Abstract
Biophysical stimuli are important to the development and maintenance of cancellous bone, but the regulatory mechanisms need to be understood. We investigated the effects of mechanical loading applied in vivo to native cancellous bone in the rabbit on bone formation and trabecular realignment. A novel device was developed to apply controlled compressive loads to cancellous bone in situ. The effect of loading on cancellous bone volume fraction and architecture was quantified. A 4 week experiment was performed in rabbits with devices implanted bilaterally. Cyclic 1 MPa pressures were applied daily to the right limb for 10, 25, or 50 cycles at 0.5 Hz, and the left limb served as the control without any applied loading. Microcomputed tomography and histomorphometry were used to characterize the cancellous tissue within a 4-mm spherical volume located below the loading core. In vivo cyclic loading significantly increased the bone volume fraction, direct trabecular thickness, mean intercept length, and mineral apposition rate in the loaded limbs compared with contralateral limbs. Insufficient evidence was found to demonstrate an effect of number of cycles on the cancellous adaptation between loaded and control limbs. Using a rabbit model, we demonstrated that mechanical loading applied to cancellous bone in situ increased bone formation and altered trabecular morphology. This in vivo model will allow further investigation of cancellous functional adaptation to controlled mechanical stimuli and the influence of mechanical loading parameters, metabolic status, and therapeutic agents.
Keywords: cancellous bone, bone adaptation, rabbit, microcomputed tomography, implant
Introduction
Mechanical loading plays an important role in the growth, development, and repair of bone tissue, but the precise mechanisms of bone adaptation to mechanical inputs are not well understood. Understanding the role that mechanical load plays in cancellous bone adaptation will aid in treating and preventing osteoporotic bone loss and in enhancing the long-term fixation of total joint arthroplasties. Therefore, studies are necessary in which the applied loading and the ensuing tissue response are both well characterized. Adaptation of the cortical diaphysis has been studied through a variety of animal models [16, 28, 32, 33] in which controlled mechanical forces are applied to the site of interest. These models have been used to examine both the mechanical stimulus and the adaptive outcome. Mechanical load parameters that influence cortical adaptation include strain magnitude, loading rate, number of cycles, and loading sessions [22, 23, 25]. High strain rates and magnitudes are particularly important [7, 22, 23]. The number of cycles is also important but only up to a threshold beyond which temporally distinct loading bouts rather than a single load of the same cumulative cycles becomes important [25]. Sex and genetic background also influence the adaptive response [1, 24, 27].
In vivo models for understanding cancellous bone adaptation are less well developed than models of cortical adaptation, possibly because loads to cancellous sites such as the metaphysis are more difficult to apply and control than loads to the cortical diaphysis [4, 9, 11]. These models often require surgical intervention to expose cancellous bone surfaces, which parallels the implantation of total joint components but may not be representative of native tissue adaptation [11, 18]. Models that do not require surgical intervention, such as the rat tail vertebra and mouse tibia loading models, load both cortical and cancellous bone [4, 6, 9]. Despite increased bone formation rates, increases in cancellous bone volume fraction were not observed following loading of rat tail vertebrae [19]. However, loading increased cancellous bone volume fraction 15% and trabecular thickness 12% in the proximal metaphysis of the mouse [9].
Isolated cancellous bone formation has been examined using tissue differentiation chambers [12, 13, 20, 31]. The type of mechanical stimuli influences whether fibrous, chondrous, or bony tissue forms and modulates the tissue architecture when bone forms. However, isolated formation de novo may not represent the adaptive response of mature cancellous bone tissue in situ. Our interest in implant fixation and adaptation of cancellous bone in vivo led us to develop a loading model to stimulate the tissue in situ in the rabbit. A rabbit model was selected for two reasons: the presence of Haversian remodeling and the substantial volume of cancellous tissue available for analysis. Prior adaptation and tissue differentiation studies have been performed in cortical bone and in tissue chambers using rabbits [2, 12, 15, 16].
We hypothesized that bone would form with cyclic loading applied directly to cancellous bone in the rabbit, and that this new bone tissue would be aligned with the loading direction. Our objectives were: (1) to develop a novel device to load the cancellous bone of the distal lateral femoral condyle in the rabbit in vivo, (2) to determine the effects of controlled loading on cancellous bone volume fraction and architecture, and (3) to investigate the role of the number of loading cycles on this response.
Materials and Methods
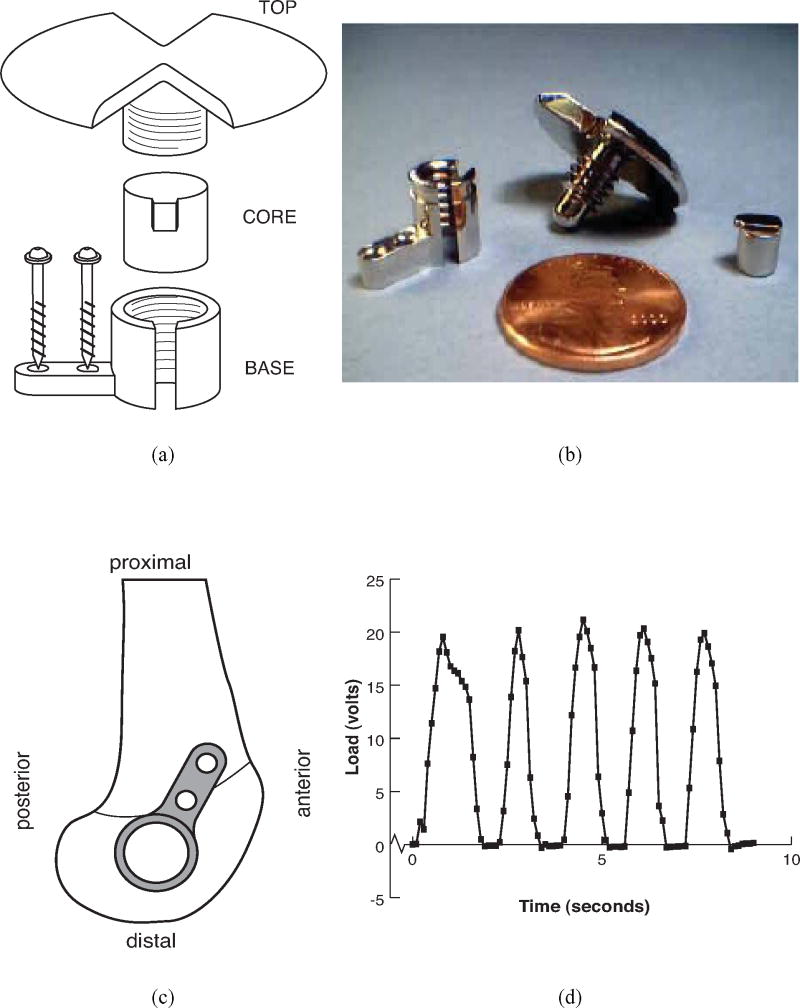
An implantable loading device for rabbits was developed to apply controlled compressive loads to cancellous bone in vivo. The design objectives were to load native cancellous bone, to allow control and characterization of the applied mechanical stimulus, and to accomplish the loading objectives while maintaining animal welfare and causing minimal discomfort. The device consisted of a stationary base mounted on the lateral femoral condyle with two bicortical screws, a movable loading core, and a top (Fig. 1). The loading core inserted into the cylindrical portion of the base; the top threaded into the base above the core. Twisting the top translated the core and compressed the underlying bone. The loading core was keyed to a slot in the base to prevent rotation of the core and to avoid transmitting shear to the underlying bone tissue. All parts were fabricated from 316L stainless steel, and the surface of the core was highly polished to discourage bone ingrowth.
Figure 1.
(a) Schematic of loading device to apply compression to native cancellous bone of the distal femoral condyle of the rabbit, (b) photograph of disassembled loading device, (c) lateral view of distal femur illustrating positioning of device, and (d) sample loading curve from instrumented device.
Each device was calibrated with an instrumented torque wrench in the laboratory. A fixture was designed to mount the device on a compressive load cell. Known torques were applied to the top with the wrench, and the compressive force under the loading core was measured for five load cycles. Sets of cores and bases were paired, calibrated in vitro, and implanted together to reduce variability of the loading. Loads were also monitored in vivo in a subset of animals by implanting instrumented loading cores, which were developed using a beam element to transmit and measure the forces applied to the core. Two miniature uniaxial strain gages (Micromeasurements EA 13-031CF-120/P; Vishay Measurements Group, Raleigh NC) were mounted on each side of the beam. The strain gages were powered by signal conditioners using a half-bridge configuration (DBK 43, IOtech, Cleveland OH). For calibration prior to implantation, load and voltage across the half bridge of the instrumented cores were recorded while a 75N sinusoidal compressive load was applied at 0.5 Hz for 10 cycles. The calibration coefficient relating voltage to load was averaged for the 10 cycles.
Surgery was performed to insert the loading devices bilaterally into the lateral condyle of the distal femora of skeletally mature male New Zealand White rabbits (8-9 months old). Each animal served as its own control with the right side subjected to compressive loads while the left side had the device surgically implanted without any subsequent loading. A standard posterior lateral surgical approach was used to expose the condyle. The base plate was secured to the cortex via two AO 1.5 mm cortical screws, after first pre-drilling using a 1.0 mm drill bit. Once the plate was securely attached, the bone was milled with a custom routing device (4.5 mm diam) to a depth of 1 mm, using constant irrigation to minimize heat necrosis. Removing the cortical bone in this manner allowed loads to be applied by the core directly to the underlying cancellous bone. The core was then inserted into the base and secured with the top piece. Bone wax was applied to the screw thread to prevent heterotopic bone formation into the threads. The wounds were thoroughly irrigated, and the skin closed in layers with resorbable chromic sutures followed by interrupted surgical steel sutures. The external wounds were treated with a local antiseptic solution.
The implantation of bilateral loading chambers required 60 minutes of surgical anesthesia (induced with a cocktail of 0.05 mg/kg atrophine sulfate, 35 mg/kg ketamine hydrochloride, and 0.5 mg/kg acetylpromazine injected intramuscularly and maintained by isoflurane inhalation). All procedures were performed aseptically, and antibiotic prophylaxis was also administered (25 mg/kg ampicillin). Analgesia was administered postoperatively (0.05 mg/kg buprenorphine). The procedure was approved and monitored by the Animal Care and Use Committee at the Hospital for Special Surgery.
An experiment was performed to examine the effect of loading cancellous bone with this device on bone formation and architecture and also to examine the effect of varying the number of applied loading cycles. A load corresponding to a peak pressure of 1 MPa was applied for different numbers of cycles to the right limb. This load was selected to correspond to the lower end of the 18-80N range previously applied to cancellous tissue in vivo [11, 18]. Three groups of animals (n=6/group) were loaded 10, 25, or 50 cycles/day at approximately 0.5 Hz for four weeks. The number of daily loading cycles was based on studies of diaphyseal cortical bone demonstrating that a low number of cycles is sufficient to elicit a response [25, 26, 28].
Loading was initiated the first day postoperatively. Loads were applied by twisting the device top manually while the rabbits were awake. All loading was performed by a single operator. The operator was trained during a pilot in vivo experiment in rabbits using loading cores instrumented with strain gages, allowing the loading waveform to be quantified and recorded (Figure 1). To confirm the operator's technique instrumented cores were loaded with and without visual feedback. A subset of rabbits received instrumented loading cores (described above). Repeatability of the loading was determined from the output data of the first, middle, and last loading cycle for each rabbit at the time of implantation. The mean coefficient of variation in load amplitude was 19.0% for 13 rabbits (95% confidence interval: 10-28%).
To assess mineral apposition rates, 0.1% calcein (15 mg/kg body mass; C0875, Sigma-Aldrich, St. Louis, MO) was given to all animals intravenously at 14 and 3 days prior to the end of the experiment. After the animals were killed, the tissues were fixed in 70% alcohol. Fourteen rabbits completed the four-week experimental period. Four animals developed infections and were removed from the study.
To assess right-left differences and to characterize the normal variability in cancellous bone mass in the rabbit distal femur, devices were also implanted in five pairs of explanted rabbit femora. Identical surgical procedures were followed as in the in vivo loading experiment. The specimens were removed, scanned by microCT, and analyzed using the methodology described below. These bones corresponded to the initial state of the control limbs. The data were not analyzed with the primary study as these samples were collected at a later time and experimental conditions were not controlled.
To confirm the response to loading found in cancellous bone with 50 cycles/day, an additional in vivo experiment was performed with 8 similar age and size rabbits. Rabbits were loaded daily with 1 MPa for 50 cycles per day for 4 weeks with the identical protocol as described above. Upon completion of the experiment, the tissues were fixed and analyzed in the same fashion as the larger study. The data were not pooled with the primary study as a single level of cycle number was examined in this follow up experiment.
Microcomputed tomography (microCT) scans of left and right lateral femoral condyles were taken at 19 μm isotropic resolution (Skyscan Model 1072, Antwerp, Belgium). Images were reconstructed and thresholded to distinguish bone voxels. A global threshold was chosen for all specimens. The sensitivity of the results to different thresholds was examined and did not influence left-right differences. Bone volume fraction (BV/TV) was assessed to determine the total amount of bone tissue present directly below the loading device for a 4-mm diameter spherical volume of interest (VOI). The top of the sphere was located at the center of the surface loaded by the device. BV/TV was calculated as the number of bone voxels divided by the total number of voxels within the VOI. For this spherical VOI, two measures of trabecular architecture were calculated: direct trabecular thickness (Tb.Th, μm) and spacing (Tb.Sp, μm). These direct trabecular measures are model independent and are more accurate measures of three-dimensional (3D) architecture than the parallel-plate based indices [17, 21]. The direct thickness was calculated throughout the VOI as the volume weighted average diameter of spheres fit to the trabecular tissue, and the direct spacing was the volume weighted average diameter of spheres fit within the marrow space [17].
The orientation of the trabecular architecture was characterized by mean intercept length (MIL), a measure of the average length of test lines through trabeculae for given orientations. The MIL is a three-dimensional measure with principal MILs corresponding to the primary trabecular orientations. MIL was measured by rotating a test grid of parallel lines in 3D through the spherical VOI for 200 randomly oriented rotations. Test grid spacing was 0.135 mm. Simmons and Hipp have shown that 200 rotations and a test grid spacing of less than or equal to 0.2 mm are adequate for calculating MIL [29]. The anisotropy tensor was then determined by least squares fitting of the MIL data to the equation of an ellipsoid [14, 36, 37]. Eigenanalysis of the anisotropy tensor provided principal structural directions and magnitudes. The principal MILs were calculated as the inverse square root of the eigenvalues and principal orientations were obtained directly from the eigenvectors. Degree of anisotropy (DA) was then calculated as the ratio of the maximum to minimum principal MILs.
Upon completion of the microCT scans, all specimens were dehydrated in graded ethanol, defatted in toluene, embedded in methylmethacrylate (03629-4, Fisher Scientific, Pittsburgh, PA), and sectioned by microtome (D-6907, Jung Polycut E, Nussloch, Germany). Three 8-μm-thick cross sections perpendicular to the loaded surface were obtained from the center of the loaded area. Sections were analyzed for percent labeled area and mineral apposition rate (MAR) using a commercial histomorphometric system (Bioquant NOVA, Nashville, TN).
MAR was analyzed for three sections in a central 4-mm × 4-mm region of interest (ROI) immediately below the loaded surface. On each section three pairs of double labels were measured for calculating the MAR (μm/day). Only pairs with two parallel labels were analyzed to assure formation was examined for the same surface. The distances between labels were measured at three different locations for each pair. The “double label distance” of a single specimen was the average across the three sections, which in turn was the average of the three measurements from each of three pairs of labels. The MAR was this mean double label distance divided by the eleven day labeling interval.
Data were analyzed for the effect of in vivo loading and number of loading cycles on bone volume and organization. For BV/TV, Tb.Th, Tb.Sp, and MAR, a two-factor repeated measures analysis of variance was performed to test for an effect of side (left=control, right= loaded) within subjects (the repeated measure) and for an effect of number of cycles between subjects. A repeated-measures analysis of covariance was performed for MIL, adjusting for BV/TV and including direction as an additional effect within subjects. If the interaction term between loading and number of cycles was significant, then a Bonferroni adjusted post-hoc comparison was performed to identify the cycle group (or groups) with a significant effect. All analyses were performed using SYSTAT (version 8, SPSS Inc., Chicago, IL) and BMDP (Program 2V, Release 7.0, BMDP Statistical Solutions, Inc., Saugus, MA). In all cases, alpha was set at 0.05. The summary descriptive statistics are presented as mean and standard deviation.
Results
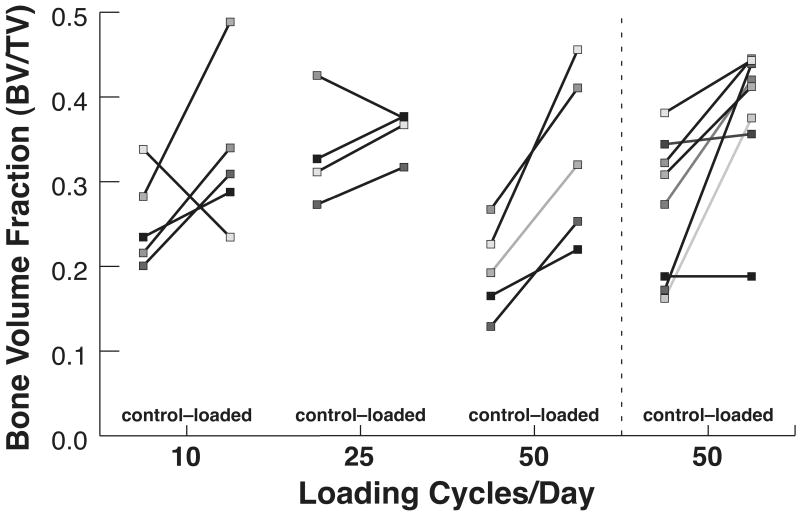
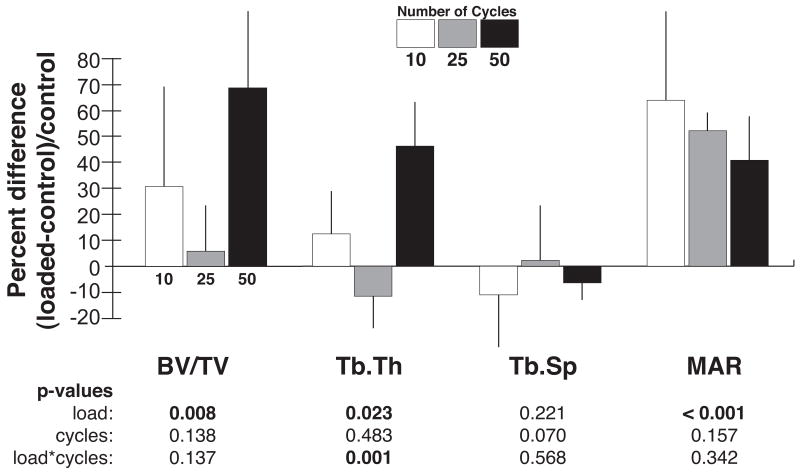
In rabbits loaded daily to peak pressures of 1 MPa for 4 weeks, a significant increase in bone volume fraction with loading was found in the tissue below the core (p=0.008, Table 1, Figure 2). In twelve of the fourteen rabbits, BV/TV was greater on the loaded side than the contralateral, control side (Figure 3). The mean percent increase of BV/TV in the VOI was 31% at 10 cycles/day, 9% at 25 cycles/day, and 70% at 50 cycles/day in loaded compared to unloaded limbs (Figure 4). The effect of load did not depend on the number of cycles. Because several rabbits did not complete the 4-week period, the power to detect effects of daily loading cycles on the increase in cancellous BV/TV was limited; approximately 10 animals would be needed per group to detect an interaction between load and number of cycles based on differences and variability found in this study.
Table 1.
Effect of loading and number of cycles on cancellous bone of the distal femoral condyle of the rabbit. Mean (SD) of bone volume fraction (BV/TV), direct trabecular thickness (Tb.Th, mm) and spacing (Tb.Sp, mm), degree of anisotropy (DA) and mineral apposition rate (MAR, μm/day).
| Group | na | BV/TV | Direct Tb.Th (mm) | Direct Tb.Sp (mm) | DA | MAR (μm/day) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| control (left) | loadedb (right) | control (left) | loadedb (right) | control (left) | loaded (right) | control (left) | loaded (right) | control (left) | loadedb (right) | ||
| 10 cycle/d | 5 | 0.264 (0.05) | 0.338 (0.11) | 0.185 (0.03) | 0.205 (0.03) | 0.630 (0.11) | 0.558 (0.15) | 1.58 (0.10) | 1.55 (0.14) | 2.22 (0.30) | 3.56 (0.50) |
| 25 cycle/d | 4 | 0.355 (0.07) | 0.366 (0.03) | 0.228 (0.04) | 0.198 (0.02) | 0.443 (0.06) | 0.445 (0.04) | 1.49 (0.12) | 1.48 (0.10) | 2.22 (0.12) | 3.38 (0.26) |
| 50 cycle/d | 5 | 0.196 (0.05) | 0.332 (0.10) | 0.155 (0.02) | 0.226 (0.04) | 0.655 (0.14) | 0.619 (0.17) | 1.51 (0.12) | 1.44 (0.21) | 2.11 (0.15) | 2.98 (0.49) |
sample size for MAR: n= 5 for 10 cycles, n = 3 for 25 cycles, n = 5 for 50 cycles.
p<0.05 for loaded vs. control in two factor ANOVA.
Figure 2.
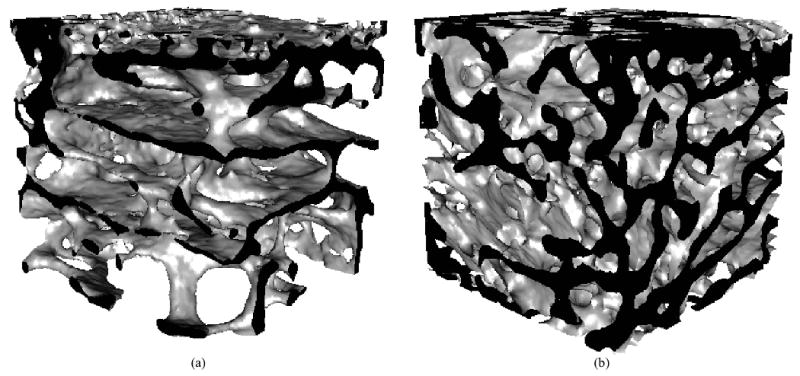
Representative 3D reconstructions from microcomputed tomography scans of tissue below the loading core for (a) unloaded (BV/TV= 0.20) and (b) loaded (BV/TV=0.30) distal rabbit femora loaded to 1 MPa at 50 cycles/day for 4 weeks. A 3.5 mm cube is shown; the top surface was in contact with the loading core. The cut edges are shown in black.
Figure 3.
Bone volume fraction for left-right pairs as a function of number of cycles for 2 loading experiments: (1) Main experiment with 3 different numbers of cycles applied daily, (2) Follow-up experiment with 50 cycles applied per day. Left = contralateral control limb; Right = loaded limb. Paired data are connected by a solid line; shading distinguishes individual pairs.
Figure 4.
Mean percent difference (SD) between loaded and control values, normalized by control value, for bone volume fraction (BV/TV), direct trabecular thickness (Tb.Th) and spacing (Tb.Sp), and mineral apposition rate (MAR). Significance from ANOVA with full-interaction indicated.
The directly measured trabecular thickness was significantly greater in loaded condyles than the contralateral limb (p=0.023). In addition the effect of loading on Tb.Th was significantly affected by the number of cycles (p=0.001, Table 1, Figure 4). When the effect of side was analyzed for the different levels of loading cycles, only the direct Tb.Th of the 50 cycles per day group was significantly increased. Tb.Sp was not significantly different between the loaded and control limbs, and the number of loading cycles had no significant effect.
The tissue architectural orientation was adapted following loading. The three principal mean intercept lengths were significantly greater in the loaded than control limb (p = 0.001). A strong, positive relationship was found between the principal MILs and BV/TV both within and across animals (p<0.0001). When an analysis of covariance was used to adjust for differences in BV/TV between the loaded and control limbs, the difference in the three principal MILs with loading remained significant (p=0.034), demonstrating that the increased MILs were the result of alignment of trabeculae as well as the increase in bone mass reflected by BV/TV. A significant dependence of principal MILs with loading on the number of cycles was found (p=0.05), and the adjusted principal MIL values were significantly greater on the loaded side for 50 cycles per day. As expected the principal MILs were strongly dependent on direction (p<0.0001). The degree of anisotropy was not significantly different with loading (Table 1).
The mineral apposition rate was significantly increased on the loaded side (p<0.0001, Table 1). No effect of the number of cycles on MAR was found. The percent of tissue surface actively forming bone during the eleven day labeling period, reflected by the percent of labeled area, did not differ between the loaded and control limbs.
The control bones examined for the initial characteristics at the time of implantation did not demonstrate any significant left-right differences in BV/TV (mean difference in BV/TV = 0.009 ± 0.09). However, substantial variation was present in BV/TV: left BV/TV = 0.249 ± 0.05 (20% coefficient of variation), right BV/TV = 0.266 ± 0.07 (26% coefficient of variation). Across the five pairs, BV/TV was more than 5% greater in the left limb of two pairs, more than 5% lower in the left limb of two pairs, and within 1% of each other in the remaining pair.
A significant effect of loading on BV/TV (p=0.010) was also seen in the second 50-cycle loading experiment that was added to confirm the first set of results: control BV/TV = 0.269 ± 0.08, loaded BV/TV = 0.385 ± 0.09.
Discussion
A novel loading device was developed to apply controlled in vivo loads to cancellous bone in the distal femur of rabbits. The effect of the applied loading on cancellous tissue was evident in this model, with loaded limbs having increased trabecular bone volume fractions, trabecular thicknesses, mean intercept lengths, and mineral apposition rates relative to the contralateral, control limbs. The difference between loaded and unloaded sides depended on number of cycles for trabecular thickness and principal MILs, with a significant effect evident for 50 cycles of applied loading per day.
Unfortunately we were unable to relate bone formation directly to parameters of the mechanical environment such as the number of cycles. Prior studies suggest that our apparent load levels will need to be converted to tissue level stimuli and examined with microstructural models [5, 11]. The results for the single loading parameter we focused on, number of daily loading cycles, were limited by our small sample size and the variability inherent to this model. However, the Tb.Th of the 50 cycles per day group was significantly increased in the loaded limbs relative to the contralateral control limbs. The variability of Tb.Th with number of loading cycles suggests a threshold could exist between 25 and 50 cycles as no effect of loading was present at 10 and 25 cycles and a significant increase was present at 50 cycles. Strain, frequency, and strain rate thresholds have been reported for cortical bone using the rodent four-point bending model [8, 34, 35]. We would also expect this behavior to be evident in BV/TV, but the sample sizes examined were small and the range of cycle numbers examined was relatively narrow.
The left-right differences measured here could arise by two distinct mechanisms: the formation of mineralized tissue in the loaded limb or the maintenance of bone in the loaded limb accompanied by loss of mineralized tissue in the control limb. The latter mechanism might be suggested by our BV/TV data for the control limbs as the mean data vary substantially (Table 1). However, the presence of double labeled surfaces and increased MAR values indicate that the applied loading generated a bone formation response. Furthermore, the greater MIL for all loaded groups supports this mechanism. In addition, substantial left-right variability in BV/TV exists in the rabbit as demonstrated in our control bone data, making our increased BV/TV in 12 of 14 rabbits remarkable (Figure 3). To further confirm this mechanism, longitudinal assessments at baseline and after four weeks would be necessary using a high-resolution in vivo microCT scan of the volume of interest.
In the flexed knee joint of the rabbit, the medial-lateral direction experiences the least loading during normal activities. In the normal rabbit condyle, the two largest orthotropic moduli in compression and the two largest principal MIL values occur in the two planes corresponding to the primary loading directions, perpendicular to the medial-lateral direction [3]. The loading in the model reported here is applied in a direction that is not habitually loaded in vivo, perpendicular to the lateral surface of the condyle. Our principal MIL data show that, even after correction for bone volume fraction, a significant difference in principal mean intercept length remained with loading.
Our model has comparatively lower surgical insult than previous studies in a canine model [18]. To load cancellous tissue in situ, our loading device was surgically placed, and then the cortical shell beneath the core was removed. A previous canine model required extensive surgery and, after 4 weeks of loading, the results were dominated by wound healing [18]. In comparison, our surgery was less injurious, and our adaptation response definitive. A related contributing difference may be the continued weight bearing by the rabbits on their femora post-implantation. The trauma of surgery presumably activated a regional acceleratory phenomenon, a local acceleration of bone remodeling [10], reflected by active bone formation below the loading core in both limbs and similar total areas of bone formation. However, this increased bone turnover activity may also have contributed to the load-related tissue modeling response in the right limbs.
Mechanisms to increase cancellous bone apparent density are highly desirable clinically. By isolating and controlling the mechanical stimulus to the cancellous tissue, our goal was to gain insights into the stimulus parameters contributing to cancellous adaptation. Strengths of this model include the ability to load cancellous bone in situ, the defined loading stimulus, and the control over the loading parameters applied to the tissue. The location, subcutaneous placement, and rotary-to-linear translation mechanism combined for a self-contained and minimally obtrusive design. The use of the contralateral limbs as controls was both a strength and a limitation. Within-subject controls helped to limit animal-based variability, but might have obscured the presence of any systemic response. In the future, both distinct controls and a larger sample size would be desirable. Another limitation is the human operator required to apply the loading. The use of an automated loading device could be more straightforward and repeatable. Nonetheless, the 19% coefficient of variability of the loading suggests reasonable consistency with manual load application and calibration. Finally, the inter-rabbit variability in BV/TV suggests a precise measure of loading throughout the experiment based on cancellous volume fraction for each individual rabbit, accounting for the changes with time would better define the in vivo loading experienced and perhaps reduce the variability in the data. Longitudinal in vivo images combined with finite element models would allow the tissue-level strains to be approximated, rather than the continuum-level approach used here [5, 11].
Given the challenges inherent to in vivo cancellous loading experiments, the results of this study are extremely promising. Loading with 50 cycles per day increased bone volume fraction accompanied by increased trabecular thickness. Further investigations with this in vivo model to examine additional loading parameters such as load level, experimental duration, and timing will greatly improve our understanding of cancellous functional adaptation. Recent data demonstrating that continuous loading may be less effective than multiple bouts or pauses between individual load cycles in cortical bone suggest additional loading approaches to consider for cancellous tissue [26, 30]. This model has the potential to provide insights into the mechanisms whereby cancellous functional adaptation occurs in vivo both for short-term gene expression and long-term mechanical stiffness and strength changes. Finally, the administration of pharmacologic agents systemically at the loading surface or as surface treatments of the loading core will allow the synergy of therapies and functional adaptation to be investigated.
Acknowledgments
The authors would like to thank Dr. Harrie Weinans, Erasmus University Rotterdam, for the use of his microcomputed tomography scanner. We thank Jianhao Lin at the Hospital for Special Surgery and Dr. David Dempster and Hua Zhou at Helen Hayes Hospital. This project was funded by the Oxnard Foundation, the National Science Foundation (BES9753164, BES9875383), a Pilot and Feasibility Award and Core Center Support from the National Institutes of Health (P30-AR46121), the Frese Foundation, and the Clark Foundation.
References
- 1.Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int. 1998;63:442–9. doi: 10.1007/s002239900554. [DOI] [PubMed] [Google Scholar]
- 2.Bostrom MP, Gamradt SC, Asnis P, Vickery BH, Hill E, Avnur Z, Waters RV. Parathyroid hormone-related protein analog RS-66271 is an effective therapy for impaired bone healing in rabbits on corticosteroid therapy. Bone. 2000;26:437–42. doi: 10.1016/S8756-3282(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 3.Bourne BC, Morgan TG, Paschalis E, van der Meulen MCH. Cancellous bone anisotropy arises from both architecture and material properties. Trans Orthop Res Soc. 2002;27:558. [Google Scholar]
- 4.Chambers TJ, Evans M, Gardner TN, Turner-Smith A, Chow JWM. Induction of bone formation in rat tail vertebrae by mechanical loading. Bone Miner. 1993;20:167–78. doi: 10.1016/s0169-6009(08)80025-6. [DOI] [PubMed] [Google Scholar]
- 5.Cheal EJ, Snyder BD, Nunamaker DM, Hayes WC. Trabecular bone remodeling around smooth and porous implants in an equine patellar model. J Biomech. 1987;20:1121–34. doi: 10.1016/0021-9290(87)90029-7. [DOI] [PubMed] [Google Scholar]
- 6.Chow JWM, Wilson AJ, Chambers TJ, Fox SW. Mechanical loading stimulates bone formation by reactivation of bone lining cells in 12-week-old rats. J Bone Miner Res. 1998;13:1760–7. doi: 10.1359/jbmr.1998.13.11.1760. [DOI] [PubMed] [Google Scholar]
- 7.Cullen DM, Smith RT, Akhter MP. Bone-loading response varies with strain magnitude and cycle number. J Appl Physiol. 2001;91:1971–6. doi: 10.1152/jappl.2001.91.5.1971. [DOI] [PubMed] [Google Scholar]
- 8.Forwood MR, Turner CH. Skeletal adaptations to mechanical usage: results from tibial loading studies in rats. Bone. 1995;17:197S–205S. doi: 10.1016/8756-3282(95)00292-l. [DOI] [PubMed] [Google Scholar]
- 9.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–8. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Frost H. Intermediary Organization of the Skeleton. Boca Raton, FL: CRC Press; 1986. [Google Scholar]
- 11.Goldstein SA, Matthews LS, Kuhn JL, Hollister SJ. Trabecular bone remodeling: an experimental model. J Biomech. 1991;24 1:135–50. doi: 10.1016/0021-9290(91)90384-y. [DOI] [PubMed] [Google Scholar]
- 12.Goodman SB, Song Y, Doshi A, Aspenberg P. Cessation of strain facilitates bone formation in the micromotion chamber implanted in the rabbit tibia. Biomaterials. 1994;15:889–93. doi: 10.1016/0142-9612(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 13.Guldberg RE, Caldwell NJ, Guo XE, Goulet RW, Hollister SJ, Goldstein SA. Mechanical stimulation of tissue repair in the hydraulic bone chamber. J Bone Miner Res. 1997;12:1295–302. doi: 10.1359/jbmr.1997.12.8.1295. [DOI] [PubMed] [Google Scholar]
- 14.Harrigan TP, Jasty M, Mann RW, Harris WH. Limitations of the continuum assumption in cancellous bone. J Biomech. 1988;21:269–75. doi: 10.1016/0021-9290(88)90257-6. [DOI] [PubMed] [Google Scholar]
- 15.Hert J, Liskova M, Landa J. Reaction of bone to mechanical stimuli. Part 1. Continuous and intermittent loading of tibia in rabbit. Folia Morph, Prague. 1971;19:290–300. [PubMed] [Google Scholar]
- 16.Hert J, Liskova M, Landrgot B. Influence of the long-term, continuous bending on the bone. An experimental study on the tibia of the rabbit. Folia Morph, Prague. 1969;17:389–99. [PubMed] [Google Scholar]
- 17.Hildebrand T, Rüegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997;185:67–75. [Google Scholar]
- 18.Hollister SJ, Guldberg RE, Juelske CL, Caldwell NJ, Richards M, Goldstein SA. Relative effects of wound healing and mechanical stimulus on early bone response to porous-coated implants. J Orthop Res. 1996;14:654–62. doi: 10.1002/jor.1100140422. [DOI] [PubMed] [Google Scholar]
- 19.Kim CH, Takai E, Zhou H, von Stechow D, Muller R, Dempster DW, Guo XE. Trabecular bone response to mechanical and parathyroid hormone stimulation: the role of mechanical microenvironment. J Bone Miner Res. 2003;18:2116–25. doi: 10.1359/jbmr.2003.18.12.2116. [DOI] [PubMed] [Google Scholar]
- 20.Moalli MR, Caldwell NJ, Patil PV, Goldstein SA. An in vivo model for investigations of mechanical signal transduction in trabecular bone. J Bone Miner Res. 2000;15:1346–53. doi: 10.1359/jbmr.2000.15.7.1346. [DOI] [PubMed] [Google Scholar]
- 21.Morgan TG, van der Meulen MCH. Comparison of three-dimensional histomorphometric techniques. J Bone Miner Res. 1999;14:S430. [Google Scholar]
- 22.Mosley JR, Lanyon LE. Strain rate as a controlling influence on adaptive modeling in response to dynamic loading of the ulna in growing male rats. Bone. 1998;23:313–8. doi: 10.1016/s8756-3282(98)00113-6. [DOI] [PubMed] [Google Scholar]
- 23.Mosley JR, March BM, Lynch J, Lanyon LE. Strain magnitude related changes in whole bone architecture in growing rats. Bone. 1997;20:191–8. doi: 10.1016/s8756-3282(96)00385-7. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen EA, Akhter MP, Cullen DM, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in C3H/HeJ mice. Calcif Tissue Int. 1999;65:41–6. doi: 10.1007/s002239900655. [DOI] [PubMed] [Google Scholar]
- 25.Robling AG, Burr DB, Turner CH. Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Miner Res. 2000;15:1596–602. doi: 10.1359/jbmr.2000.15.8.1596. [DOI] [PubMed] [Google Scholar]
- 26.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–54. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 27.Robling AG, Li J, Shultz KL, Beamer WG, Turner CH. Evidence for a skeletal mechanosensitivity gene on mouse chromosome 4. FASEB J. 2003;17:324–6. doi: 10.1096/fj.02-0393fje. [DOI] [PubMed] [Google Scholar]
- 28.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Jt Surg. 1984;66-A:397–402. [PubMed] [Google Scholar]
- 29.Simmons CA, Hipp JA. Method-based differences in the automated analysis of the three-dimensional morphology of trabecular bone. J Bone Miner Res. 1997;12:942–7. doi: 10.1359/jbmr.1997.12.6.942. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17:1613–20. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tägil M, Aspenberg P. Cartilage induction by controlled mechanical stimulation in vivo. J Orthop Res. 1999;17:200–4. doi: 10.1002/jor.1100170208. [DOI] [PubMed] [Google Scholar]
- 32.Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcif Tissue Int. 1994;54:241–7. doi: 10.1007/BF00301686. [DOI] [PubMed] [Google Scholar]
- 33.Turner CH, Akhter MP, Raab DM, Kimmel DB, Recker RR. A noninvasive, in vivo model for studying strain adaptive bone modeling. Bone. 1991;12:73–9. doi: 10.1016/8756-3282(91)90003-2. [DOI] [PubMed] [Google Scholar]
- 34.Turner CH, Forwood MR, Otter MW. Mechanotransduction in bone: do bone cells act as sensors of fluid flow? FASEB J. 1994;8:875–8. doi: 10.1096/fasebj.8.11.8070637. [DOI] [PubMed] [Google Scholar]
- 35.Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9:87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- 36.Underwood EE. Quantitative Stereology. Reading, MA: Addison-Wesley; 1970. [Google Scholar]
- 37.Whitehouse WJ. The quantitative morphology of anisotropic trabecular bone. J Microsc. 1974;101:153–268. doi: 10.1111/j.1365-2818.1974.tb03878.x. [DOI] [PubMed] [Google Scholar]