Abstract
OBJECTIVE: To determine the incidence and causes of skin reactions to the synthetic pentasaccharide fondaparinux.
PATIENTS AND METHODS: Patients who received prophylactic/therapeutic subcutaneous fondaparinux treatment for more than 7 days were prospectively examined for cutaneous adverse effects between September 1, 2008, and April 30, 2009. When indicated, other procedures, such as skin biopsy, allergy testing, and clinical/laboratory assessment for thrombosis and heparin-induced thrombocytopenia, were performed.
RESULTS: Overall, 231 patients were enrolled. No patient developed typical delayed type IV hypersensitivity (DTH) erythematous skin lesions. However, one female patient experienced abdominal pruritus at sites of injection. Histology revealed a mild lymphohistiocytic infiltrate, confirming a DTH reaction. Heparin-induced thrombocytopenia, as another possible underlying pathomechanism for cutaneous lesions, was ruled out clinically and serologically. Hence, the overall incidence of fondaparinux-induced allergic skin lesions was 0.4% (95% confidence interval, 0.01%-2.4%). No cross-allergies were observed in patients with DTH reaction to heparins.
CONCLUSION: Fondaparinux has a low allergenic potential. The incidence of allergic cutaneous DTH reactions is almost 20 times lower compared to that with commonly used heparins. These results, together with the known low prevalence of secondary thrombotic events or heparin-induced thrombocytopenia during fondaparinux therapy, suggest that in selected patients fondaparinux might substantially improve patient care, therapeutic safety, and cost-effectiveness of anticoagulant therapy.
Trial Registration: clinicaltrials.gov identifier: NCT00510432
The incidence of allergic cutaneous delayed type IV hypersensitivity reactions to fondaparinux is almost 20 times lower compared to that with commonly used heparins.
BMI = body mass index; DTH = delayed type IV hypersensitivity; DVT = deep venous thrombosis; HIT = heparin-induced thrombocytopenia; LMWH = low-molecular-weight heparin; PE = pulmonary embolism; UFH = unfractionated heparin
The synthetic pentasaccharide fondaparinux is an ultra-low-molecular-weight (1.728 kDa) selective factor Xa inhibitor. Its pentasaccharide sequence resembles the antithrombin-binding site of heparins. Similar to heparins, its anticoagulatory function is exerted indirectly via enhancement (300-fold) of the inhibitory activity of the serine protease inhibitor antithrombin.1 Fondaparinux and heparins are both well-established drugs for prophylaxis and treatment of venous thromboembolic diseases and treatment of acute coronary syndrome.2,3
Besides bleeding complications, heparin-induced skin lesions are the most frequent adverse effects of subcutaneous heparin therapy.4-6 Patients typically present with itching and eczematous plaques that occur predominantly at heparin injection sites; however, presentations can vary (Figure 1, top).4,5 In a recent prospective investigation of patients receiving subcutaneous heparin therapy,7 we observed an incidence of 7.5% for heparin-induced skin lesions that could be classified as lymphocyte-mediated delayed type IV hypersensitivity (DTH) reactions (Figure 2, A). Because fondaparinux is a synthetic molecule, heparins are obtained from porcine gut processing, and the potential for sensitization seems to decrease with lower molecular weights (fondaparinux, 1.728 kDa; low-molecular-weight heparin [LMWH], 3-10 kDa; unfractionated heparin [UFH], 3-30 kDa),8 we hypothesized that fondaparinux should have a low allergenic potential. This assumption was also supported by several case reports, even in patients with multiple cross-allergies to various heparins.9,10 Nevertheless, possible allergic reactions to fondaparinux have been reported.11,12 However, to date, no clinical data exist regarding the incidence and etiology of fondaparinux-induced skin lesions. Because heparin-induced skin lesions may also frequently result from heparin-induced thrombocytopenia (HIT)13,14 caused by antigenic platelet factor 4/heparin complexes,15 we performed HIT diagnostics in patients with fondaparinux-induced skin lesions.
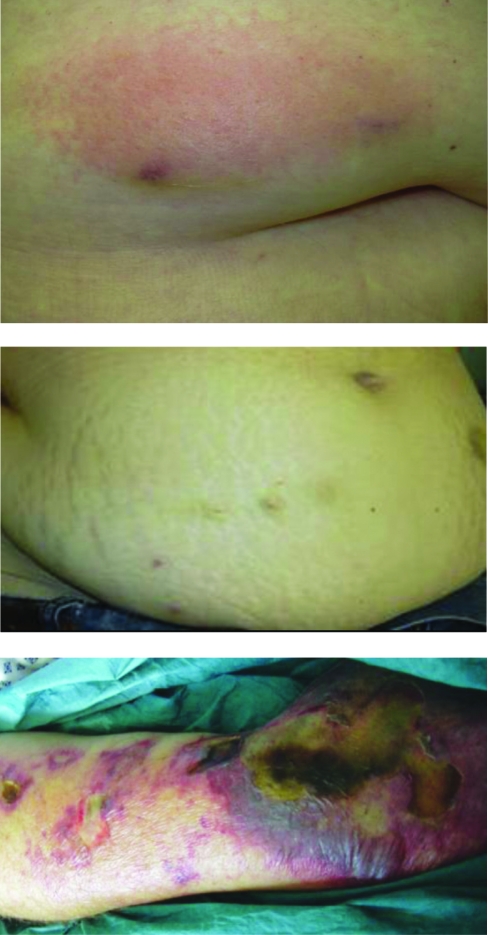
FIGURE 1.
Top, Typical presentation of a patient with delayed type IV hypersensitivity (DTH) reaction to nadroparin. The patient had pruritus at the injection areas and appearance of a single red plaque, with few papules at the heparin injection site 4 to 6 hours after subcutaneous application. Middle, Clinical presentation of the only study patient with a DTH reaction to fondaparinux. The patient had pruritus at the injection areas, but the skin appeared normal without erythematous lesions. Bottom, Clinical presentation of a patient with cutaneous necrosis due to underlying heparin-induced thrombocytopenia. Histologic study confirmed microthromboses in the necrotic areas.
FIGURE 2.
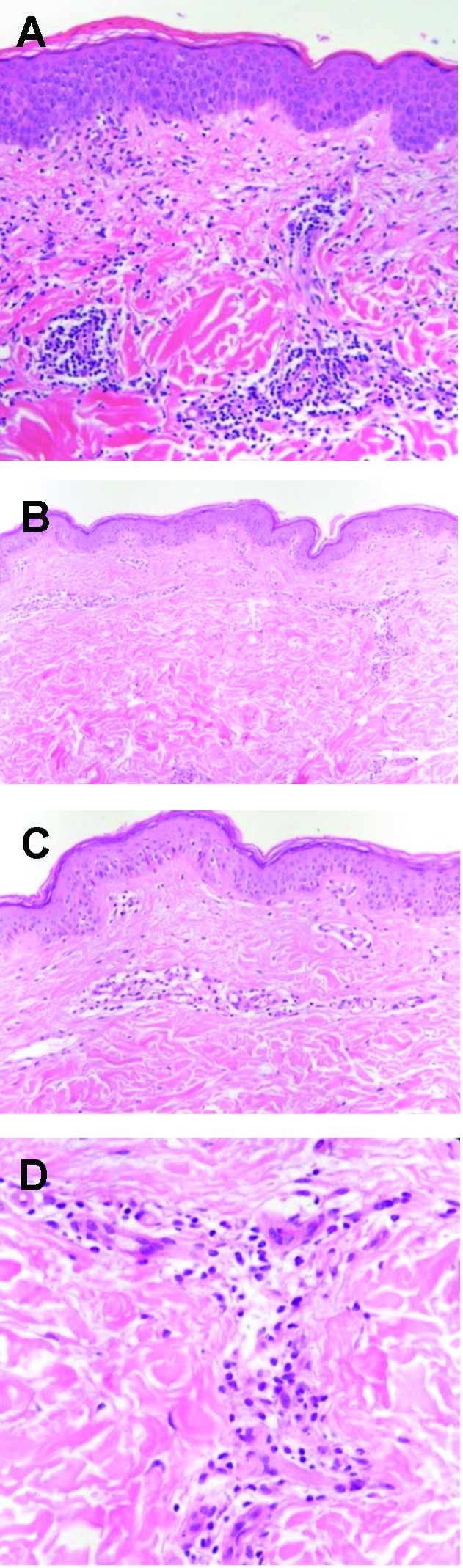
A, Skin biopsy specimen from a patient with typical delayed type IV hypersensitivity (DTH) reaction to heparin, showing epidermal spongiosis and dermal infiltration with lymphocytes and eosinophils, characteristic of a DTH reaction (hematoxylin-eosin; original magnification, x200). B-D, Skin biopsy specimens from the only study patient with a DTH reaction to fondaparinux, showing a mild, mainly perivascular dermal infiltration, predominantly with lymphocytes and to a low degree with eosinophils, typical of a DTH reaction. There are no microthromboses in dermal vessels; these would be suggestive of the presence of heparin-induced thrombocytopenia (B-D, hematoxylineosin; original magnification, x100, x200, x400, respectively).
PATIENTS AND METHODS
From September 1, 2008, through April 30, 2009, hospitalized patients at the J. W. Goethe University Hospital in Frankfurt am Main, Germany, who received subcutaneous anticoagulant therapy with fondaparinux sodium (Arixtra, GlaxoSmithKline, Munich, Germany) were screened prospectively and consecutively for study eligibility. In-patients from the departments of internal medicine, dermatology, surgery, and gynecology treated with fondaparinux were seen 3 to 4 times per week until discharged. Those aged 18 years or older who had received prophylactic or therapeutic fondaparinux treatment for a minimum duration of 7 days were eligible for study participation. Doses given were 1.5 mg/d, 2.5 mg/d, 5 mg/d, 7.5 mg/d, or 10 mg/d. Patients with a history of DTH reactions to fondaparinux, those with preexisting skin disorders involving potential fondaparinux injection areas, and those undergoing immunosuppressive therapy were excluded. Patients with a known DTH reaction to heparins were not excluded. Outcome measures were incidence and causes of fondaparinux-induced skin reactions. Investigations and procedures were approved by the local ethics committee of the J. W. Goethe University (16/07). The study was registered at clinicaltrials.gov (NCT00510432) and performed in accordance with the Declaration of Helsinki. All enrolled patients who received prophylactic or therapeutic fondaparinux provided written informed consent for study participation.
Study Procedures
Eligible patients were evaluated by 1 or more investigators; initially, the medical history, including age, sex, reason for admission, indication for fondaparinux therapy, pregnancy, use of female sex hormones, and previous exposure to anticoagulant therapy, was obtained from the patients. Information regarding current anticoagulant therapy (preparation, duration, dose) as well as height and weight for calculation of body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]) were obtained from patients' clinical records. This was followed by clinical examination of the local fondaparinux injection sites for skin lesions and/or pruritus, as well as a full body examination of the skin. If a fondaparinux-induced skin lesion was suspected, the patient was evaluated by a second investigator. Skin biopsy specimens from suspected fondparinux-induced lesions were obtained with the patient under local anesthesia and routinely processed for hematoxylin-eosin staining. Sections were evaluated by 2 experienced dermatopathologists (M.W. and R.K.). The presence of spongiosis and leukocyte infiltration (lymphocytes and eosinophils in particular) was considered to represent a DTH reaction.7
A skin lesion resulting from HIT was diagnosed histologically if dermal vessels were occluded. To rule out HIT, all patients with suspected fondaparinux-induced skin lesions underwent platelet count monitoring and were tested for HIT antibodies at onset of skin lesions and again 7±1 days afterward (platelet factor 4/heparin–enzyme-linked immunosorbent assay [Asserachrom HPIA Diagnostica Stago, Paris, France] and heparin-induced platelet activation assay) (as described elsewhere16) to capture cases of delayed antibody development. To rule out any thromboembolic complications that might possibly occur during HIT, all patients, and notably those with suspected fondaparinux-induced skin lesions, were screened clinically for signs of new or progressive deep venous thrombosis (DVT) according to the Wells score.17 To rule out HIT, a clinical pretest probability score18 was applied. Patients also underwent further diagnostics, ie, ultrasonography and laboratory testing, if needed. If HIT was excluded, all patients with skin lesions underwent allergologic testing with undiluted original drug formulations, as described elsewhere.8
Statistical Analyses
Age, BMI, and the time of onset of skin lesions while patients were receiving fondaparinux therapy were calculated as mean ± SD or median and range. Sample size calculation was performed with Bias 8.4.6. software (Epsilon, Hochheim Darmstadt, Germany). A sample size of at least 168 patients was calculated to determine the incidence of suspected fondaparinux-induced skin lesions with a precision of at least ±1.5% with a 95% confidence interval.
RESULTS
Of 244 patients screened, 231 were enrolled. The reasons for exclusion included preexisting inflammatory skin diseases with involvement of possible injection areas (n=3) and loss to follow-up (n=10). No patient was excluded because of antecedent allergy to fondaparinux.
Of the 231 study patients, 115 were women, and 116 were men. The mean ± SD age of the patients was 59.4±18.8 years (women, 62.0±18.6 years; men, 56.7±18.7 years). The mean ± SD BMI was 26.2±5.9 (women, 26.4±6.3; men, 26.0±5.5). The median duration of fondaparinux therapy was 11 days (range, 7-274 days). Of the 231 patients, 153 (66.2%) received anticoagulant therapy for a prophylactic indication (Table 1). Of these 153 patients, 152 received 2.5 mg of fondaparinux subcutaneously once daily; 1 patient received an adjusted dose of 1.5 mg of fondaparinux because of underlying renal insufficiency. Of the 231 patients, 74 (32.0%) received an adjusted therapeutic dose of fondaparinux according to body weight: 1 patient (1.4%) was treated with 5 mg, 66 (89.2%) were treated with 7.5 mg, and 1 (1.4%) with 10 mg of fondaparinux subcutaneously once daily. Six women (8.1%) were therapeutically treated with a reduced dose of 2.5 mg: 1 with DVT and pulmonary embolism (PE) due to underlying thrombocytopenia (this patient was enrolled twice), 1 with digital artery occlusions and antiphospholipid syndrome due to chronic bleeding that led to anemia, 1 with subclavian vein thrombosis due to malignant hemorrhagic pericardial effusion, 1 with PE due to hemorrhagic cystitis, and 2 with minor peripheral superficial thrombophlebitis. In 3 patients, a change from a prophylactic to a therapeutic dose of fondaparinux was necessary: in 2 the change was due to a new postoperative DVT after multiple bone fractures, and in 1 with DVT the dose was slowly increased because of a progressively decreasing bleeding risk after recovery from polytrauma. In one patient, a therapeutic dose of fondaparinux was started because of initial clinical suspicion of PE, but this dose was later changed to a prophylactic dose after exclusion of thromboembolism.
TABLE.
Characteristics of the Study Cohort
Low Incidence of Cutaneous Reactions to Fondaparinux
None of the 231 patients experienced local erythema characteristic of a DTH reaction at the fondaparinux injection sites. One 44-year-old woman (height, 1.63 m; weight, 93.0 kg; BMI, 35.0) who received fondaparinux therapy at 7.5 mg once daily because of postoperative DVT experienced a slight but persistent itching of the abdominal wall at the injection sites and at surrounding areas starting on day 9 after therapy initiation. Clinically, the skin looked normal without erythematous lesions at the injection sites (Figure 1, middle). To rule out any subclinical manifestation of fondaparinux-induced DTH reaction, a skin biopsy was performed. Histologic study revealed a mild lymphohistiocytic infiltrate that confirmed a mild DTH reaction to fondaparinux (Figure 2, B-D). HIT was ruled out serologically and by the histologic absence of thrombotic microvascular dermal occlusions. Fondaparinux administration was continued temporarily, while phenprocoumon therapy was initiated. In the patient's subsequent clinical course, no further skin reactions were observed. A DTH reaction was subsequently confirmed by subcutaneous skin provocation with fondaparinux; pruritus without erythematous skin lesions recurred at fondaparinux injection sites. Because a recent investigation indicated a low sensitivity of intracutaneous allergy testing for DTH reactions to heparins, we discontinued the practice of obtaining further intracutaneous allergy testing.19
Of the 231 patients, 155 (67.1%) had a former exposure to heparins in their medical history; 6 (2.6%) of the 231 patients had a reexposure to fondaparinux. None of the patients in these 2 subgroups developed a DTH reaction.
In summary, the overall incidence of DTH reactions to fondaparinux therapy was 0.4% (1/231; 95% confidence interval, 0.01%-2.4%). There was no association between DTH reactions to fondaparinux and HIT. The only patient who had a fondaparinux-induced DTH reaction had negative findings on 2 tests for HIT and did not develop other sequelae of HIT. The clinical pretest probability score for HIT was 1, and the Wells score for DVT was 0.
Lack of Cross-reactivity With Fondaparinux in 5 Patients With Known Cutaneous Heparin Allergy
Of the 231 patients, 5 (2.2%) had reported a history of cutaneous DTH reactions: 4 patients had had a reaction to nadroparin and 1 to danaparoid. During therapy with fondaparinux, none of these patients developed a cutaneous DTH reaction.
Low Prevalence of new or Progressive Thrombosis and Lack of HIT With Fondaparinux Therapy
According to the Wells score,17 we identified 26 (11.3%) of 231 patients with more than 3 points for a high clinical DVT probability. In 23 (88.5%) of these 26 patients, new or progressive DVT was ruled out sonographically. Two (7.7%) of these 26 patients developed a new thrombosis postoperatively after polytrauma and multiple bone fractures despite prophylactic fondaparinux therapy. Testing for HIT was negative in both patients. A good clinical outcome was achieved after the fondaparinux dose was increased to a therapeutic dose. One (3.9%) of 26 patients developed a progressive thrombosis due to intentional subtherapeutic dosing because of a malignant hemorrhagic pericardial effusion. Testing for HIT was negative.
None of the remaining study patients developed a clinically overt HIT. None of the patients had a high score according to the well-established pretest probability score for HIT.18
DISCUSSION
In the current study, we prospectively examined the incidence of allergic cutaneous adverse reactions to the subcutaneously administered pentasaccharide fondaparinux. Overall, the incidence was 0.4%. This is substantially lower than the incidence of DTH reactions to heparins, which is at least 7.5%.7 Therefore, fondaparinux-induced allergic skin lesions should be considered an infrequent adverse drug reaction compared with heparins. The median duration of exposure to fondaparinux as well as the sex and age characteristics in the current study were similar to those of the aforementioned study.7 In our study cohort, recurrent exposure to fondaparinux, former exposure to heparins, or long-term fondaparinux therapy did not influence the rate of fondaparinux-induced DTH reactions Thus, our data strongly support our initial hypothesis that fondaparinux has a lower sensitizing potential compared with the porcine gut–derived UFHs and LMWHs.
Structural Considerations
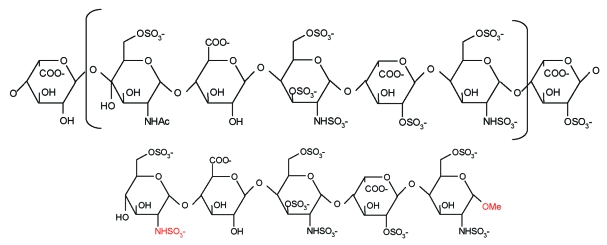
The lower sensitizing potential of fondaparinux might be due to its synthetic origin, which also provides a high intercharge and intracharge consistency compared with the heparins. Heparins represent a mixture of polysaccharide chains of different lengths and molecular weights ranging from 3000 to 10,000 Da (LMWH) and from 3000 to 30,000 Da (UFH). These polysaccharides contain the antithrombin-binding pentasaccharide sequence in up to 30% of molecules,20 and only polysaccharide chains with a critical length of at least 15 to 18 saccharides show an interaction with thrombin via a thrombin-binding site,21 which is less specific than that of the antithrombin-binding domain. Thus, the variable, polyanionic, negatively charged polysaccharide chains of heparins are likely to generate nonspecific binding reactions with other molecules and may induce sensitization. The minimal carbohydrate chain required for binding and activation of antithrombin is a pentasaccharide sequence.21 The synthetic pentasaccharide fondaparinux differs from heparins only by the presence of an N-sulfate group instead of an N-acetyl group at monosaccharide unit D and by introduction of a methyl group that stabilizes the reactive aldehyde at the reducing end of monosaccharide unit H21 (Figure 3). This increases the affinity toward antithrombin (dissociation constant, 36 nM) as well as anti-Xa activity compared with the natural heparin pentasaccharide22; thus, the ultra-low-molecular-weight pentasaccharide fondaparinux (1.728 kDa) is almost exclusively bound to antithrombin (>94%).23 Together with its low charge density due to lower degree of sulfation compared with heparins,24 fondaparinux is physically too short to exhibit the aforementioned interactions with thrombin and possibly with other proteins as well. Thus, fondaparinux might not serve as a hapten and might bind less effectively to other dermal proteins to acquire full antigenicity to be recognized and processed by dendritic cells, which is a required step for induction of DTH reactions. Additionally, these structural properties might explain the low incidence of HIT with fondaparinux therapy, which hinders binding with platelet factor 4 and subsequent generation of the pathogenic complex for induction of HIT.24
FIGURE 3.
Molecular structures of the antithrombin-binding pentasaccharide sequence of heparin (top) and fondaparinux (bottom).
The expected lower antigenicity of fondaparinux corresponds to our own observations that the sensitization potential decreases with decreasing molecular weight of an anticoagulant.8,25 The overlap in the polysaccharide composition of the different LMWHs and UFHs might explain the high degree of cross-reactivity among the different heparins,4,5,26 and thus the high degree of tolerance to fondaparinux therapy in patients with multiple cross-allergic reactions to heparins9,10,26 and in our 5 study patients with a known cutaneous heparin allergy. Therefore, we hypothesize that the allergenic epitope might not consist of the pentasaccharide sequence that the fondaparinux molecule shares with the different heparins. It is possible that molecular weight–dependent quarternary structures of ultralarge multimeric lattices of colinked heparin molecules27,28 that could not be detected among fondaparinux molecules28 are responsible for the graduated antigenicity of heparins.
Clinical Considerations
A lower antigenicity should not be the only rationale for choosing an appropriate anticoagulant. Instead, we propose an individualized anticoagulant regimen that takes into account positive and adverse side-effect profiles of each drug and clinical risk profiles of each patient to improve therapeutic safety. Because female sex, obesity, and long duration of therapy are risk factors for DTH reactions to heparins,4,7 fondaparinux treatment should be warranted in these patients. Also, in cases of immediate or previous DTH reactions to heparins, fondaparinux should be preferred because of the high degree of cross-allergies among different heparins.4 Because pregnant women taking heparins are more likely to experience DTH reactions and because approved therapeutic options for such women are limited,7,29,30 more data on fondaparinux therapy, which is not approved for use during pregnancy,31 are desirable.
In patients with malignant diseases, UFH therapy with nadroparin or dalteparin, rather than fondaparinux therapy, might be more beneficial because these drugs decrease P-selectin–mediated metastasis.32 Patients with a high risk of bleeding complications or renal insufficiency and those undergoing surgery should receive UFH therapy because of its shorter half-life and because its effects can be antagonized/reversed by protamine. Furthermore, intravenously administered UFH has been shown to be tolerated in patients who have a DTH reaction to subcutaneously administered heparins.33 Patients with a long-term indication for subcutaneous anticoagulant therapy, patients with underlying osteoporosis, or those who experience bone fracture might benefit more from fondaparinux than from LMWH therapy because in vitro data have shown that fondaparinux does not inhibit the proliferation of osteoblasts.34
Although our data suggest a negligible incidence of fondaparinux-induced skin lesions, we still recommend application of the easy-to-perform and clinically well-established pretest probability score for HIT18 in cases of skin lesions because they are considered one of the main sequelae of HIT18 and may progress to necrosis (Figure 1, bottom) and because it remains uncertain whether fondaparinux therapy can induce HIT.35,36
Limitations
Because our study consisted of only 5 patients with a known DTH reaction to LMWH/danaparoid, we cannot conclude with certainty that fondaparinux has no cross-allergies with heparins; however, several case reports4,5,26 and our own observations of more than 30 patients with DTH reactions to heparins (M.S., E.L.-L., R.J.L., unpublished data, December 15, 2009) and tolerance to fondaparinux suggest it is possible. To minimize possible biases, several investigators (M.S., J.S., H.K., M.W., R.K., E.L.-L., R.J.L.) performed examinations in duplicate and confirmed the findings with various methods, ie, platelet factor 4/heparin–enzyme-linked immunosorbent assay, heparin-induced activation assay, platelet count monitoring, clinical assessment and follow-up examinations, allergy testing, and histologic studies.
CONCLUSION
The incidence of DTH reactions during therapy with the heparin-derived synthetic anticoagulant fondaparinux (0.4%) is significantly lower compared to that with UFH or LMWH (7.5%). Because of the low allergenic potential, the known lower risk of HIT, and a favorable outcome regarding thromboembolic complications compared with the heparins, fondaparinux should be considered if an individualized anticoagulant regimen is being chosen to improve patient care, enhance therapeutic safety, and increase cost-effectiveness.
REFERENCES
- 1.Petitou M, Lormeau JC, Choay J. Chemical synthesis of glycosaminoglycans: new approaches to antithrombotic drugs. Nature. 1991;350(6319, suppl):30-33 [DOI] [PubMed] [Google Scholar]
- 2.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6, suppl):454S-545S [DOI] [PubMed] [Google Scholar]
- 3.Harrington RA, Becker RC, Cannon CP, et al. Antithrombotic therapy for non-ST-segment elevation acute coronary syndromes: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6, suppl):670S-707S [DOI] [PubMed] [Google Scholar]
- 4.Ludwig RJ, Schindewolf M, Utikal J, Lindhoff-Last E, Boehncke WH. Management of cutaneous type IV hypersensitivity reactions induced by heparin. Thromb Haemost. 2006;96(5):611-617 [PubMed] [Google Scholar]
- 5.Trautmann A, Seitz CS. Heparin allergy: delayed-type non-IgE-mediated allergic hypersensitivity to subcutaneous heparin injection. Immunol Allergy Clin North Am. 2009;29(3):469-480 [DOI] [PubMed] [Google Scholar]
- 6.Scherer K, Tsakiris DA, Bircher AJ. Hypersensitivity reactions to anticoagulant drugs. Curr Pharm Des. 2008;14(27):2863-2873 [DOI] [PubMed] [Google Scholar]
- 7.Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. CMAJ. 2009;181(8):477-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludwig RJ, Schindewolf M, Alban S, Kaufmann R, Lindhoff-Last E, Boehncke WH. Molecular weight determines the frequency of delayed type hypersensitivity reactions to heparin and synthetic oligosaccharides. Thromb Haemost. 2005;94(6):1265-1269 [DOI] [PubMed] [Google Scholar]
- 9.Schindewolf M, Ludwig RJ, Wolter M, et al. Tolerance of fondaparinux in patients with generalized contact dermatitis to heparin. J Eur Acad Dermatol Venereol. 2008;22(3):378-380 [DOI] [PubMed] [Google Scholar]
- 10.Sacher C, Hunzelmann N. Tolerance to the synthetic pentasaccharide fondaparinux in heparin sensitization. Allergy. 2003;58(12):1318-1319 [DOI] [PubMed] [Google Scholar]
- 11.Hirsch K, Ludwig RJ, Lindhoff-Last E, Kaufmann R, Boehncke WH. Intolerance of fondaparinux in a patient allergic to heparins. Contact Dermatitis. 2004;50(6):383-384 [DOI] [PubMed] [Google Scholar]
- 12.Utikal J, Peitsch WK, Booken D, et al. Hypersensitivity to the pentasaccharide fondaparinux in patients with delayed-type heparin allergy. Thromb Haemost. 2005;94(4):895-896 [DOI] [PubMed] [Google Scholar]
- 13.Warkentin TE. Heparin-induced skin lesions. Br J Haematol. 1996;92(2):494-497 [DOI] [PubMed] [Google Scholar]
- 14.Harenberg J, Huhle G, Wang L, Hoffmann U, Bayerl C, Kerowgan M. Association of heparin-induced skin lesions, intracutaneous tests, and heparin-induced IgG. Allergy. 1999;54(5):473-477 [DOI] [PubMed] [Google Scholar]
- 15.Visentin GP, Ford SE, Scott JP, Aster RH. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J Clin Invest. 1994;93(1):81-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakchoul T, Giptner A, Najaoui A, Bein G, Santoso S, Sachs UJ. Prospective evaluation of PF4/heparin immunoassays for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7(8):1260-1265 [DOI] [PubMed] [Google Scholar]
- 17.Wells PS, Hirsh J, Anderson DR, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345(8961):1326-1330 [DOI] [PubMed] [Google Scholar]
- 18.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4(4):759-765 [DOI] [PubMed] [Google Scholar]
- 19.Schindewolf M, Ludwig RJ, Wolter M, et al. Diagnosis of heparin-induced delayed type hypersensitivity. Phlebologie. 2010;39(4):226-231 [Google Scholar]
- 20.Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1, suppl):64S-94S [DOI] [PubMed] [Google Scholar]
- 21.van Boeckel CA, Petitou M. The unique antithrombin III binding domain of heparin: a lead to new synthetic antithrombotics. Angew Chem Int Ed Engl. 1993:32(12):1671-1690 [Google Scholar]
- 22.Petitou M, van Boeckel CA. A synthetic antithrombin III binding pentasaccharide is now a drug! what comes next? Angew Chem Int Ed Engl. 2004;43(24):3118-3133 [DOI] [PubMed] [Google Scholar]
- 23.Paolucci F, Clavies MC, Donat F, Necciari J. Fondaparinux sodium mechanism of action: identification of specific binding to purified and human plasma-derived proteins. Clin Pharmacokinet. 2002;41(suppl 2):11-18 [DOI] [PubMed] [Google Scholar]
- 24.Greinacher A, Alban S, Dummel V, Franz G, Mueller-Eckhardt C. Characterization of the structural requirements for a carbohydrate based anticoagulant with a reduced risk of inducing the immunological type of heparin-associated thrombocytopenia. Thromb Haemost. 1995;74(3):886-892 [PubMed] [Google Scholar]
- 25.Ludwig RJ, Schindewolf M, Lindhoff-Last E, Boehncke WH. The influence of heparin's molecular weight and the incidence of delayed type hypersensitivity reactions revisited; [in response to Grims et al, Br J Dermatol 2007;157:514-17.] Br J Dermatol. 2008;158(4):849-851 [DOI] [PubMed] [Google Scholar]
- 26.Jappe U, Juschka U, Kuner N, Hausen BM, Krohn K. Fondaparinux: a suitable alternative in cases of delayed-type allergy to heparins and semisynthetic heparinoids? a study of 7 cases. Contact Dermatitis. 2004;51(2):67-72 [DOI] [PubMed] [Google Scholar]
- 27.Rauova L, Poncz M, McKenzie SE, et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105(1):131-138 [DOI] [PubMed] [Google Scholar]
- 28.Greinacher A, Gopinadhan M, Gunther JU, et al. Close approximation of two platelet factor 4 tetramers by charge neutralization forms the antigens recognized by HIT antibodies. Arterioscler Thromb Vasc Biol. 2006;26(10):2386-2393 [DOI] [PubMed] [Google Scholar]
- 29.Bank I, Libourel EJ, Middeldorp S, Van Der Meer J, Buller HR. High rate of skin complications due to low-molecular-weight heparins in pregnant women. J Thromb Haemost. 2003;1(4):859-861 [DOI] [PubMed] [Google Scholar]
- 30.Schindewolf M, Magnani HN, Lindhoff-Last E. Danaparoid in pregnancy in cases of heparin intolerance—use in 59 cases [in German]. Hamostaseologie. 2007;27(2):89-97 [PubMed] [Google Scholar]
- 31.Dempfle CE. Minor transplacental passage of fondaparinux in vivo. N Engl J Med. 2004;350(18):1914-1915 [DOI] [PubMed] [Google Scholar]
- 32.Ludwig RJ, Alban S, Bistrian R, et al. The ability of different forms of heparins to suppress P-selectin function in vitro correlates to their inhibitory capacity on bloodborne metastasis in vivo. Thromb Haemost. 2006;95(3):535-540 [DOI] [PubMed] [Google Scholar]
- 33.Boehncke WH, Weber L, Gall H. Tolerance to intravenous administration of heparin and heparinoid in a patient with delayed-type hypersensitivity to heparins and heparinoids. Contact Dermatitis. 1996;35(2):73-75 [DOI] [PubMed] [Google Scholar]
- 34.Handschin AE, Trentz OA, Hoerstrup SP, Kock HJ, Wanner GA, Trentz O. Effect of low molecular weight heparin (dalteparin) and fondaparinux (Arixtra) on human osteoblasts in vitro. Br J Surg. 2005;92(2):177-183 [DOI] [PubMed] [Google Scholar]
- 35.Schindewolf M, Lindhoff-Last E. Fondaparinux-related thrombocytopenia in a patient with former HIT. [Response to Rota, et al. (Thromb Haemost 2008;99:779-781) letter reply]. Thromb Haemost. 2008;100(1):168-169 [DOI] [PubMed] [Google Scholar]
- 36.Warkentin TE, Maurer BT, Aster RH. Heparin-induced thrombocytopenia associated with fondaparinux. N Engl J Med. 2007;356(25):2653-2655 [DOI] [PubMed] [Google Scholar]