Abstract
Human adipose stem cells (hASCs) can differentiate into a variety of phenotypes. Native extracellular matrix (e.g., demineralized bone matrix or small intestinal submucosa) can influence the growth and differentiation of stem cells. The hypothesis of this study was that a novel ligament-derived matrix (LDM) would enhance expression of a ligamentous phenotype in hASCs compared to collagen gel alone. LDM prepared using phosphate-buffered saline or 0.1% peracetic acid was mixed with collagen gel (COL) and was evaluated for its ability to induce proliferation, differentiation, and extracellular matrix synthesis in hASCs over 28 days in culture at different seeding densities (0, 0.25 × 106, 1 × 106, or 2 × 106 hASC/mL). Biochemical and gene expression data were analyzed using analysis of variance. Fisher's least significant difference test was used to determine differences between treatments following analysis of variance. hASCs in either LDM or COL demonstrated changes in gene expression consistent with ligament development. hASCs cultured with LDM demonstrated more dsDNA content, sulfated-glycosaminoglycan accumulation, and type I and III collagen synthesis, and released more sulfated-glycosaminoglycan and collagen into the medium compared to hASCs in COL (p ≤ 0.05). Increased seeding density increased DNA content incrementally over 28 days in culture for LDM but not COL constructs (p ≤ 0.05). These findings suggest that LDM can stimulate a ligament phenotype by hASCs, and may provide a novel scaffold material for ligament engineering applications.
Introduction
The functional repair of tendon and ligament injury remains a major challenge, and current surgical treatments are limited by high failure rates, failure to restore normal tissue attachments, donor-site morbidity for autografts, risk of disease transmission for allografts, prolonged healing times, and incomplete ligamentization.1,2 Functional tissue engineering utilizes a combination of scaffolds, cells, and bioactive molecules to replace or repair injured tissues with a primary goal of restoring biomechanical function,3 and there is particular emphasis on the rotator cuff and anterior cruciate ligament as key targets in this area.1 To this end, a number of cell sources and biomaterials have been investigated for use in tissue engineered tendon and ligament constructs. Cell sources have included tendon and ligament fibroblasts, bone-marrow-derived mesenchymal stem cells (MSCs), adipose-derived stem cells (ASCs), tendon sheath fibroblasts, and multipotent tendon progenitor cells.4–7 Growth factors are often necessary to enhance differentiation of these cells toward a tendon or ligament phenotype and to promote tissue formation, and previous studies have investigated the roles of transforming growth factor β (TGF-β), insulin-like growth factor, epidermal growth factor (EGF), basic fibroblastic growth factor (bFGF), vascular endothelial growth factor, bone morphogenetic proteins (BMPs)-12, -13, and -14, and autologous platelet concentrate.6–17 However, BMP-12, -13, and -14 can induce cartilage and bone formation within injured tendon in a dose-dependent manner, and sequential application of growth factors may be useful if an in vitro bioreactor is used, but may be difficult to apply if used directly in vivo.10,13,15,18
A variety of biomaterials have been investigated for their ability to support tendon or ligament tissue engineering, principally focusing on collagen, gelatin, polyurethane, polylactic and polyglycolic acids, poly(ɛ-caprolactone), polylactide-co-glycolide, and silk.19–29 Purified collagen gel in particular has been investigated extensively as a substrate or cell delivery substrate for repair of tendon and ligament defects and supports cell migration, proliferation, alignment, glycosaminoglycan and collagen synthesis, and increased expression of genes associated with tendon and ligament.30–42 Further, there is growing evidence that native extracellular matrices may possess additional biological factors that can enhance cell growth and differentiation in the absence of exogenous growth factors. For example, extensive work by Urist in the 1960s led to the identification and subsequent clinical development of demineralized bone matrix as an osteoinductive agent,43,44 and recently cartilage-derived extracellular matrix (ECM) was found to induce chondrogenesis in MSCs and ASCs.45,46 Other investigators have evaluated biologic scaffolds, such as small intestinal submucosa, dermis, fascia lata, pericardium, and decellularized tendon or tendon slices for repair of tendon and ligament.5,47–62
While these other tissue matrices have shown some ability to induce tendon or ligament formation, the ECM components from native tendon appear to be critical for differentiation because depletion of key ECM components from tendon impairs tendon formation by tendon progenitor cells.4 Decorin inhibits type I collagen fibrillogenesis during repair, but is critical for normal fibrillogenesis in tendon development when expression increases rapidly and then decreases to a steady state.63 Biglycan expression on the other hand decreases during tendon development, suggesting a critical temporal switch between biglycan and decorin expression for collagen fibrillogenesis.63 Conversely, during tendon healing, biglycan expression increases and decorin expression decreases.64 These data suggest that even though the processes involved in tendon formation remain to be fully elucidated, ECM components specific to tendon play a critical role in tendon development and repair. Therefore, ECM from tendon and ligament is a logical choice for repair of tendon and ligament.65 ECM derived from tendon or ligament, which can be manipulated to produce a mechanically functional scaffold while promoting rapid cellular infiltration and proliferation and tissue-specific ECM synthesis, could make a valuable contribution to tissue engineering of tendon and ligament.
The goal of this study was to develop a scaffold prepared from native ligament using a combination of pulverization and lyophilization, and to evaluate the influence of this ligament-derived matrix (LDM) on the growth and differentiation of human adipose stem cells (hASCs). We hypothesize that LDM would enhance expression of a ligamentous phenotype by hASCs compared to culture in collagen gel alone. Further, we examined whether hASCs would respond differently to LDM prepared by disinfection and decellularization with 0.1% peracetic acid (PAA) compared to that prepared using phosphate-buffered saline (PBS). Cells were seeded into constructs containing type I collagen gel or additionally containing LDM powder prepared using either 0.1% PAA or PBS. Constructs were cultured in vitro for up to 28 days and evaluated for cellular proliferation and development of a ligament phenotype by sulfated-glycosaminoglycan (s-GAG) and collagen content, immunofluorescence, histology, and by quantitative real-time PCR using a panel of markers associated with ligament development.
Materials and Methods
Cell culture
hASCs were isolated from lipoaspirate surgical waste by collagenase digestion66 from three female donors (age, 42–61; body–mass index, 22.3–24.9) with approval from the Duke University Institutional Review Board. Cells were expanded in monolayer on tissue culture plastic through passage 4 in an expansion medium consisting of Dulbecco's modified Eagle's medium/F12 (Lonza, Walkersville, MD), 10% fetal bovine serum (Zen-Bio, Research Triangle Park, NC), 1% penicillin–streptomycin–Fungizone (Invitrogen, Carlsbad, CA), 0.25 ng/mL TGF-β1 (R&D Systems, Minneapolis, MN), 5 ng/mL EGF (Roche Diagnostics, Indianapolis, IN), and 1 ng/mL bFGF (Roche Diagnostics), which was changed every other day. Cells were trypsinized (0.05% trypsin–ethylenediaminetetraacetic acid; Invitrogen) at 90% confluence and passaged.
Construct preparation
Anterior cruciate ligament (ACL) was harvested from six adult female porcine knee joints within 12 h of slaughter, and minced into ∼1-mm3 pieces using scalpel blades. One gram aliquots were loaded into a 6750 Spex SamplePrep Freezer Mill (Spex CertiPrep, Metuchen, NJ) and precooled for 3 min in liquid nitrogen. Minced ACL was pulverized for five cycles of 1 min duration at 15 Hz with 2 min of additional cooling between cycles. The resulting ACL powder was resuspended (0.1 g/mL) in PBS (pH 7.4) and stirred overnight at 4°C or subjected to additional disinfection and decellularization using 0.1% PAA and stirred overnight at 4°C. The suspensions were frozen (−80°C) and lyophilized (VirTis Benchtop SLC freeze dryer; SP Industries, Warminster, PA) at −48°C and 100 mTorr for 24 h. Pulverization was repeated using the freezer mill, after 3 min of precool and two cycles of 1 min duration at 5 Hz with 2 min of additional cooling between cycles. LDM prepared from PBS- or PAA-treated ACL was divided into aliquots and sterilized with ethylene oxide. Aliquots of LDM were not evaluated for ethylene oxide residue or contaminants after sterilization. PBS- or PAA-treated LDM aliquots were mixed with type I rat tail collagen (High Concentration Rat Tail Collagen; BD Biosciences, Bedford, MA) prepared and gelled according to manufacturer's instructions to a final collagen concentration of 2 mg/mL, and a PBS- or PAA-LDM concentration of 25 mg/mL, the highest concentration of LDM that permitted transfer and mixing. A control group was prepared using 2 mg/mL type I rat tail collagen alone. During mixing, the three groups were seeded with passage 4 hASCs from each of the three donors at 0, 0.25 × 106, 1 × 106, or 2 × 106 hASC/mL. The resulting gels were immediately transferred into the barrel of modified 1 mL syringes, and allowed to solidify at 37°C for 1 h.39 The constructs were removed from the syringe and cultured at 37°C in 5% CO2 for 0, 7, 14, or 28 days in 12-well plates coated with 2% agarose (to prevent cell adherence on tissue culture plastic) in 2 mL Advanced Dulbecco's modified Eagle's medium (Invitrogen), 10% fetal bovine serum (Zen-Bio), 1% penicillin–streptomycin–Fungizone (Invitrogen), 4 mM L-glutamine (Invitrogen), and 1.5 mM L-ascorbic acid-2-phosphate (Sigma, St. Louis, MO) with no added growth factors, and the medium was changed every other day.
LDM particle size
Samples of PAA- and PBS-treated LDM powder were critical point dried in CO2, and then sputter coated with gold. The prepared powder was viewed with a Philips 501 scanning electron microscope. Two representative images were taken of each sample and overlaid with a digital grid. Particle length and width were measured using GNU Image Manipulation Program 2.4.2 freeware for particles in the image that lay underneath points of intersection of lines on the grid. Intersections were not counted if there was no particle under the point, and intersections were evenly and randomly distributed over the entire image. The size of 140 particles was measured for each type of LDM.
dsDNA, s-GAG, and collagen quantitation
On days 0, 7, 14, and 28, constructs from each treatment group were harvested, dried in an oven until constant weight, and then digested for 18 h in papain (125 μg/mL) at 60°C. dsDNA content was quantified using the Picogreen Assay (Invitrogen). s-GAG content was quantified spectrophotometrically using 1,9-dimethylmethylene blue (DMMB) dye (pH 3.0) and bovine chondroitin-4-sulfate as a standard.67 The hydroxyproline assay was used to determine total collagen content, and a conversion factor of 1:7.46 was used to convert hydroxyproline to collagen.68 dsDNA, s-GAG, and collagen content of 1 mL aliquots of the cell culture medium was quantified after papain digestion using the same assays on days 0, 2, 4, 6, 10, 14, 20, and 28 of culture.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from constructs seeded with 1 × 106 hASCs/mL from each treatment group using TRIzol (Invitrogen) followed by further purification using the RNeasy Mini Clean Up protocol and on-column DNAse treatment (Qiagen, Valencia, CA) on days 0, 7, and 14 of culture according to instructions provided by the manufacturer. RNA was quantified spectrophotometrically using the Nanodrop ND-1000 (Nanodrop Technologies, Wilmington, DE). Equal amounts of RNA were reverse transcribed using the Superscript VILO cDNA Synthesis Kit (Invitrogen). Equal aliquots of each cDNA sample were pooled and used to generate serial dilutions for standard curves from which efficiency was calculated for each gene of interest. Real-time PCR was performed on an iCycler (Biorad, Hercules, CA) using Express qPCR SuperMix (Invitrogen). Commercially available primer probes (Applied Biosystems, Foster City, CA) or custom-designed primer probes were used to compare transcript levels for nine different genes: 18S rRNA (endogenous control, assay ID Hs99999901_s1), type I collagen (COL1A1, assay ID Hs00164004_m1), type II collagen (COL2A1A forward primer 5′-GAGACAGCATGACGCCGAG-3′; reverse primer 5′-GCGGATGCTCTCAATCTGGT-3′; probe 5′-FAM-TGGATGCCACACTCAAGTCCCTCAAC-TAMRA-3′),69 type III collagen (COL3A1, assay ID Hs00164103_m1), type X collagen (COL-10A1, assay ID Hs00166657_m1), biglycan (BGN, assay ID Hs00959143_m1), decorin (DCN, assay ID Hs00266491_m1), tenomodulin (TNMD, assay ID Hs00223332_m1), and tenascin C (TNC, assay ID Hs00233648_m1). Expression of type II and X collagen was examined to confirm that hASCs were not undergoing chondrogenic differentiation.
Data from each gene of interest for each sample were corrected for efficiency and normalized to expression of 18S, and then expressed as fold-change relative to the level of gene expression in 1 million P4 hASCs from each donor at day 0.70
Histology and immunohistochemistry
Unseeded constructs and constructs seeded with 1 × 106 hASCs/mL from each biological donor were harvested after 28 days of culture, fixed overnight at 4°C in 4% paraformaldehyde, dehydrated, cleared, embedded in paraffin, cut into 5-μm-thick sections, and mounted on slides. A section of human tensor fascia lata was used as positive control. For hematoxylin and eosin staining, sections were deparaffinized, rehydrated, stained with Harris hematoxylin, differentiated with 1% acid alcohol, and counterstained with eosin-phloxine B solution. For safranin-O/fast green staining, sections were deparaffinized, rehydrated, incubated in 1% acid alcohol, stained with 0.02% fast green, rinsed in 1% acetic acid, and then stained with 0.1% Safranin O. For immunofluorescence labeling of human type I and III collagen deposition in the constructs, sections were deparaffinized and rehydrated, and then subjected to epitope retrieval using 0.1% pepsin in 0.01 N HCl at room temperature for 20 min. Sections were blocked with 10% normal goat serum for 20 min, and then negative controls were incubated with 1.5% normal goat serum in PBS. Adjacent sections on the same slide were incubated with a 1:50 dilution of mouse monoclonal IgM antibody to human-specific type III collagen (sc-59901; Santa Cruz Biotechnology, Santa Cruz, CA) or a 1:50 dilution of mouse monoclonal IgG antibody to human-specific type I collagen (sc-80760; Santa Cruz Biotechnology) in 1.5% normal goat serum in PBS for 40 min at room temperature. After washing in PBS, sections were incubated in a 1:100 dilution of goat anti-mouse IgG-fluorescein isothiocyanate (FITC) (sc-2082; Santa Cruz Biotechnology) or a 1:100 dilution of goat anti-mouse IgG-FITC (sc-2010; Santa Cruz Biotechnology), respectively, for 45 min. Sections were washed in PBS and then mounted in 90% glycerol/PBS and examined under a Zeiss LSM 510 Confocal Microscope (Carl Zeiss, Thornwood, NY).
Statistical analysis
LDM particle size data were reported as mean ± SD and analyzed by t-test. Biochemical and gene expression data were reported as mean ± standard error of the mean, tested for normality, and analyzed using analysis of variance. Fisher's least significant difference test was used to determine differences between treatments following analysis of variance. Significance was reported at the 95% confidence level for all analyses (α = 0.05).
Results
Length and width (mean ± SD) of particles of PBS-LDM were significantly smaller (length, 60.19 ± 48.86 μm; width, 23.96 ± 19.01 μm) than particles of PAA-LDM (length, 114.14 ± 136.99 μm [p < 0.00005]; width, 38.78 ± 57.94 μm [p < 0.005]). Dry mass of constructs containing collagen gel alone was significantly less than those containing either PBS- or PAA-LDM (Supplemental Fig. S1, available online at www.liebertonline.com). Constructs containing collagen gel alone contracted more over 28 days in culture than those containing either type of LDM.
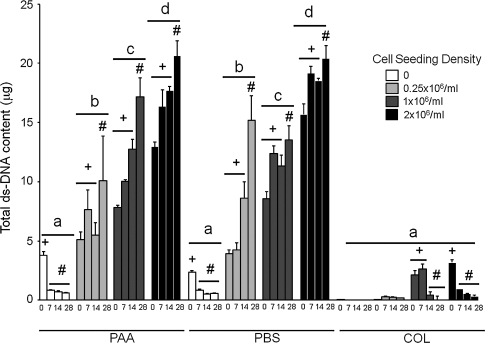
hASC-seeded constructs containing PAA- or PBS-LDM contained more dsDNA than all constructs containing collagen gel alone. dsDNA content increased with initial seeding density for constructs containing PAA- and PBS-LDM. hASC-seeded constructs treated with either type of LDM had increased dsDNA content at day 28 of culture compared to days 0–14. Constructs containing collagen gel alone and seeded with 1 × 106 or 2 × 106 hASCs/mL had decreased dsDNA content after 14 and 28 days of culture compared to day 0. dsDNA was not completely removed from PAA- or PBS-LDM, but there was a significant reduction in dsDNA content of LDM constructs not seeded with cells after 7 days in culture, and dsDNA content of unseeded LDM constructs was not different to all seeding densities containing collagen gel alone (Fig. 1). dsDNA release into the cell culture medium was increased at day 2 for constructs containing PAA- or PBS-LDM compared to all other time points, and to constructs containing collagen gel alone. Constructs containing PAA-LDM released more dsDNA into the medium than those containing PBS-LDM on day 2 of culture (Supplemental Fig. S2, available online at www.liebertonline.com).
FIG. 1.
Total dsDNA content (μg) of constructs prepared using peracetic acid (PAA)-ligament-derived matrix (LDM) (PAA), phosphate-buffered saline (PBS)-LDM (PBS), or collagen gel alone (COL), seeded with 0, 0.25 × 106, 1 × 106, or 2 × 106 human adipose stem cells (hASCs)/mL and harvested at day 0, 7, 14, or 28 of culture. The presence of LDM induced a significant increase in DNA content as compared to collagen gel alone. Groups having different letters are significantly different from other groups across all time points. Groups having different symbols (# and+) over time for different seeding densities and treatments are significantly different (p ≤ 0.05; n = 3 biologic donors, mean ± standard error of the mean [SEM]).
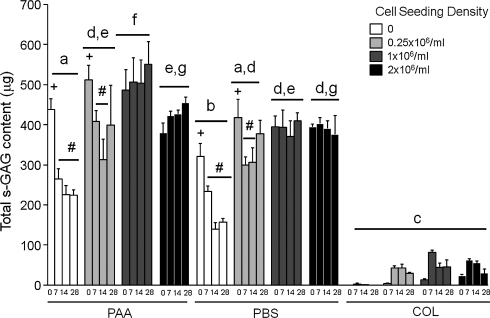
s-GAG content of constructs prepared using collagen gel alone was significantly less than constructs prepared using either type of LDM. Unseeded constructs containing either type of LDM contained less s-GAG than those seeded with hASCs, but unseeded constructs prepared using PAA-LDM contained significantly more s-GAG than those prepared with PBS-LDM. s-GAG content in unseeded LDM constructs decreased from days 7 to 28 of culture, and this was also true for days 7–14 of culture for LDM constructs seeded with 0.25 × 106 hASCs/mL. Constructs prepared with PAA-LDM and seeded with 1 million hASCs/mL contained more s-GAG than any other group, but other than this, seeding density did not affect s-GAG content over 28 days in culture (Fig. 2). s-GAG release into the culture medium was significantly less from constructs containing collagen gel alone than those containing either PAA- or PBS-LDM. Constructs containing PAA-LDM released more s-GAG than those containing PBS-LDM on day 2, but by day 4 of culture there was no difference between type of LDM and release of s-GAG. Unseeded LDM constructs and those seeded with 0.25 × 106 hASCs/mL released significantly more s-GAG into the medium than constructs containing collagen gel alone only until day 6 of culture, whereas those seeded with 1 × 106 or 2 × 106 hASCs/mL released more s-GAG into the medium than constructs containing collagen gel alone until later time points (Supplemental Fig. S3, available online at www.liebertonline.com).
FIG. 2.
Total sulfated-glycosaminoglycan (s-GAG) content (μg) of constructs prepared using PAA-LDM (PAA), PBS-LDM (PBS), or collagen gel alone (COL), seeded with 0, 0.25 × 106, 1 × 106, or 2 × 106 hASCs/mL and harvested at day 0, 7, 14, or 28 of culture. Constructs containing LDM showed higher overall sulfated-glycosaminoglycan content, but did not exhibit consistent changes over time in culture. Groups having different letters are significantly different from other groups across all time points. Groups having different symbols (# and +) over time for different seeding densities and treatments are significantly different (p ≤ 0.05; n = 3 biologic donors, mean ± SEM).
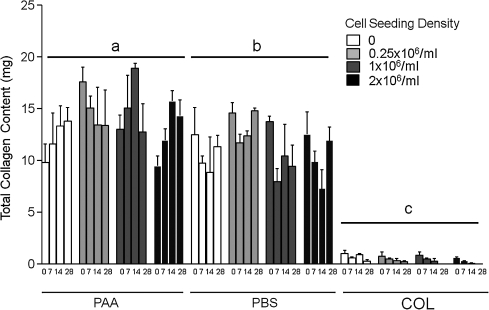
Collagen content of PAA-LDM constructs for all seeding densities was greater than the collagen content of all seeding densities of PBS-LDM constructs. Constructs prepared with collagen gel alone had the lowest collagen content of all treatment groups (Fig. 3).
FIG. 3.
Total collagen content (mg) of constructs prepared using PAA-LDM (PAA), PBS-LDM (PBS), or collagen gel alone (COL), seeded with 0, 0.25 × 106, 1 × 106, or 2 × 106 hASCs/mL and harvested at day 0, 7, 14, or 28 of culture. Constructs containing LDM showed significantly higher collagen content as compared to collagen gel alone. Groups having different letters are significantly different from other groups across all time points (p ≤ 0.05; n = 3 biologic donors, mean ± SEM).
The earliest and greatest increase in collagen release into the medium was seen after 2 days of culture for constructs prepared using PAA-LDM. Peak collagen release into the medium was delayed and was significantly increased from day 6 to 14 of culture for constructs prepared using PBS-LDM and seeded with 1 × 106 or 2 × 106 hASCs/mL. Collagen release through the culture period from constructs at all seeding densities prepared using collagen gel alone was significantly less than LDM constructs seeded with either 1 × 106 or 2 × 106 hASCs/mL. Unseeded constructs prepared with either type of LDM briefly released collagen into the medium on days 0–4 of culture, and LDM constructs seeded with 0.25 × 106 hASCs/mL released similar levels of collagen into the medium as did cell-seeded collagen gel constructs (Supplemental Fig. S4, available online at www.liebertonline.com).
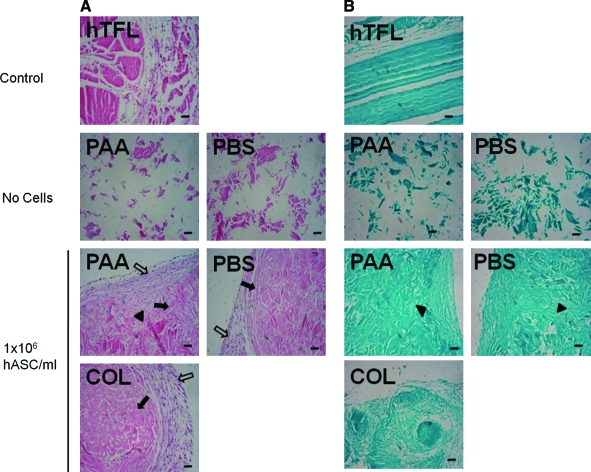
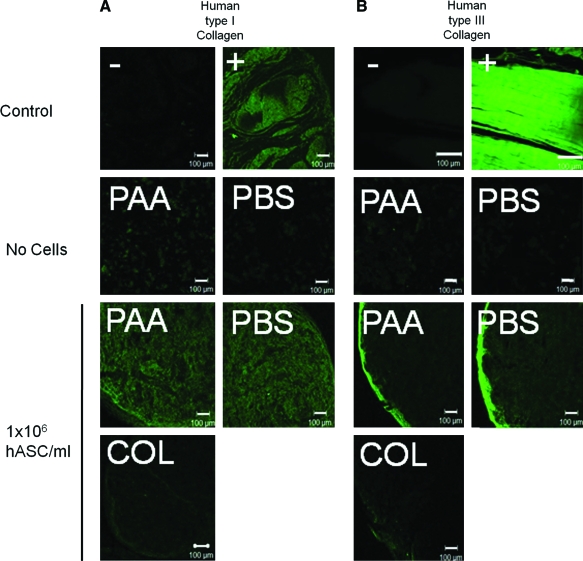
Hematoxylin and eosin staining of constructs seeded with 1 × 106 hASCs/mL demonstrated development of two distinct cell populations: an inner cell population within what appeared to be the original components of the construct, and an outer capsular population of cells that surrounded the original components of the construct and in some regions appeared to be extending into the original construct components. Safranin O/fast green staining yielded very little evidence of proteoglycan, consistent with normal tendon, but collagen staining was more intense in LDM constructs seeded with 1 × 106 hASCs/mL than in constructs prepared with collagen gel alone (Fig. 4). For human type I and III collagen immunofluorescence, lack of cross reactivity of the primary antibodies with porcine type I or type III collagen in the LDM or rat type I collagen was demonstrated by lack of positive staining of unseeded LDM constructs. In hASC-seeded LDM constructs, strong positive staining for human type I collagen was identified centrally, and strong positive staining for human type III collagen was identified around the periphery of the constructs. In hASC-seeded constructs prepared using collagen gel alone, no positive staining for human type I collagen was identified, and staining for human type III collagen was much less intense than for LDM-treated constructs (Fig. 5).
FIG. 4.
Histology sections stained with (A) hematoxylin and eosin or (B) safranin-O/fast green for control tendon (human tensor fascia lata [hTFL]), unseeded PAA-LDM (PAA), and PBS-LDM (PBS) constructs, or constructs prepared with PAA, PBS, or collagen gel alone (COL) and seeded with 1 × 106 hASCs/mL. Two distinct cell populations are evident: an inner cell population within the original components of the construct (closed arrows), and an outer capsular population of cells (open arrows). In some regions cells from the outer capsule extend into the original construct components (closed arrowheads) (5 μm sections, 100 × magnification, scale bar = 100 μm). Color images available online at www.liebertonline.com/ten.
FIG. 5.
Positive (+) and negative (−) control tendon, unseeded PAA-LDM (PAA) and PBS-LDM (PBS) constructs, or constructs prepared with PAA, PBS, or collagen gel alone (COL) and seeded with 1 × 106 hASCs/mL stained for human type I collagen (A) or human type III collagen (B). Cell-seeded constructs showed significant labeling for human type I collagen throughout the construct, whereas human type III collagen was located peripherally (5 μm sections, 100 × magnification, scale bar = 100 μm). Color images available online at www.liebertonline.com/ten.
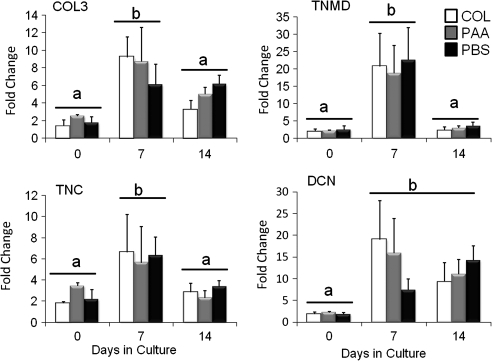
Gene expression data were not significantly different between either LDM group or constructs prepared using collagen gel alone. Expression of type III collagen, tenomodulin, and tenascin C was increased at day 7 of culture compared to days 0 and 14. Expression of decorin was elevated at both days 7 and 14 compared to day 0 (Fig. 6). Expression of biglycan did not change significantly over 14 days of culture, and expression of type I collagen was decreased at day 14 compared to days 0 and 7 (data not shown). Expression of type II and X collagen was identified at low levels in hASCs, and did not change with treatment or with time in culture (data not shown).
FIG. 6.
Fold change in gene expression of type III collagen (COL3), tenomodulin (TNMD), tenascin C (TNC), or decorin (DCN) for constructs prepared with PAA-LDM (PAA), PBS-LDM (PBS), or with collagen gel alone (COL), seeded with 1 × 106 hASCs/mL and cultured for 0, 7, or 14 days compared to expression of these genes in 1 × 106 P4 hASCs. Expression levels for all of these genes were increased at day 7 compared to day 0, and COL3, TNMD, and TNC levels were reduced to day 0 values by day 14. Data normalized to 18S house-keeping gene. Groups having different letters are significantly different (p ≤ 0.05; n = 3 biologic donors, mean ± SEM).
Discussion
The findings of this study show that the presence of native pulverized and lyophilized ligament powder induced ligamentous differentiation of ASCs by measures of cell proliferation, gene expression, protein synthesis, and immunofluorescence. Cell proliferation and protein synthesis were enhanced to a greater extent by the presence of LDM than by collagen gel alone. Although the mechanisms of induction of this differentiation remain to be determined, the results suggest that the presence of native ECM molecules can serve to promote a ligamentous phenotype in ASCs.
Gene expression data from this study suggest that hASCs cultured in collagen gel express several markers characteristic of a tendon or ligament phenotype with or without the addition of LDM. Although there is no single specific marker for a tendon or ligament phenotype, tenomodulin, tenascin C, biglycan, decorin, and type I and III collagen are all expressed in tendon and ligament.71–74 The increase in decorin expression with no change in biglycan expression seen in the current study is more consistent with a pattern of tendon or ligament development rather than repair.63,64 The reason for a decrease in type I collagen expression over the first 14 days in culture is unknown but may be related to the high initial collagen content of the ECM or the lack of any mechanical loading of these constructs. Similar decreases in collagen expression have been reported by other investigators for tenocytes seeded in collagen gel compared to tissue culture plastic, even in the presence of static loading.39 Treatment of cells with bFGF downregulates type I collagen expression, and treatment of human MSCs with BMP-14 causes an initial increase in expression, followed by downregulation of type I collagen expression.75,76 Flexor tendon tenocytes subjected to shear stress also demonstrated downregulation of type I collagen expression,77 as did bone marrow stem cells seeded on a composite of tendon slices.56
The presence of LDM significantly increased cell proliferation and matrix synthesis. Although the mechanism of this effect is unknown, the complexity of ECM and its importance in regulation of cell behavior through a variety of mechanisms is becoming increasingly recognized.78 The influence of ECM on cell behavior may be due to the release of soluble mediators from the tissue, or to direct cell–matrix interactions. Various components of tendon and ligament ECM, including thrombospondin-4,79 decorin,80 versican,81 tenascin,82 and fibrillin-1,83 also contain matrikines, peptides contained within ECM proteins that bind cell surface receptors, including those from the growth factor receptor family.84–86 In ASCs, EGF stimulates proliferation and migration.87 Both tenascin C and versican contain matrikines for EGF motifs, which bind directly to the EGF receptor; fibrillin-1 possesses 46 EGF-like repeats and similar matrikines in versican interact with the EGF receptor to promote chondrocyte proliferation and inhibit mesenchymal chondrogenesis.86,88–90
ECM components of tendon and ligament can also regulate growth factor activity directly. Heparan sulfate is a minor ECM proteoglycan in adult ovine posterior cruciate ligament and patellar tendon, but constitutes 6%–8% of the total proteoglycan content of avian embryonic tendon.91,92 Heparan sulfate binds to bFGF, an interaction that is required for fibroblast growth and differentiation,93,94 supporting the idea that heparan sulfate may be an important proteoglycan in developing tendon. bFGF also stimulates proliferation and the ability to differentiate in adipose stem cells.95–97 Biglycan and decorin both inhibit the activity of TGF-β, even though TGF-β1 stimulates synthesis of biglycan and decorin.98,99 In ASCs, TGF-β inhibits adipogenesis,100 and is key in the regulation of proliferation and differentiation, particularly in chondrogenesis.101 TGF-β also stimulates the synthesis of several other components of tendon and ligament, including collagen and fibronectin.102 Biglycan and fibromodulin both interact with BMP-2 signaling pathways to suppress ectopic ossification in tendon.4 The ECM is an abundant source of motifs for binding of cell surface integrins that provide a means of connecting the ECM to the cell cytoskeleton a wide variety of signal transduction events.103 In the ECM, integrins bind three major groups of ECM ligand, laminin, collagen, and arginine–glycine–aspartate (RGD)-containing motifs. Some integrin subunit combinations in cells exhibit highly specific ligand binding to specific components of the ECM to regulate critical functions in ECM organization. For example, the αVβ3, α5β1, and αVβ6 integrins regulate microfibril assembly by binding RGD peptides in fibrillin-1, a glycoprotein present in tendon.104 Whether the presence of fibrillin-1 and other glycoproteins specific to tendon or ligament ECM is required for successful microfibril assembly in tissue-engineered tendon or ligament is unknown. However, mutations in the fibrillin-1 gene in the region of the RGD ligands and matrikines downregulate collagen type I, III, and V expression in the ECM, and may be responsible for increased matrix metalloprotease activity seen in Marfan syndrome, a disorder characterized by hypermobility of distal joints.105–107 Alternatively, the presence of LDM may change the physical properties of the collagen gel that reduce cell-mediated contraction and provide increased surface area for matrix deposition and cell adhesion.
The method of preparation influenced the baseline characteristics of the unseeded LDM constructs. Unseeded PAA-LDM constructs contained similar amounts of dsDNA and collagen but more s-GAG within the construct and released more DNA, s-GAG, and collagen into the medium in the first 2–4 days of culture than unseeded PBS-LDM constructs. The size of PAA-LDM particles was significantly greater than PBS-LDM; thus, for equal amounts of powder, the larger particle size of powder prepared using PAA would be expected to have a smaller total surface area and therefore have increased retention of LDM components compared to powder prepared using PBS rather than the increased release that was seen in this study. The reason for the difference between powder preparation techniques is unknown, but PAA modifies protein structure through oxidation, and may have rendered s-GAG, collagen, or collagen fragments more susceptible to release from the scaffold.108 Decellularization of biologic scaffolds is essential to prevent adverse immune reactions caused by xenogenic or allogenic cellular antigenic epitopes, and disinfection or sterilization is required to remove microbial contamination.65,109 PAA disinfects via oxidation and is sporicidal, fungicidal, antiviral, and antibacterial, essential properties for any agent used to process any biological material for in vivo use.109 PAA (0.1%–0.15%) is commonly used to decellularize biologic scaffolds, though it does not completely remove all DNA present.110,111 In this study, there was no benefit to the use of 0.1% PAA for decellularization purposes when compared to immersion in liquid nitrogen and mechanical pulverization followed by resuspension in PBS. Further, even though PAA-LDM constructs released more dsDNA into the culture medium at day 2 after seeding for all seeding densities compared to PBS-LDM constructs, these results could not distinguish between greater loss of residual native porcine dsDNA from the PAA-LDM scaffolds and loss of human dsDNA from cell death associated with cell seeding onto the PAA-LDM scaffolds. It is therefore possible that PBS-LDM constructs resulted in less cell death after seeding than PAA-LDM constructs. Further studies are necessary to determine the optimal method of decellularization for preparation of LDM.
Increased seeding density resulted in incremental increases in DNA content of the scaffolds over 28 days in culture, consistent with increasing cell number, but there was no difference between treatment with PAA-LDM or PBS-LDM. There was no effect of seeding density on collagen content of the constructs; however, PAA-LDM constructs contained more collagen than PBS-LDM constructs. The only effect of LDM treatment on s-GAG content of the scaffolds was seen at a seeding density of 1 × 106 hASCs/mL when PAA-LDM contained more s-GAG over 28 days in culture than PBS-LDM or other seeding densities for PAA-LDM constructs. In both PAA- and PBS-LDM constructs seeded with 0.25 × 106 hASCs/mL there was increased release of s-GAG into the medium at days 2–4 followed by a decrease in s-GAG content at days 7 and 14 of culture compared to day 0, suggesting that cells seeded at this density were less able to retain s-GAG in the constructs. In comparison, a seeding density of 1 × 106 or 2 × 106 hASC/mL maintained s-GAG content in the constructs through 28 days in culture compared to unseeded constructs, and release of s-GAG into the medium through days 10–20 of culture was greater for the 1 × 106 or 2 × 106 hASC/mL seeding density than for other seeding densities, suggesting that synthesis was increased in these groups, but that the additional s-GAG produced was not retained in the construct. Collagen release into the medium was greatest at the 2 × 106 hASCs/mL seeding density even though no additional collagen was retained in the constructs, suggesting little if any additional benefit of seeding with 2 × 106 hASC/mL compared to 1 × 106 hASC/mL. The increased s-GAG content of PAA-LDM after construct preparation and s-GAG synthesis at some seeding densities, combined with the increased collagen content of constructs containing PAA-LDM, suggests that 0.1% PAA may the preferred method of preparing LDM, but there is no apparent benefit to the use of 0.1% PAA for decellularization of the LDM. Therefore, future studies may wish to evaluate alternative methods of decellularization and the effect of preparation method on cytotoxicity and on retention of matrix within the LDM. Of clinical relevance, the particulate nature of the LDM prepared in this study also lends itself with minimal further modification to resuspension and intralesional delivery by percutaneous injection directly into a focal tendon or ligament lesion in a tendon or ligament, such as occurs in Achilles tendon injury.65,112
In summary, our findings support the hypothesis that a scaffold derived from native ligament ECM can influence the growth and differentiation of ASCs. The beneficial effects of LDM on hASC proliferation and matrix synthesis seen in this study may be due to a combination of matrikines, growth factors, or enhanced presentation of ligands from the LDM, as well as the provision of increased surface area for cell attachment. However, 0.1% PAA was not clearly better than PBS for preparation of LDM; therefore, further research is necessary to determine the ideal method of preparation. LDM may provide a novel approach to enhancing tendon and ligament engineering by accelerating ECM synthesis in the absence of exogenous growth factors.
Supplementary Material
Footnotes
This study has been presented as an abstract titled “Effect of Native Ligament Extracellular Matrix on Human Adipose Stem Cells” in the International Tendon and Ligament Symposium IX, Las Vegas, Nevada, February 21, 2009.
Acknowledgments
This work was funded by an unrestricted fellowship grant from Synthes and NIH Grant AR48852. The authors acknowledge the technical assistance of Adrienne Blount and Jared Beller.
Disclosure Statement
No competing financial interests exist.
References
- 1.Functional Tissue Engineering Conference Group. Evaluation criteria for musculoskeletal and craniofacial tissue engineering constructs: a conference report. Tissue Eng Part A. 2008;14:2089. doi: 10.1089/ten.tea.2007.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheffler S.U. Unterhauser F.N. Weiler A. Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16:834. doi: 10.1007/s00167-008-0560-8. [DOI] [PubMed] [Google Scholar]
- 3.Butler D.L. Goldstein S.A. Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 4.Bi Y. Ehirchiou D. Kilts T.M. Inkson C.A. Embree M.C. Sonoyama W. Li L. Leet A.I. Seo B.M. Zhang L. Shi S. Young M.F. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 5.Kryger G.S. Chong A.K. Costa M. Pham H. Bates S.J. Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg [Am] 2007;32:597. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Marui T. Niyibizi C. Georgescu H.I. Cao M. Kavalkovich K.W. Levine R.E. Woo S.L. Effect of growth factors on matrix synthesis by ligament fibroblasts. J Orthop Res. 1997;15:18. doi: 10.1002/jor.1100150104. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt C.C. Georgescu H.I. Kwoh C.K. Blomstrom G.L. Engle C.P. Larkin L.A. Evans C.H. Woo S.L. Effect of growth factors on the proliferation of fibroblasts from the medial collateral and anterior cruciate ligaments. J Orthop Res. 1995;13:184. doi: 10.1002/jor.1100130206. [DOI] [PubMed] [Google Scholar]
- 8.Abreu E.L. Leigh D. Derwin K.A. Effect of altered mechanical load conditions on the structure and function of cultured tendon fascicles. J Orthop Res. 2008;26:364. doi: 10.1002/jor.20520. [DOI] [PubMed] [Google Scholar]
- 9.Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. Int Orthop. 2007;31:783. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forslund C. Aspenberg P. CDMP-2 induces bone or tendon-like tissue depending on mechanical stimulation. J Orthop Res. 2002;20:1170. doi: 10.1016/S0736-0266(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 11.Forslund C. Rueger D. Aspenberg P. A comparative dose-response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J Orthop Res. 2003;21:617. doi: 10.1016/S0736-0266(03)00010-X. [DOI] [PubMed] [Google Scholar]
- 12.Meaney Murray M. Rice K. Wright R.J. Spector M. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res. 2003;21:238. doi: 10.1016/S0736-0266(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 13.Moreau J. Chen J. Kaplan D. Altman G. Sequential growth factor stimulation of bone marrow stromal cells in extended culture. Tissue Eng. 2006;12:2905. doi: 10.1089/ten.2006.12.2905. [DOI] [PubMed] [Google Scholar]
- 14.Moreau J.E. Chen J. Bramono D.S. Volloch V. Chernoff H. Vunjak-Novakovic G. Richmond J.C. Kaplan D.L. Altman G.H. Growth factor induced fibroblast differentiation from human bone marrow stromal cells in vitro. J Orthop Res. 2005;23:164. doi: 10.1016/j.orthres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Moreau J.E. Chen J. Horan R.L. Kaplan D.L. Altman G.H. Sequential growth factor application in bone marrow stromal cell ligament engineering. Tissue Eng. 2005;11:1887. doi: 10.1089/ten.2005.11.1887. [DOI] [PubMed] [Google Scholar]
- 16.Wong Y.P. Fu S.C. Cheuk Y.C. Lee K.M. Wong M.W. Chan K.M. Bone morphogenetic protein 13 stimulates cell proliferation and production of collagen in human patellar tendon fibroblasts. Acta Orthop. 2005;76:421. [PubMed] [Google Scholar]
- 17.Zhang F. Liu H. Stile F. Lei M.P. Pang Y. Oswald T.M. Beck J. Dorsett-Martin W. Lineaweaver W.C. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg. 2003;112:1613. doi: 10.1097/01.PRS.0000086772.72535.A4. [DOI] [PubMed] [Google Scholar]
- 18.Forslund C. Aspenberg P. Improved healing of transected rabbit Achilles tendon after a single injection of cartilage-derived morphogenetic protein-2. Am J Sports Med. 2003;31:555. doi: 10.1177/03635465030310041301. [DOI] [PubMed] [Google Scholar]
- 19.Altman G.H. Horan R.L. Weitzel P. Richmond J.C. The use of long-term bioresorbable scaffolds for anterior cruciate ligament repair. J Am Acad Orthop Surg. 2008;16:177. doi: 10.5435/00124635-200804000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Ayres C.E. Bowlin G.L. Pizinger R. Taylor L.T. Keen C.A. Simpson D.G. Incremental changes in anisotropy induce incremental changes in the material properties of electrospun scaffolds. Acta Biomater. 2007;3:651. doi: 10.1016/j.actbio.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper J.A., Jr. Sahota J.S. Gorum W.J., 2nd Carter J. Doty S.B. Laurencin C.T. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci USA. 2007;104:3049. doi: 10.1073/pnas.0608837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bashur C.A. Shaffer R.D. Dahlgren L.A. Guelcher S.A. Goldstein A.S. Effect of fiber diameter and alignment of electrospun polyurethane meshes on mesenchymal progenitor cells. Tissue Eng Part A. 2009;15:2435. doi: 10.1089/ten.tea.2008.0295. [DOI] [PubMed] [Google Scholar]
- 23.Boland E.D. Matthews J.A. Pawlowski K.J. Simpson D.G. Wnek G.E. Bowlin G.L. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front Biosci. 2004;9:1422. doi: 10.2741/1313. [DOI] [PubMed] [Google Scholar]
- 24.Kumbar S.G. James R. Nukavarapu S.P. Laurencin C.T. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3:034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 25.Laurencin C.T. Freeman J.W. Ligament tissue engineering: an evolutionary materials science approach. Biomaterials. 2005;26:7530. doi: 10.1016/j.biomaterials.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 26.Li W.J. Mauck R.L. Cooper J.A. Yuan X. Tuan R.S. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40:1686. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H.H. Cooper J.A., Jr. Manuel S. Freeman J.W. Attawia M.A. Ko F.K. Laurencin C.T. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 28.Mauck R.L. Baker B.M. Nerurkar N.L. Burdick J.A. Li W.J. Tuan R.S. Elliott D.M. Engineering on the straight and narrow: the mechanics of nanofibrous assemblies for fiber-reinforced tissue regeneration. Tissue Eng Part B Rev. 2009 doi: 10.1089/ten.teb.2008.0652. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vunjak-Novakovic G. Altman G. Horan R. Kaplan D.L. Tissue engineering of ligaments. Annu Rev Biomed Eng. 2004;6:131. doi: 10.1146/annurev.bioeng.6.040803.140037. [DOI] [PubMed] [Google Scholar]
- 30.Awad H.A. Boivin G.P. Dressler M.R. Smith F.N. Young R.G. Butler D.L. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 31.Awad H.A. Butler D.L. Harris M.T. Ibrahim R.E. Wu Y. Young R.G. Kadiyala S. Boivin G.P. In vitro characterization of mesenchymal stem cell-seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J Biomed Mater Res. 2000;51:233. doi: 10.1002/(sici)1097-4636(200008)51:2<233::aid-jbm12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 32.Berry C.C. Shelton J.C. Lee D.A. Cell-generated forces influence the viability, metabolism and mechanical properties of fibroblast-seeded collagen gel constructs. J Tissue Eng Regen Med. 2009;3:43. doi: 10.1002/term.133. [DOI] [PubMed] [Google Scholar]
- 33.Butler D.L. Juncosa-Melvin N. Boivin G.P. Galloway M.T. Shearn J.T. Gooch C. Awad H. Functional tissue engineering for tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 34.Gentleman E. Lay A.N. Dickerson D.A. Nauman E.A. Livesay G.A. Dee K.C. Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials. 2003;24:3805. doi: 10.1016/s0142-9612(03)00206-0. [DOI] [PubMed] [Google Scholar]
- 35.Huang D. Chang T.R. Aggarwal A. Lee R.C. Ehrlich H.P. Mechanisms and dynamics of mechanical strengthening in ligament-equivalent fibroblast-populated collagen matrices. Ann Biomed Eng. 1993;21:289. doi: 10.1007/BF02368184. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson M. Fufa D. Abreu E.L. Kevy S. Murray M.M. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regen. 2008;16:370. doi: 10.1111/j.1524-475X.2008.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juncosa-Melvin N. Boivin G.P. Galloway M.T. Gooch C. West J.R. Butler D.L. Effects of cell-to-collagen ratio in stem cell-seeded constructs for Achilles tendon repair. Tissue Eng. 2006;12:681. doi: 10.1089/ten.2006.12.681. [DOI] [PubMed] [Google Scholar]
- 38.Kuo C.K. Tuan R.S. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 39.Lamberti P.M. Wezeman F.H. Biologic behavior of an in vitro hydrated collagen gel-human tenocyte tendon model. Clin Orthop Relat Res. 2002;397:414. doi: 10.1097/00003086-200204000-00049. [DOI] [PubMed] [Google Scholar]
- 40.Murray M.M. Martin S.D. Spector M. Migration of cells from human anterior cruciate ligament explants into collagen-glycosaminoglycan scaffolds. J Orthop Res. 2000;18:557. doi: 10.1002/jor.1100180407. [DOI] [PubMed] [Google Scholar]
- 41.Noth U. Schupp K. Heymer A. Kall S. Jakob F. Schutze N. Baumann B. Barthel T. Eulert J. Hendrich C. Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy. 2005;7:447. doi: 10.1080/14653240500319093. [DOI] [PubMed] [Google Scholar]
- 42.Palmer M.P. Abreu E.L. Mastrangelo A. Murray M.M. Injection temperature significantly affects in vitro and in vivo performance of collagen-platelet scaffolds. J Orthop Res. 2009;27:964. doi: 10.1002/jor.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Covey D.C. Albright J.A. Clinical induction of bone repair with demineralized bone matrix or a bone morphogenetic protein. Orthop Rev. 1989;18:857. [PubMed] [Google Scholar]
- 44.Urist M.R. Bone: formation by autoinduction. Science. 1965;150:893. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 45.Cheng N.C. Estes B.T. Awad H.A. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2008;15:231. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Q. Peng J. Guo Q. Huang J. Zhang L. Yao J. Yang F. Wang S. Xu W. Wang A. Lu S. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 47.Adams J.E. Zobitz M.E. Reach J.S., Jr. An K.N. Steinmann S.P. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Almarza A.J. Yang G. Woo S.L. Nguyen T. Abramowitch S.D. Positive changes in bone marrow-derived cells in response to culture on an aligned bioscaffold. Tissue Eng Part A. 2008;14:1489. doi: 10.1089/ten.tea.2007.0422. [DOI] [PubMed] [Google Scholar]
- 49.Badylak S. Arnoczky S. Plouhar P. Haut R. Mendenhall V. Clarke R. Horvath C. Naturally occurring extracellular matrix as a scaffold for musculoskeletal repair. Clin Orthop Relat Res. 1999;367:S333. doi: 10.1097/00003086-199910001-00032. [DOI] [PubMed] [Google Scholar]
- 50.Derwin K.A. Baker A.R. Spragg R.K. Leigh D.R. Farhat W. Iannotti J.P. Regional variability, processing methods, and biophysical properties of human fascia lata extracellular matrix. J Biomed Mater Res A. 2008;84:500. doi: 10.1002/jbm.a.31455. [DOI] [PubMed] [Google Scholar]
- 51.Gilbert T.W. Stewart-Akers A.M. Simmons-Byrd A. Badylak S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 52.Karaoglu S. Fisher M.B. Woo S.L. Fu Y.C. Liang R. Abramowitch S.D. Use of a bioscaffold to improve healing of a patellar tendon defect after graft harvest for ACL reconstruction: a study in rabbits. J Orthop Res. 2008;26:255. doi: 10.1002/jor.20471. [DOI] [PubMed] [Google Scholar]
- 53.Liang R. Woo S.L. Takakura Y. Moon D.K. Jia F. Abramowitch S.D. Long-term effects of porcine small intestine submucosa on the healing of medial collateral ligament: a functional tissue engineering study. J Orthop Res. 2006;24:811. doi: 10.1002/jor.20080. [DOI] [PubMed] [Google Scholar]
- 54.Musahl V. Abramowitch S.D. Gilbert T.W. Tsuda E. Wang J.H. Badylak S.F. Woo S.L. The use of porcine small intestinal submucosa to enhance the healing of the medial collateral ligament—a functional tissue engineering study in rabbits. J Orthop Res. 2004;22:214. doi: 10.1016/S0736-0266(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 55.Zantop T. Gilbert T.W. Yoder M.C. Badylak S.F. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. J Orthop Res. 2006;24:1299. doi: 10.1002/jor.20071. [DOI] [PubMed] [Google Scholar]
- 56.Omae H. Zhao C. Sun Y.L. An K.N. Amadio P.C. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27:937. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blum B.E. Burgess A.V. Special segment: soft tissue matrices—one form of acellular human dermis for use in tendon and ligament repairs in the foot and ankle. Foot Ankle Spec. 2009;2:235. doi: 10.1177/1938640009347455. [DOI] [PubMed] [Google Scholar]
- 58.Androjna C. Gatica J.E. Belovich J.M. Derwin K.A. Oxygen diffusion through natural extracellular matrices: implications for estimating “critical thickness” values in tendon tissue engineering. Tissue Eng Part A. 2008;14:559. doi: 10.1089/tea.2006.0361. [DOI] [PubMed] [Google Scholar]
- 59.Barber F.A. Herbert M.A. Coons D.A. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22:534. doi: 10.1016/j.arthro.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Kummer F.J. Iesaka K. The role of graft materials in suture augmentation for tendon repairs and reattachment. J Biomed Mater Res B Appl Biomater. 2005;74:789. doi: 10.1002/jbm.b.30296. [DOI] [PubMed] [Google Scholar]
- 61.Cook J.L. Fox D.B. Kuroki K. Jayo M. De Deyne P.G. In vitro and in vivo comparison of five biomaterials used for orthopedic soft tissue augmentation. Am J Vet Res. 2008;69:148. doi: 10.2460/ajvr.69.1.148. [DOI] [PubMed] [Google Scholar]
- 62.Badhe S.P. Lawrence T.M. Smith F.D. Lunn P.G. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg. 2008;17:35S. doi: 10.1016/j.jse.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Zhang G. Ezura Y. Chervoneva I. Robinson P.S. Beason D.P. Carine E.T. Soslowsky L.J. Iozzo R.V. Birk D.E. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 64.Berglund M. Reno C. Hart D.A. Wiig M. Patterns of mRNA expression for matrix molecules and growth factors in flexor tendon injury: differences in the regulation between tendon and tendon sheath. J Hand Surg [Am] 2006;31:1279. doi: 10.1016/j.jhsa.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Badylak S.F. Freytes D.O. Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 66.Dubois S.G. Floyd E.Z. Zvonic S. Kilroy G. Wu X. Carling S. Halvorsen Y.D. Ravussin E. Gimble J.M. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol. 2008;449:69. doi: 10.1007/978-1-60327-169-1_5. [DOI] [PubMed] [Google Scholar]
- 67.Enobakhare B.O. Bader D.L. Lee D.A. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243:189. doi: 10.1006/abio.1996.0502. [DOI] [PubMed] [Google Scholar]
- 68.Neidert M.R. Lee E.S. Oegema T.R. Tranquillo R.T. Enhanced fibrin remodeling in vitro with TGF-beta1, insulin and plasmin for improved tissue-equivalents. Biomaterials. 2002;23:3717. doi: 10.1016/s0142-9612(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 69.Mehlhorn A.T. Niemeyer P. Kaiser S. Finkenzeller G. Stark G.B. Sudkamp N.P. Schmal H. Differential expression pattern of extracellular matrix molecules during chondrogenesis of mesenchymal stem cells from bone marrow and adipose tissue. Tissue Eng. 2006;12:2853. doi: 10.1089/ten.2006.12.2853. [DOI] [PubMed] [Google Scholar]
- 70.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banos C.C. Thomas A.H. Kuo C.K. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res C Embryo Today. 2008;84:228. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- 72.Riou J.F. Umbhauer M. Shi D.L. Boucaut J.C. Tenascin: a potential modulator of cell-extracellular matrix interactions during vertebrate embryogenesis. Biol Cell. 1992;75:1. doi: 10.1016/0248-4900(92)90118-k. [DOI] [PubMed] [Google Scholar]
- 73.Robinson P.S. Huang T.F. Kazam E. Iozzo R.V. Birk D.E. Soslowsky L.J. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127:181. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- 74.Shukunami C. Takimoto A. Oro M. Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 75.Koch H. Jadlowiec J.A. Fu F.H. Nonn J. Merk H.R. Hollinger J.O. Campbell P.G. [The effect of growth/differentiation factor-5 (GDF-5) on genotype and phenotype in human adult mesenchymal stem cells] Z Orthop Ihre Grenzgeb. 2004;142:248. doi: 10.1055/s-2004-822612. [DOI] [PubMed] [Google Scholar]
- 76.Palmon A. Roos H. Edel J. Zax B. Savion N. Grosskop A. Pitaru S. Inverse dose- and time-dependent effect of basic fibroblast growth factor on the gene expression of collagen type I and matrix metalloproteinase-1 by periodontal ligament cells in culture. J Periodontol. 2000;71:974. doi: 10.1902/jop.2000.71.6.974. [DOI] [PubMed] [Google Scholar]
- 77.Fong K.D. Trindade M.C. Wang Z. Nacamuli R.P. Pham H. Fang T.D. Song H.M. Smith R.L. Longaker M.T. Chang J. Microarray analysis of mechanical shear effects on flexor tendon cells. Plast Reconstr Surg. 2005;116:1393. doi: 10.1097/01.prs.0000182345.86453.4f. [DOI] [PubMed] [Google Scholar]
- 78.Huxley-Jones J. Pinney J.W. Archer J. Robertson D.L. Boot-Handford R.P. Back to basics—how the evolution of the extracellular matrix underpinned vertebrate evolution. Int J Exp Pathol. 2009;90:95. doi: 10.1111/j.1365-2613.2008.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hauser N. Paulsson M. Kale A.A. DiCesare P.E. Tendon extracellular matrix contains pentameric thrombospondin-4 (TSP-4) FEBS Lett. 1995;368:307. doi: 10.1016/0014-5793(95)00675-y. [DOI] [PubMed] [Google Scholar]
- 80.Pringle G.A. Dodd C.M. Immunoelectron microscopic localization of the core protein of decorin near the d and e bands of tendon collagen fibrils by use of monoclonal antibodies. J Histochem Cytochem. 1990;38:1405. doi: 10.1177/38.10.1698203. [DOI] [PubMed] [Google Scholar]
- 81.Robbins J.R. Vogel K.G. Regional expression of mRNA for proteoglycans and collagen in tendon. Eur J Cell Biol. 1994;64:264. [PubMed] [Google Scholar]
- 82.Hurle J.M. Hinchliffe J.R. Ros M.A. Critchlow M.A. Genis-Galvez J.M. The extracellular matrix architecture relating to myotendinous pattern formation in the distal part of the developing chick limb: an ultrastructural, histochemical and immunocytochemical analysis. Cell Differ Dev. 1989;27:103. doi: 10.1016/0922-3371(89)90740-5. [DOI] [PubMed] [Google Scholar]
- 83.Ritty T.M. Ditsios K. Starcher B.C. Distribution of the elastic fiber and associated proteins in flexor tendon reflects function. Anat Rec. 2002;268:430. doi: 10.1002/ar.10175. [DOI] [PubMed] [Google Scholar]
- 84.Maquart F.X. Pasco S. Ramont L. Hornebeck W. Monboisse J.C. An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit Rev Oncol Hematol. 2004;49:199. doi: 10.1016/j.critrevonc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 85.Schwarz R.I. Bissell M.J. Dependence of the differentiated state on the cellular environment: modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci USA. 1977;74:4453. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swindle C.S. Tran K.T. Johnson T.D. Banerjee P. Mayes A.M. Griffith L. Wells A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol. 2001;154:459. doi: 10.1083/jcb.200103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baer P.C. Schubert R. Bereiter-Hahn J. Ploser M. Geiger H. Expression of a functional epidermal growth factor receptor on human adipose-derived mesenchymal stem cells and its signaling mechanism. Eur J Cell Biol. 2009;88:273. doi: 10.1016/j.ejcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 88.Pereira L. D'Alessio M. Ramirez F. Lynch J.R. Sykes B. Pangilinan T. Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum Mol Genet. 1993;2:961. doi: 10.1093/hmg/2.7.961. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y. Cao L. Kiani C. Yang B.L. Hu W. Yang B.B. Promotion of chondrocyte proliferation by versican mediated by G1 domain and EGF-like motifs. J Cell Biochem. 1999;73:445. [PubMed] [Google Scholar]
- 90.Zhang Y. Cao L. Kiani C.G. Yang B.L. Yang B.B. The G3 domain of versican inhibits mesenchymal chondrogenesis via the epidermal growth factor-like motifs. J Biol Chem. 1998;273:33054. doi: 10.1074/jbc.273.49.33054. [DOI] [PubMed] [Google Scholar]
- 91.Gassler N. Tugtekin I. Decker B. Bosch U. Delbruck A. Changes in the extracellular matrix of the autogenous patellar tendon graft after posterior cruciate ligament reconstruction: a biochemical study in sheep. Matrix Biol. 1994;14:87. doi: 10.1016/0945-053x(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 92.Ninomiya Y. Nagai Y. Modulation of glycosaminoglycan synthesis during cell growth as observed in an embryonic chick tendon cell culture. J Biochem. 1979;86:111. [PubMed] [Google Scholar]
- 93.Rapraeger A.C. Krufka A. Olwin B.B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 94.Walker A. Turnbull J.E. Gallagher J.T. Specific heparan sulfate saccharides mediate the activity of basic fibroblast growth factor. J Biol Chem. 1994;269:931. [PubMed] [Google Scholar]
- 95.Chiou M. Xu Y. Longaker M.T. Mitogenic and chondrogenic effects of fibroblast growth factor-2 in adipose-derived mesenchymal cells. Biochem Biophys Res Commun. 2006;343:644. doi: 10.1016/j.bbrc.2006.02.171. [DOI] [PubMed] [Google Scholar]
- 96.Quarto N. Longaker M.T. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 2006;12:1405. doi: 10.1089/ten.2006.12.1405. [DOI] [PubMed] [Google Scholar]
- 97.Safford K.M. Hicok K.C. Safford S.D. Halvorsen Y.D. Wilkison W.O. Gimble J.M. Rice H.E. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294:371. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 98.Border W.A. Okuda S. Languino L.R. Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990;37:689. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- 99.Yamaguchi Y. Mann D.M. Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 100.Petruschke T. Rohrig K. Hauner H. Transforming growth factor beta (TGF-beta) inhibits the differentiation of human adipocyte precursor cells in primary culture. Int J Obes Relat Metab Disord. 1994;18:532. [PubMed] [Google Scholar]
- 101.Erickson G.R. Gimble J.M. Franklin D.M. Rice H.E. Awad H. Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 102.Ignotz R.A. Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337. [PubMed] [Google Scholar]
- 103.Takada Y. Ye X. Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsuruga E. Sato A. Ueki T. Nakashima K. Nakatomi Y. Ishikawa H. Yajima T. Sawa Y. Integrin alphavbeta3 regulates microfibril assembly in human periodontal ligament cells. Tissue Cell. 2009;41:85. doi: 10.1016/j.tice.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 105.Booms P. Pregla R. Ney A. Barthel F. Reinhardt D.P. Pletschacher A. Mundlos S. Robinson P.N. RGD-containing fibrillin-1 fragments upregulate matrix metalloproteinase expression in cell culture: a potential factor in the pathogenesis of the Marfan syndrome. Hum Genet. 2005;116:51. doi: 10.1007/s00439-004-1194-7. [DOI] [PubMed] [Google Scholar]
- 106.Christner P.J. Ayitey S. Extracellular matrix containing mutated fibrillin-1 (Fbn1) down regulates Col1a1, Col1a2, Col3a1, Col5a1, and Col5a2 mRNA levels in Tsk/+and Tsk/Tsk embryonic fibroblasts. Amino Acids. 2006;30:445. doi: 10.1007/s00726-005-0265-y. [DOI] [PubMed] [Google Scholar]
- 107.Voermans N.C. Bonnemann C.G. Hamel B.C. Jungbluth H. van Engelen B.G. Joint hypermobility as a distinctive feature in the differential diagnosis of myopathies. J Neurol. 2009;256:13. doi: 10.1007/s00415-009-0105-1. [DOI] [PubMed] [Google Scholar]
- 108.Hodde J. Janis A. Ernst D. Zopf D. Sherman D. Johnson C. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med. 2007;18:537. doi: 10.1007/s10856-007-2300-x. [DOI] [PubMed] [Google Scholar]
- 109.Lomas R.J. Huang Q. Pegg D.E. Kearney J.N. Application of a high-level peracetic acid disinfection protocol to re-process antibiotic disinfected skin allografts. Cell Tissue Bank. 2004;5:23. doi: 10.1023/b:catb.0000022236.14311.b9. [DOI] [PubMed] [Google Scholar]
- 110.Aurora A. McCarron J. Iannotti J.P. Derwin K. Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg. 2007;16:S171. doi: 10.1016/j.jse.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 111.Gilbert T.W. Sellaro T.L. Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 112.Schepsis A.A. Jones H. Haas A.L. Achilles tendon disorders in athletes. Am J Sports Med. 2002;30:287. doi: 10.1177/03635465020300022501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.