Abstract
In MKN1 gastric cancer cells, lysophosphatidic acid (LPA) upregulates expression of sphingosine kinase 1 (SphK1) and its downregulation or inhibition suppresses LPA-mediated proliferation. Although LPA activates numerous signaling pathways downstream of its receptors, including ERK1/2, p38, JNK, and Akt, and the transactivation of the EGF receptor, pharmacological and molecular approaches demonstrated that only activation of ERK1, in addition to the CCAAT/enhancer-binding protein β (C/EBPβ) transcription factor, is involved in transcriptional upregulation of SphK1 by LPA. Our data implicate ERK1 as an important mediator of LPA signaling leading to upregulation of SphK1 and point to SphK1 and S1P production as potential therapeutic targets in gastric cancer.
Keywords: Lysophosphatidic Acid, Sphingosine Kinase, Gastric Cancer, Proliferation
1. Introduction
Lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) and their respective families of G protein-coupled receptors (GPCRs) have been implicated in progression of gastric cancer [1,2], the second most common cause of cancer related deaths. Binding of either of these lysophospholipids to their cognate receptors on gastric cancer cells transactivates the epidermal growth factor receptor (EGFR) [3,4], whose activation or overexpression correlates with poor clinical outcomes [5]. Moreover, recent studies suggest that sphingosine kinase 1 (SphK1), one of the two isoenzymes that produce S1P, is up-regulated in gastric cancer lesions and is associated with gastric cancer progression and poor survival of patients [2]. In this regard, we have previously shown that LPA markedly enhanced SphK1 mRNA and protein in MKN1 gastric cancer cells via activation of the LPA1 receptor [6], which may underlie the metastatic behavior of gastric cancer [7]. Moreover, down-regulation of SphK1 attenuated LPA-stimulated migration and invasion of MNK1 cells, suggesting that SphK1 is a convergence point of multiple cell surface receptors for three different ligands, LPA, EGF, and S1P, which have all been implicated in regulation of motility and invasiveness of gastric cancer cells [6].
In this study, we report that LPA-stimulated proliferation of MKN1 gastric cancer cells also requires increased expression of SphK1. We examined the signaling pathways responsible for transcriptional upregulation of SphK1 by LPA and demonstrated that activation of ERK1 and the transcription factor CCAAT enhancer-binding protein β (C/EBPβ) are critical to the proliferative response of gastric cancer cells to LPA.
2. Materials and methods
2.1. Reagents
Ki16425 and recombinant EGF were purchased from Sigma-Aldrich (St. Louis, MO). LPA was obtained from Avanti Polar Lipids (Alabaster, AL). Antibodies against phospho-ERK1/2 (Thr202/Tyr204), phospho-Akt (Ser473), Akt, ERK1, ERK2, phospho-C/EBPβ (Thr235), and β-tubulin were from Cell Signaling (Danvers, MA). Anti-C/EBPβ antibody was from Santa Cruz Biotech (Santa Cruz, CA).
2.2. Cell culture and transfection
The MKN1 human gastric cancer cell line was obtained from the Riken Cell Bank (Tsukuba, Japan). Cells were cultured and transfected with siRNA exactly as previously described [6].
2.3. Proliferation assays
Cell proliferation was monitored with WST reagent (Roche Applied Science, Indianapolis, IN). Cells were incubated with 2% WST in serum-free DMEM without phenol red for 30 min. Absorbance was measured at 450 nm with background subtraction at 630 nm with a plate reader. Alternatively, cell proliferation was quantified with 5% crystal violet (Fisher, Middletown, VA) and absorbance was measured at 570 nm.
2.4. Quantitative real-time PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) and reverse-transcribed with the high-capacity cDNA Archive Kit (Applied Biosystems, Foster, CA). QPCR was performed with an ABI PRISM 7900HT (Applied Biosystems) as described [6].
2.5. Western blotting
Equal amounts of proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes. Immunoreactive signals were visualized by enhanced chemiluminescence using SuperSignal West Pico Chemiluminescent Substrate (Pierce/Thermo Fisher, Rockford, IL) [6].
2.6. Statistical analysis
Experiments were repeated at least three times with consistent results. Results were analyzed for statistical significance with the Student’s t test for unpaired samples and P < 0.05 was considered significant.
3. Results and discussion
3.1. LPA stimulates proliferation of MKN1 cells
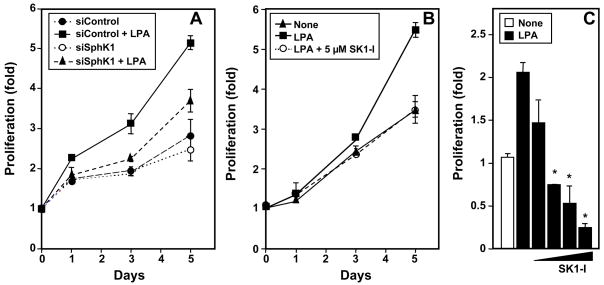
We have previously shown in gastric cancer cells that LPA markedly stimulated expression of SphK1 [6], which has been linked to proliferation and is upregulated in many cancers, including gastric cancer [2,8]. Therefore, it was of interest to examine the role of SphK1 expression in LPA-induced proliferation of MKN1 cells. In agreement with previous studies [9], LPA stimulated growth of MKN1 cells (Fig. 1A). Downregulation of SphK1 in MKN1 cells by transfection with siRNA, which decreased its expression by 70% or more (data not shown), reduced the stimulatory effect on proliferation of LPA by more than 60% without significantly affecting the basal growth rate (Fig. 1A). A pharmacological approach was next used to substantiate the involvement of SphK1. Treatment with the isozyme-specific SphK1 inhibitor, SK1-I [10,11], also suppressed LPA-induced proliferation in a time- (Fig. 1B) and dose-dependent manner (Fig. 1C). These results support the notion that SphK1 is critical for LPA-induced gastric cancer cell proliferation.
Fig. 1. LPA induced proliferation of MKN1 cells requires SphK1.
(A) MKN1 cells transfected with siControl or siSphK1 were stimulated without or with 10 μM LPA and cell growth determined with WST-1 on the indicated days. (B) MKN1 cells were cultured in the absence or presence of LPA (10 μM) and 5 μM SK1-I and cell growth determined with WST-1 on the indicated days. (C) Cells were cultured for 5 days in the absence or presence of LPA (10 μM) without or with 1, 3, 5, and 10 μM SK1-I and proliferation determined by crystal violet staining. Data are expressed as fold ± SD of triplicate determinations. *, P < 0.05, compared to LPA treated cells.
3.2. LPA signaling pathways involved in upregulation of SphK1
MKN1 cells express LPA1, LPA2, and LPA3 receptors [7], of which the LPA1 receptor has been suggested to play a major role in LPA-induced proliferation [12]. In agreement, we have previously shown that an LPA1 receptor antagonist or down-regulation of its expression prevented SphK1 up-regulation by LPA in MKN1 cells [6]. Furthermore, enhanced SphK1 expression by LPA was also observed in other types of human cancer cells and was strictly dependent on the presence of the LPA1 receptor [6]. Taken together, our results suggest that upregulation of SphK1 via activation of LPA1 is a critical event in LPA mediated proliferation.
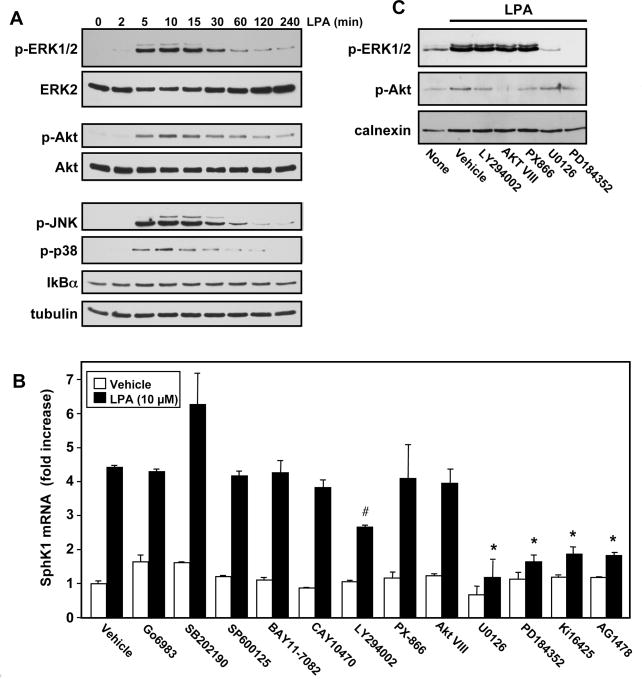
Therefore, it was of interest to examine which of the signaling pathways downstream of LPA1 is involved in regulating expression of SphK1. In agreement with previous studies [13,14], LPA rapidly activated several members of the MAPK pathway, including ERK1/2, JNK1/2, and p38, as well as Akt, determined by western blotting with phospho-specific antibodies (Fig. 2A). In contrast, the NF-κB pathway was not activated, as shown by the lack of reduction in levels of IkBα after treatment with LPA.
Fig. 2. Effects of inhibitors of LPA signaling pathways on SphK1 upregulation.
(A) MKN1 cells were stimulated with 1 μM LPA for the indicated times, equal amounts of cell lysates were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. Blots were stripped and immunoblotted with anti-ERK2, anti-Akt, and anti-tubulin to ensure equal loading and transfer. (B) MKN1 cells were pretreated with vehicle or with Ki16425 (10 μM), AG1478 (1 μM), Go6983 (10 μM), SB202190 (1 μM), SP600125 (10 μM), BAY11-7082 (1 μM), CAY10470 (1 μM), LY294002 (10 μM), PX-866 (100 nM), Akt VIII (10 μM), U0126 (10 μM), or PD184352 (10 μM), for 1 hour, and subsequently stimulated with LPA (10 μM) for 6 hours. RNA was isolated, reverse-transcribed, and SphK1 mRNA measured by QPCR. Data are expressed as fold induction after normalization to GAPDH. *, P < 0.001, #, P< 0.05 compared to LPA treated cells. (C) Cultures were pretreated with the indicated inhibitors for 1 hour prior to stimulation with LPA (10 μM) for 20 min. Equal amounts of lysate proteins were analyzed by immunoblotting with anti-phospho-Akt and anti-phospho-ERK1/2 antibodies. Blots were stripped and reprobed with anti-calnexin antibody to ensure equal loading and transfer.
We next examined the effects of a variety of inhibitors that perturb LPA stimulated signaling pathways on SphK1 expression. Consistent with our previous results [6], both Ki16425, a LPA1 receptor antagonist, and the EGFR tyrosine kinase inhibitor AG1478 prevented the increase in SphK1 expression induced by LPA (Fig. 2B). However, upregulation of SphK1 by LPA was not blocked by Go6983, a broad-spectrum protein kinase C inhibitor, SB202190, a selective p38 inhibitor, or SP600125, a JNK inhibitor (Fig. 2B). Similarly, BAY11-7082 and CAY10470, established NF-κB inhibitors, had no effect on LPA-induced SphK1 upregulation (Fig. 2B). LY294002, an inhibitor of PI3K, slightly inhibited LPA-stimulated expression of SphK1. This is likely due to non-specific effects of LY294002 [15], as PX-866, an irreversible and potent PI3K inhibitor that reduced Akt activation by LPA to a similar extent (Fig. 2C), had no effect on SphK1 upregulation (Fig. 2B). Moreover, Akt VIII, a potent and selective Akt1/2 inhibitor, which completely blocked LPA-induced Akt activation (Fig. 2C), did not decrease LPA-induced upregulation of SphK1 (Fig. 2B). Importantly, both U0126, a highly selective inhibitor of MEK1 and MEK2, and PD184352, a selective and non-competitive inhibitor of MEK1, completely abolished the increase in SphK1 expression (Fig. 2B) and as expected, drastically blocked LPA induced ERK1/2 phosphorylation without affecting Akt phosphorylation (Fig. 2C).
3.3. ERK1 is critical for LPA-induced expression of SphK1
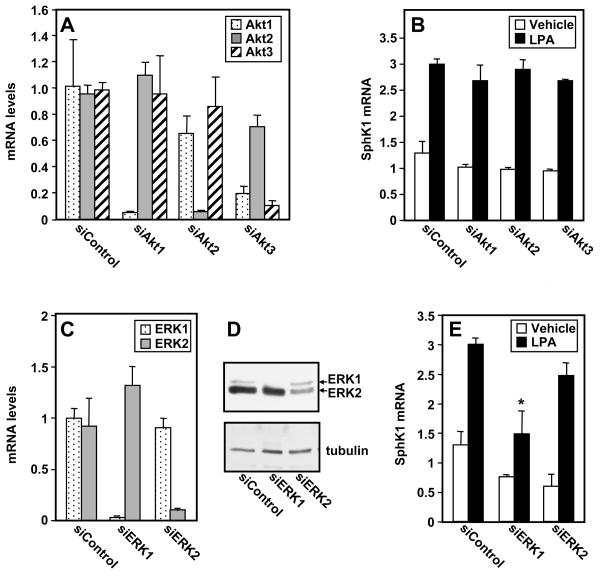
To unequivocally determine if Akt activation by LPA is involved in SphK1 upregulation in MKN1 cells, its expression was downregulated. MKN1 cells express Akt isoforms 1, 2 and 3. Specific siRNA mediated knockdown of each of these isoforms achieved greater than 80% reduction in their mRNA levels (Fig. 3A), although siRNA targeted to Akt3 also reduced expression of the major isoform, Akt1. Nevertheless, downregulation of any of the Akt isoforms did not significantly decrease LPA-induced upregulation of SphK1 expression (Fig. 3B). These results indicate that activation of Akt by LPA is not required for its effect on SphK1 induction.
Fig. 3. ERK1 is important for upregulation of SphK1 by LPA.
(A) MKN1 cells were transfected with ON TARGET plus SMART pool siRNA targeted to Akt-1, Akt-2, Akt-3 or with control siRNA. RNA was isolated and mRNA levels were measured by QPCR. (B) Duplicate cultures were stimulated for 6 hours without or with LPA and SphK1 mRNA measured by QPCR. (C,D) MKN1 cells were transfected with ON TARGET plus SMART pool siRNA targeted to ERK1, ERK2 or control siRNA. (C) RNA was isolated and mRNA levels were measured by QPCR. (D) Western blotting analysis of ERK1/2 expression. (E) Duplicate cultures were stimulated for 6 hours without or with LPA and SphK1 mRNA measured by QPCR. (A,B,C,E) Data are expressed as fold change after normalization to GAPDH. *, P < 0.05, compared to LPA stimulated siControl.
To conclusively demonstrate the involvement of ERK1/2, as was suggested by the effects of the MEK1/2 inhibitors (Fig. 2B), we used siRNA to downregulate ERK1 and ERK2 individually. Transfection with either siRNA targeted to ERK1 or ERK2 reduced the levels of the respective mRNAs by greater than 85% without decreasing the other isoform (Fig. 3C). Similarly, immunoblotting revealed that protein levels of ERK1 or ERK2 were specifically and significantly reduced (Fig. 3D). Interestingly, however, only downregulation of ERK1 but not ERK2 significantly reduced upregulation of SphK1 by LPA (Fig. 3E)
3.4. LPA induces transcriptional activation of SphK1 via C/EBPβ
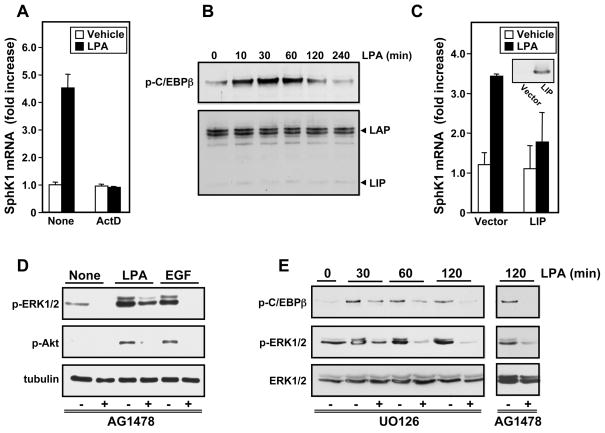
Pretreatment with actinomycin D, which halts new RNA synthesis, completely blocked the LPA-induced upregulation of SphK1 expression (Fig. 4A), indicating that LPA increases SphK1 by stimulation of its transcription rather than by increasing mRNA stability. As the human sphk1 promoter region contains a binding site for the transcription factor C/EBPβ, which is known to be phosphorylated and activated by ERK [16,17], we examined its role in the regulation of SphK1 expression by LPA. In agreement with previous studies [13,14], in MKN1 cells, LPA stimulated phosphorylation of C/EBPβ at Thr235 (Fig. 4B), which enhances its transcription activity. A significant increase C/EBPβ phosphorylation was observed within 10 min and was sustained for 2 hours. Anti-C/EBPβ antibody detected the 38 kDa liver activating protein (LAP) protein, the major form of C/EBPβ, which is a transcriptional activator, while the 20 kDa liver inhibitory protein (LIP) was barely noticeable (Fig. 4B). Moreover, overexpression of LIP, a dominant-negative, truncated form of C/EBPβ, completely suppressed LPA-induced SphK1 upregulation (Fig. 4C), suggesting that C/EBPβ is important for regulation of SphK1 expression.
Fig. 4. Involvement of C/EPBβ in LPA-induced transcriptional upregulation of SphK1.
(A) MKN1 cells were pretreated for 1 hour with actinomycin D (5 μg/ml) and then stimulated for 6 hours without or with LPA and SphK1 mRNA measured by QPCR. (B) Cells were stimulated with LPA for the indicated times and equal amounts of lysates analyzed by immunoblotting with anti-phospho-C/EPBβ (Thr235) antibody or with antiC/EPBβ antibody. The LAP and LIP subunits are indicated. (C) MKN1 cells transfected with vector or LIP were stimulated without or with LPA for 6 hours and SphK1 mRNA measured by QPCR (Insert: western blotting demonstrating LIP expression). (D,E) MKN1 cells were pretreated for 30 min with vehicle, AG1478 (1 μM) (D,E), or U0126 (10 μM) (E), as indicated. Cells were then stimulated for 5 min with vehicle, LPA (10 μM), or EGF (10 ng/ml) (D), or for the designated times (E), as indicated. Proteins were analyzed by immunoblotting with antibodies against phospho-ERK1/2, phospho-Akt, tubulin, phospho-C/EPBβ (Thr235), or ERK1/2.
Because activation of C/EBPβ in other cell types is downstream of LPA-induced transactivation of EGFR [13,14], and we have previously shown that in MKN1 cells LPA transactivates EGFR which in turn is required for increased expression of SphK1 ([6] and Fig. 2C), we examined the role of EGFR transactivation in ERK1/2 activation and subsequent phosphorylation of C/EBPβ. Pretreatment with the EGFR tyrosine kinase inhibitor AG1478 blocked LPA-stimulated phosphorylation of ERK1 and attenuated phosphorylation of ERK2 (Fig. 4D). Moreover, LPA-induced phosphorylation of C/EBPβ was also markedly reduced by AG1478 as well as by U0126 (Fig. 4E), confirming that C/EBPβ is downstream of EGFR transactivation and ERK1 activation by LPA.
4. Conclusions
SphK1, which is critical for cell growth and survival, is overexpressed in many types of cancers yet very little is known about its transcriptional regulation. Based solely on the effects of pharmacological inhibitors, it has previously been reported that both ERK1/2 and PI3K/Akt pathways are involved in regulation of sphk1 gene expression [18,19]. Here we have shown that although LPA activates multiple downstream signaling pathways, transcriptional upregulation of SphK1 is dependent solely on ERK1, which in turn phosphorylates C/EBPβ leading to its activation and regulation of transcription of SphK1. Transactivation of the EGFR by LPA also specifically contributes to ERK1 activation. While activation of Akt by LPA was completely dependent on transactivation of EGFR, Akt was dispensable for LPA-stimulated SphK1 upregulation. Although the ERK1/2 pathway was also required for enhanced transcription of sphk1 in response to other stimuli, different transcription factors, particularly Sp1 and AP-2, have been implicated [19,20]. Our results shed light on the regulation of SphK1 transcription, which is highly expressed in gastric cancer and correlates with poor prognosis [2], and provide further support for the notion that SphK1 might be a useful therapeutic target.
Acknowledgments
This work was supported by National Institutes of Health Grants R37GM043880 and R01CA61774 (S.S.), BIRCWH K12HD055881 and Susan G. Komen for the Cure Career Catalyst Research Grant. KG090510 (K.T.), and RO1CA102196 (X. Fang). MN was supported by the SUMITOMO Life Social Welfare Services Foundation.
Abbreviations
- C/EBPβ
CCAAT/enhancer-binding protein β
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ERK1/2
extracellular-signal-regulated kinase 1/2
- LPA
lysophosphatidic acid
- QPCR
quantitative real-time polymerase chain reaction
- siRNA
small interfering RNA
- S1P
sphingosine-1-phosphate
- S1PR
S1P receptor
- SphK1
sphingosine kinase type 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, Takuwa Y, Nagawa H. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–1711. [PubMed] [Google Scholar]
- 2.Li W, Yu CP, Xia JT, Zhang L, Weng GX, Zheng HQ, Kong QL, Hu LJ, Zeng MS, Zeng YX, Li M, Li J, Song LB. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- 3.Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Yatomi Y, Nagawa H. Sphingosine 1-phosphate transactivates c-Met as well as epidermal growth factor receptor (EGFR) in human gastric cancer cells. FEBS Lett. 2004;577:333–338. doi: 10.1016/j.febslet.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Nagawa H. Lysophosphatidic acid transactivates both c-Met and epidermal growth factor receptor, and induces cyclooxygenase-2 expression in human colon cancer LoVo cells. World J Gastroenterol. 2005;11:5638–5643. doi: 10.3748/wjg.v11.i36.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galizia G, Lieto E, Orditura M, Castellano P, Mura AL, Imperatore V, Pinto M, Zamboli A, De Vita F, Ferraraccio F. Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J Surg. 2007;31:1458–1468. doi: 10.1007/s00268-007-9016-4. [DOI] [PubMed] [Google Scholar]
- 6.Shida D, Fang X, Kordula T, Takabe K, Lepine S, Alvarez SE, Milstien S, Spiegel S. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008;68:6569–6577. doi: 10.1158/0008-5472.CAN-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shida D, Kitayama J, Yamaguchi H, Hama K, Aoki J, Arai H, Yamashita H, Mori K, Sako A, Konishi T, Watanabe T, Sakai T, Suzuki R, Ohta H, Takuwa Y, Nagawa H. Dual mode regulation of migration by lysophosphatidic acid in human gastric cancer cells. Exp Cell Res. 2004;301:168–178. doi: 10.1016/j.yexcr.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shida D, Kitayama J, Yamaguchi H, Yamashita H, Mori K, Watanabe T, Nagawa H. Lysophospholipids transactivate HER2/neu (erbB-2) in human gastric cancer cells. Biochem Biophys Res Commun. 2005;327:907–914. doi: 10.1016/j.bbrc.2004.12.088. [DOI] [PubMed] [Google Scholar]
- 10.Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant S, Spiegel S. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, Zipkin RE, Dent P, Kordula T, Milstien S, Spiegel S. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyesanya RA, Lee ZP, Wu J, Chen J, Song Y, Mukherjee A, Dent P, Kordula T, Zhou H, Fang X. Transcriptional and post-transcriptional mechanisms for lysophosphatidic acid-induced cyclooxygenase-2 expression in ovarian cancer cells. FASEB J. 2008;22:2639–2651. doi: 10.1096/fj.07-101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He D, Natarajan V, Stern R, Gorshkova IA, Solway J, Spannhake EW, Zhao Y. Lysophosphatidic acid-induced transactivation of epidermal growth factor receptor regulates cyclo-oxygenase-2 expression and prostaglandin E(2) release via C/EBPbeta in human bronchial epithelial cells. Biochem J. 2008;412:153–162. doi: 10.1042/BJ20071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima T, Kinoshita S, Sasagawa T, Sasaki K, Naruto M, Kishimoto T, Akira S. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc Natl Acad Sci USA. 1993;90:2207–2211. doi: 10.1073/pnas.90.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piwien-Pilipuk G, MacDougald O, Schwartz J. Dual regulation of phosphorylation and dephosphorylation of C/EBPbeta modulate its transcriptional activation and DNA binding in response to growth hormone. J Biol Chem. 2002;277:44557–44565. doi: 10.1074/jbc.M206886200. [DOI] [PubMed] [Google Scholar]
- 18.Doll F, Pfeilschifter J, Huwiler A. The epidermal growth factor stimulates sphingosine kinase-1 expression and activity in the human mammary carcinoma cell line MCF7. Biochim Biophys Acta. 2005;1738:72–81. doi: 10.1016/j.bbalip.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Murakami M, Ichihara M, Sobue S, Kikuchi R, Ito H, Kimura A, Iwasaki T, Takagi A, Kojima T, Takahashi M, Suzuki M, Banno Y, Nozawa Y, Murate T. RET signaling-induced SPHK1 gene expression plays a role in both GDNF-induced differentiation and MEN2-type oncogenesis. J Neurochem. 2007;102:1585–1594. doi: 10.1111/j.1471-4159.2007.04673.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakade Y, Banno Y, KTK, Hagiwara K, Sobue S, Koda M, Suzuki M, Kojima T, Takagi A, Asano H, Nozawa Y, Murate T. Regulation of sphingosine kinase 1 gene expression by protein kinase C in a human leukemia cell line, MEG-O1. Biochim Biophys Acta. 2003;1635:104–116. doi: 10.1016/j.bbalip.2003.11.001. [DOI] [PubMed] [Google Scholar]