameters.
Birth characteristics were retrieved from medical birth records (33). SES at birth (B-SES) was defined according to the occupation of the head-of-household. At around age 58, participants were invited for a data-collection protocol that included anthropometry and a standardized interview, which yielded information on education level, current SES (C-SES), medical history, use of medication, and lifestyle. Head circumference was measured as the distance around the largest part of the head with a flexible tape measure. Educational level was measured on a 10-point scale (Table S2). C-SES was defined according to the International Index of Occupational Status 92, which is based on the participant's or their partner's occupation, whichever status is highest (34). We asked the participant about current smoking and whether he or she consumed alcohol. We considered drinking at least one glass of alcohol per week a positive answer. As a measure of depression and anxiety symptoms, we took the total score on the Hospital Anxiety and Depression Scale (35).
Cognitive Function.
We measured cognition in four domains: general intelligence, episodic memory, perceptual motor learning, and selective attention.
Alice Heim test, fourth version.
The AH4 (36) measures general intelligence. This test was chosen because it was previously used in a study to measure the association between birth weight (a summary measure of prenatal nutritional status) and cognitive function at adult age (37). The AH4 comprises 65 verbal and mathematical reasoning items of increasing difficulty. We measured the time it took the participant to complete each test item in seconds and the number of correct and incorrect responses. We calculated the percentage of correct responses.
Paragraph encoding and recall.
Paragraph encoding and recall measures episodic memory. We chose this test because it is easily administered and frequently used in memory research. Two paragraphs were orally presented (prerecorded on tape). Participants were told to remember as many story elements as possible and asked to reproduce the story immediately and 30 min later. The number of correctly retrieved elements was recorded (immediate memory) and the percentage retained from the immediate to the delayed condition was calculated (retrieval).
Mirror drawing task.
The mirror drawing task measures perceptual motor learning. This task was part of a psychosocial stress protocol performed in the cohort. Participants were instructed to copy a star-like shape that was only visible to them in a mirror. They were encouraged to copy as many stars as possible in 5 min. We measured the number of rounds completed and number of errors made and calculated the number of errors per round.
Stroop-like task.
The Stroop-like task measures executive function, specifically selective attention. This task was again part of a psychosocial stress protocol performed in the cohort. We used a short computerized version of a single trial Stroop-like task (13, 14). A name of a color was presented in one of four different ink colors (i.e., the word “blue” printed in yellow ink). Participants had 5 s to name the color of the ink and to choose the correct option out of four names of colors printed in different ink colors. Total test time was 5 min. Time of responding to each item in seconds was recorded, as well as the number of correct and incorrect answers. We calculated the percentage of correct answers.
Statistical Analyses.
We calculated Spearman's ρ or point biserial correlations between the results on the cognitive tests, birth, and adult characteristics (Table 2). To assess associations between prenatal famine exposure and cognitive functioning, we applied linear-regression analysis. We adjusted for sex in all analyses. In additional analyses of statistically significant associations between prenatal famine exposure and cognitive test results, we corrected for potential confounders. As potential confounders, we included birth and adult characteristics that showed a correlation with the test outcomes. Variables with a skewed distribution were log-transformed before analyses and are given as medians and interquartile ranges. Transformation did not normalize the distribution of the variables number of rounds and errors on the mirror task and percentage-correct on the Stroop task. We therefore ranked the scores on these variables and used these in the regression model. In secondary analyses, we tried to rule out the influence of very fast reaction times to the Stroop stimuli as a result of indifferent or inattentive task performance. We therefore excluded participants with faster than average reaction times but also less than 40% correct responses. We considered differences to be statistically significant with P-values ≤ 0.05.
Supplementary Material
Acknowledgments
We thank the participants for their willing cooperation. This study was funded by The Netherlands Heart Foundation (Grants 2001B087, 2003B165, and 2007B083), the Academic Medical Centre (Amsterdam, The Netherlands), the Medical Research Council (United Kingdom), and the European Science Foundation/The Netherlands Organization for Scientific Research.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 16757.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009459107/-/DCSupplemental.
References
- 1.Fontana L, Partridge L, Longo VD. Extending healthy life span—From yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillette-Guyonnet S, Vellas B. Caloric restriction and brain function. Curr Opin Clin Nutr Metab Care. 2008;11:686–692. doi: 10.1097/MCO.0b013e328313968f. [DOI] [PubMed] [Google Scholar]
- 3.Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30:15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Simonson M, Chow BF. Maze studies on progeny of underfed mother rats. J Nutr. 1970;100:685–690. doi: 10.1093/jn/100.6.685. [DOI] [PubMed] [Google Scholar]
- 5.Bush M, Leathwood PD. Effect of different regimens of early malnutrition on behavioural development and adult avoidance learning in Swiss white mice. Br J Nutr. 1975;33:373–385. doi: 10.1079/bjn19750042. [DOI] [PubMed] [Google Scholar]
- 6.Rogers PJ, Tonkiss J, Smart JL. Incidental learning is impaired during early-life undernutrition. Dev Psychobiol. 1986;19:113–124. doi: 10.1002/dev.420190204. [DOI] [PubMed] [Google Scholar]
- 7.Ranade SC, et al. Different types of nutritional deficiencies affect different domains of spatial memory function checked in a radial arm maze. Neuroscience. 2008;152:859–866. doi: 10.1016/j.neuroscience.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Colombo J, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–1267. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 9.Bhate V, et al. Vitamin B12 status of pregnant Indian women and cognitive function in their 9-year-old children. Food Nutr Bull. 2008;29:249–254. doi: 10.1177/156482650802900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hibbeln JR, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 11.Stein Z, Susser M, Saenger G, Marolla F. Nutrition and mental performance. Science. 1972;178:708–713. doi: 10.1126/science.178.4062.708. [DOI] [PubMed] [Google Scholar]
- 12.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 14.MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 15.Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: Costs and potential benefits. Prog Brain Res. 2008;169:353–363. doi: 10.1016/S0079-6123(07)00022-2. [DOI] [PubMed] [Google Scholar]
- 16.Balota DA, et al. Predicting conversion to dementia of the Alzheimer's type in a healthy control sample: the power of errors in Stroop color naming. Psychol Aging. 2010;25:208–218. doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Susser E, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 18.Hoek HW, et al. Schizoid personality disorder after prenatal exposure to famine. Am J Psychiatry. 1996;153:1637–1639. doi: 10.1176/ajp.153.12.1637. [DOI] [PubMed] [Google Scholar]
- 19.Hulshoff Pol HE, et al. Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry. 2000;157:1170–1172. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- 20.Henik A, Salo R. Schizophrenia and the stroop effect. Behav Cogn Neurosci Rev. 2004;3:42–59. doi: 10.1177/1534582304263252. [DOI] [PubMed] [Google Scholar]
- 21.Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154:1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- 22.Fukui T, Sugita K, Sato Y, Takeuchi T, Tsukagoshi H. Cognitive functions in subjects with incidental cerebral hyperintensities. Eur Neurol. 1994;34:272–276. doi: 10.1159/000117055. [DOI] [PubMed] [Google Scholar]
- 23.van Swieten JC, Staal S, Kappelle LJ, Derix MM, van Gijn J. Are white matter lesions directly associated with cognitive impairment in patients with lacunar infarcts? J Neurol. 1996;243:196–200. doi: 10.1007/BF02444014. [DOI] [PubMed] [Google Scholar]
- 24.Stein Z, Susser M, Saenger G, Morolla F. Famine and Human Development. The Dutch Hunger Winter of 1944–45. New York: Oxford University Press; 1975. [Google Scholar]
- 25.Panza F, et al. Cognitive frailty: Predementia syndrome and vascular risk factors. Neurobiol Aging. 2006;27:933–940. doi: 10.1016/j.neurobiolaging.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 26.de la Torre JC. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 27.Breteler MM, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds MD, Johnston JM, Dodge HH, DeKosky ST, Ganguli M. Small head size is related to low Mini-Mental State Examination scores in a community sample of nondemented older adults. Neurology. 1999;53:228–229. doi: 10.1212/wnl.53.1.228. [DOI] [PubMed] [Google Scholar]
- 29.Ravelli ACJ, van Der Meulen JHP, Osmond C, Barker DJP, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 30.Salo R, Henik A, Robertson LC. Interpreting Stroop interference: An analysis of differences between task versions. Neuropsychology. 2001;15:462–471. doi: 10.1037//0894-4105.15.4.462. [DOI] [PubMed] [Google Scholar]
- 31.National Bureau of Food Distribution in Wartime Rations per day period 1 Oct 1944– 22 July 1945 for the western Netherlands. 1945 (Translated from the Dutch) [Google Scholar]
- 32.Burger GCE, Sandstead HR, Drummond JC. Malnutrition and Starvation in Western Netherlands, September 1944 to July 1945. Part I and II. The Hague: General State Printing Office; 1948. [Google Scholar]
- 33.Ravelli ACJ, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 34.Bakker B, Sieben I. International Socio-Economic Index of occupational status (1992) Soc Wetenschappen. 1997;40:1–22. (Translated from the Dutch) [Google Scholar]
- 35.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 36.Heim A. AH4 Group Test of General Intelligence. Windsor, United Kingdom: NFER-Nelson Publishing Company Ltd; 1970. [Google Scholar]
- 37.Martyn CN, Gale CR, Sayer AA, Fall C. Growth in utero and cognitive function in adult life: follow up study of people born between 1920 and 1943. BMJ. 1996;312:1393–1396. doi: 10.1136/bmj.312.7043.1393a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


