Abstract
Blood vessels control all stages of tumor development and therapy by defining the physicochemical and cellular state of the tumor microenvironment. However, no pathologically relevant culture systems currently exist that recapitulate the associated cellular and convective mass transfer processes that are implicated in tumor angiogenesis. By integrating tissue engineering and microfluidic technologies, it will be possible to develop tumor-mimetic culture environments with embedded microvascular structures. Utilization of these microfluidic tumor models will help reveal the importance of the transport of chemical and cellular factors in tumor angiogenesis, and provide a test bed that may ultimately improve current strategies to antiangiogenic therapy.
Introduction
Insights from the study of tumor-inherent mechanisms that lead to increased vascularization have long been exploited to improve angiogenesis in engineered or diseased tissues. For example, spatiotemporally controlled delivery of proangiogenic factors and vascular cells represents a common approach to induce therapeutic angiogenesis.1,2 Now, tissue engineers may return the favor to the cancer biologists by providing new culture platforms that will help to dissect further the angiogenic processes in tumors. Advanced in vitro platforms that recapitulate both the macro- and microscale physiology of solid tumors may reveal new mechanisms and effects implicated in tumor angiogenesis. Specifically, three-dimensional (3D) culture systems that integrate vascular structure, a key microphysiological component of surrounding host tissues and of advanced tumors themselves, will enable pathologically relevant testing of hypotheses and therapies.
Blood vessels not only provide a foundation from which neovascularization of developing tumor occurs, but also constitute an interface for the exchange of chemical and cellular factors between tumor and host tissue.3,4 Specifically, convective mass transfer regulates hydration, exchange of metabolites, body temperature, and the transmission of chemical signals (e.g., growth factors and chemotherapeutic drugs).3,5 Further, perfusion processes control the transport, recruitment, and replication of secondary cell types that may be critical to tumorigenesis, metastasis, and the efficacy of chemotherapies. For example, capillaries guide the transport of bone-marrow-derived progenitor cells6 and immune cells7,8 and constitute supportive niches that control the activation and maintenance of cancer stem cells.9 In these roles, vascular structures define the physicochemical and cellular state of a tumor at all stages of development (pre- and postangiogenesis) and set important criteria for the design of successful therapeutic interventions.
Conventional 3D culture systems enable the recapitulation of certain characteristics of tumors such as gradients of oxygen tension, 3D cell–cell and cell–matrix interactions10,11; however, they exhibit limitations in their ability to couple local cell behavior with convective mass transfer to systemic sources of morphogens and cells. Conventional microfluidic systems for cell culture, on the other hand, provide fine control of the physical environment of cells living within the fluid-filled space defined by the microchannels12,13; however, they fail to provide exchange of solutes and cells with a bulk tissue, specific barrier properties of the endothelium, and potential for angiogenic progression. The appropriate integration of tissue engineering strategies and microfluidics has the potential to overcome these limitations and transform in vitro approaches for the study of cancer.
Here, we present a vision of this fusion of tissue engineering and microfluidic technologies (Fig. 1), and explore the challenges and opportunities associated with the development of microfluidic tumor models.
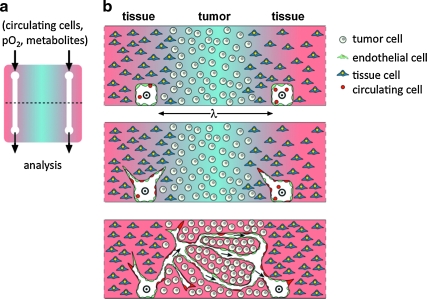
FIG. 1.
Vision of a microfluidic tumor model. (a) Top view of model with a pair of microchannels embedded in a slab of cell-seeded matrix. Composition of fluid is defined at inlet and analyzed at outlet. Dashed line indicates position of cross-sectional views shown in (b). (b) Cross-sectional views of scaffold in (a) indicating possible temporal progression, from top to bottom. Flow through channels is indicated with ⊙ and arrows. Cells are shown in well-defined locations: tumor and host tissue in the bulk of the scaffold, endothelial cells lining channels, and circulating cells (e.g., endothelial progenitor cells) in the flow and integrated into the developing endothelium. Blue shading indicates gradients of soluble factors (e.g., oxygen or metabolites).
Engineering Design Considerations
As suggested in Figure 1, microfabrication can be exploited to generate the initial conditions of a tumor model with well-defined microstructure in both the matrix and the cellular composition.14,15 The inclusion of microchannels within the 3D matrix maintains the benefits inherent to 3D culture—for example, spontaneous emergence of metabolite gradients and cell–matrix interactions—while providing access to the bulk of the developing tissue. The potential benefits of these conduits include spatially resolved delivery and extraction of solutes to control and monitor the biochemistry of the tumor's microenvironment; growth of an endothelium in an appropriate architecture to act as a biologically specific interface between the tumor and the blood volume16; delivery of circulating cells such as bone-marrow-derived endothelial progenitor cells (EPCs) to study their attachment, integration, and influence on the angiogenic process17; and definition of physiologically relevant hydraulic stresses—for example, wall tension and shear stress on the endothelium—to study their roles in angiogenesis, tumor development, and drug delivery.5,18,19 An ideal model will also accommodate significant morphological changes during culture. In particular, recapitulation of sprouting angiogenesis in this platform would allow for real-time observations of the entire angiogenic progression while maintaining unprecedented control of physical, chemical, and biological parameters via the externally controlled perfusion. Such studies could shed light on outstanding questions related to the origins of hypervascularization, high vessel permeability, and the migration of cells into and out of the tumor during immune response and metastasis.5,20
The realization of this vision presents significant engineering challenges. Paramount to this effort is the development of microfabrication techniques in appropriate materials to define micropatterned structures and distributions of cells in 3D. Advances in lithography14,21 and printing technologies22 for processing cell-seeded hydrogels provide a foundation for this work, yet continued refinement of the spatial resolution of fully 3D fabrication processes and of material characteristics (e.g., to allow for cellular remodeling with the maintenance of structural integrity) are desirable. Additional challenges include the necessity for microfluidic tumor models to be compatible with microscopy to allow for in situ and histological analysis, as well as for the selective harvest of cells for genotyping.
Biological Design Considerations
By controlling the cellular composition of the microfluidic tumor models, it will be possible to adjust the complexity of the culture system to the specific question to be addressed. For example, biologically relevant conditions can be recreated by including mixed cell populations consisting of tumor cells in the bulk, stroma cells in the periphery, endothelial cells lining the vessel, and immune cells being transported and recruited via the biomimetic perfusion (Fig. 1b). Additionally, pericytes, important functional components of the tumor vasculature, may also be incorporated into these systems.23 By selectively adding or subtracting complexity, it will be possible to investigate responses of individual cell types in the presence and absence of specific cellular mediators, studies not possible with in vivo analysis. This approach will enable analysis of potential circulating molecular and cellular components that promote tumor angiogenesis, via, for example, the recruitment of bone-marrow-derived progenitor cells.6,24 These circulating progenitor cells are known to modulate tumor progression, but it remains unclear when, how, and where these cells act. While EPCs differentiate into mature endothelial cells and incorporate into vascular networks, hematopoietic progenitor cells preferentially localize to the perivascular space.25 Perfusion of these cells into microfluidic, humanized tumor models may allow for the elucidation of the underlying cellular and molecular mediators and spatiotemporal regulation of their tumorigenic behavior. The cell types to be used for the development of microfluidic tumor models can be primary cells, cell lines, or patient-derived cells. This universality enhances the prognostic value of these models by enabling the comparison of well-characterized cell behaviors with actual patient samples. Further, the use of patient-derived cells will permit individualized drug testing, which may improve therapeutic outcomes by identifying and excluding (non-) effective treatment options before the actual start of the therapy.
To further enhance the tractability of microfluidic tumors, it will be beneficial to develop these systems from artificial extracellular matrices. Beyond their role in maintaining the structural integrity of the microfluidic channels, these matrices must also provide appropriate spatiotemporal presentation of signaling molecules (e.g., sequestration and cell-demanded release of growth factors) and allow for cell adhesion and matrix remodeling to permit the invasion of tumorigenic and normal cells, both of which are critical for tumor formation.26,27 Further, the biomaterials that form the microfluidic scaffold will have to recapitulate and withstand mechanical cues such as enhanced matrix stiffness and interstitial pressure, respectively, that modulate cell signaling via mechanoreceptor signal transduction.28
Future Perspectives
A number of requirements will need to be met to allow for routine application of microfluidic tumor models in academia and industry. For example, scaling up of the fabrication process will be required to enable multiplex analyses of cell behavior and drug response. To this end, microfluidic tumors may be developed as tool boxes in which individual cellular and molecular components of the tumor microenvironment can be selectively added depending on the specific scientific questions to be answered. Further, it will be necessary to develop approaches in which the biomimetic vascular channels can be connected to other body compartments to study systemic effects related to different experimental conditions or treatment regimes. For example, anastomosis of the developed vascular structures with the host vasculature following in vivo implantation may warrant such studies.29 Alternatively, the development of tumors-on-a-chip strategies may be ideal to test the efficacy and negative side effects of therapeutic strategies.30 In particular, such a platform could help to optimize important aspects of antiangiogenic therapies ranging from the development of the drug itself to the actual treatment of patients (e.g., chemical efficacy, choice of delivery vehicle, appropriate temporal profile of delivery, intratumoral localization of the drug, and efficacy of normalization of low quality vessels5).
In summary, microfluidic tumor models are applicable to studying a wide range of questions related to tumorigenesis, tumor vascularization, and antiangiogenic therapy. They have significant potential to enhance progress in the fields of cancer biology, tissue engineering, and microfluidics, which collectively may yield new universal or individualized anticancer therapies.
Acknowledgments
The authors acknowledge funding from the Cornell Nanobiotechnology Center (supported by the STC Program of the National Science Foundation under Agreement No. ECS-9876771), the Beckman Foundation, NYSTAR, the Morgan Fund for Tissue Engineering, and NIH (RC1 CA146065 and 1U54 CA143876-01). The authors would also like to thank Scott Verbridge, Nakwon Choi, Ying Zheng, and Vivek Mittal for their assistance with experiments and helpful discussions.
Disclosure Statement
No competing financial interests exist.
References
- 1.Fischbach C. Mooney D.J. Polymers for pro- and anti-angiogenic therapy. Biomaterials. 2007;28:2069. doi: 10.1016/j.biomaterials.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Moon J.J. West J.L. Vascularization of engineered tissues: approaches to promote angiogenesis in biomaterials. Curr Top Med Chem. 2008;8:300. doi: 10.2174/156802608783790983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerbel R.S. Molecular origins of cancer: tumor angiogenesis. N Engl J Med. 2008;358:2039. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D. Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Jain R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 6.Lyden D. Hattori K. Dias S. Costa C. Blaikie P. Butros L. Chadburn A. Heissig B. Marks W. Witte L. Wu Y. Hicklin D. Zhu Z.P. Hackett N.R. Crystal R.G. Moore M.A.S. Hajjar K.A. Manova K. Benezra R. Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 7.Hiratsuka S. Watanabe A. Sakurai Y. Akashi-Takamura S. Ishibashi S. Miyake K. Shibuya M. Akira S. Aburatani H. Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nature Cell Biology. 2008;10:1349–U229. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 8.De Larco J.E. Wuertz B.R.K. Furcht L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese C. Poppleton H. Kocak M. Hogg T.L. Fuller C. Hamner B. Oh E.Y. Gaber M.W. Finklestein D. Allen M. Frank A. Bayazitov I.T. Zakharenko S.S. Gajjar A. Davidoff A. Gilbertson R.J. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Pampaloni F. Reynaud E.G. Stelzer E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 11.Lee G.Y. Kenny P.A. Lee E.H. Bissell M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Ali J. Sorger P.K. Jensen K.F. Cells on chips. Nature. 2006;442:403. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- 13.Tourovskaia A. Figueroa-Masot X. Folch A. Differentiation-on-a-chip: a microfluidic platform for long-term cell culture studies. Lab Chip. 2005;5:14. doi: 10.1039/b405719h. [DOI] [PubMed] [Google Scholar]
- 14.Choi N.W. Cabodi M. Held B. Gleghorn J.P. Bonassar L.J. Stroock A.D. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 15.Nelson C.M. VanDuijn M.M. Inman J.L. Fletcher D.A. Bissell M.J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrobak K.M. Potter D.R. Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Gao D.C. Nolan D.J. Mellick A.S. Bambino K. McDonnell K. Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 18.Boucher Y. Leunig M. Jain R.K. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56:4264. [PubMed] [Google Scholar]
- 19.Helm C.L.E. Fleury M.E. Zisch A.H. Boschetti F. Swartz M.A. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci U S A. 2005;102:15779. doi: 10.1073/pnas.0503681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmeliet P. Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 21.Cabodi M. Choi N.W. Gleghorn J.P. Lee C.S.D. Bonassar L.J. Stroock A.D. A microfluidic biomaterial. J Am Chem Soc. 2005;127:13788. doi: 10.1021/ja054820t. [DOI] [PubMed] [Google Scholar]
- 22.Cohen D.L. Malone E. Lipson H. Bonassar L.J. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12:1325. doi: 10.1089/ten.2006.12.1325. [DOI] [PubMed] [Google Scholar]
- 23.Bergers G. Song S. Meyer-Morse N. Bergsland E. Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Investig. 2003;111:1287. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaked Y. Ciarrocchi A. Franco M. Lee C.R. Man S. Cheung A.M. Hicklin D.J. Chaplin D. Foster F.S. Benezra R. Kerbel R.S. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 25.Rafii S. Lyden D. Benezra R. Hattori K. Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 26.Fischbach C. Kong H.J. Hsiong S. Yuen W. Mooney D.J. Cancer cell angiogenic capability is regulated by 3-D culture and integrin engagement. Proc Natl Acad Sci U S A. 2009;106:399. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 28.Butcher D.T. Alliston T. Weaver V.M. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X.F. Aledia A.S. Ghajar C.M. Griffith C.K. Putnam A.J. Hughes C.C.W. George S.C. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15:1363. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sin A. Chin K.C. Jamil M.F. Kostov Y. Rao G. Shuler M.L. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol Prog. 2004;20:338. doi: 10.1021/bp034077d. [DOI] [PubMed] [Google Scholar]