Abstract
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant plasma cell disorder that is associated with a lifelong risk of multiple myeloma. We conducted a systematic review of all studies investigating the prevalence and incidence of MGUS in the online database PubMed. The review was conducted from January 6, 2009, through January 15, 2010. The following MeSH search headings were used: monoclonal gammopathy, benign and prevalence; monoclonal gammopathy, benign and incidence; paraproteinemia and prevalence; and paraproteinemia and incidence. Articles were limited to those written in English and published by January 2009. Fourteen studies that met prespecified criteria were included and systematically assessed to identify the most accurate prevalence estimates of MGUS based on age, sex, and race. On the basis of our systematic review, we estimate that the crude prevalence of MGUS in those older than 50 years is 3.2% in a predominantly white population. Studies in white and Japanese populations demonstrate a clear increase in prevalence with age. The prevalence is also affected by sex: 3.7% and 2.9% in white men and women, respectively; and 2.8% and 1.6% in Japanese men and women, respectively. Additionally, MGUS is significantly more prevalent in black people (5.9%-8.4%) than in white people (3.0%-3.6%). We conclude that MGUS is a common premalignant plasma cell disorder in the general population of those older than 50 years. The prevalence increases with age and is affected by race, sex, family history, immunosuppression, and pesticide exposure. These results are important for counseling, clinical care, and the design of clinical studies in high-risk populations.
HIV = human immunodeficiency virus; IMWG = International Myeloma Working Group; MGUS = monoclonal gammopathy of undetermined significance
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant, asymptomatic disorder characterized by monoclonal plasma cell proliferation in bone marrow with absence of end-organ damage.1 Although historically considered a benign condition, patients with MGUS have a lifelong risk of multiple myeloma, an incurable plasma cell malignancy with a median survival of approximately 4 to 5 years.2-4 Patients with MGUS are also at risk of related disorders, such as light-chain amyloidosis and macroglobulinemia. Conditions such as osteoporosis, hip fractures, and peripheral neuropathy are also associated with MGUS.5
The rate at which MGUS progresses to multiple myeloma or a related disorder is 1% per year.6,7 The probability of progression at 25 years of follow-up is approximately 30%. However, after accounting for competing causes of death, true life-time probability of progression is lower (11%).8 The risk of progression with MGUS does not diminish over time and persists even in patients whose condition has remained stable for decades.7,9 This fixed risk of progression regardless of duration of disease suggests a random 2-hit model of progression rather than a cumulative damage accumulation model in which the risk of progression would be expected to increase with duration of MGUS. The main risk factors for progression of clinical MGUS are size and type of serum M protein and presence of an abnormal serum free light chain ratio.10
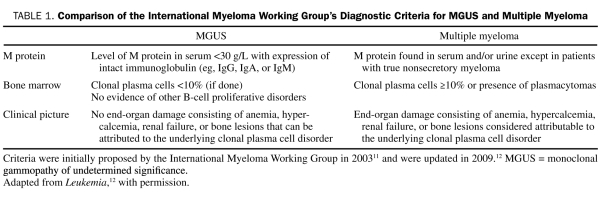
The International Myeloma Working Group (IMWG) has established a precise definition of MGUS (Table 1).11,12 This definition allows clinicians to diagnose MGUS using routinely available investigations. Despite uniform diagnostic criteria, prevalence and incidence estimates of MGUS vary considerably. Because MGUS is the premalignant stage that precedes multiple myeloma, accurate studies of the descriptive epidemiology of MGUS are necessary to provide assessments of disease burden. These investigations may elucidate etiologies of MGUS and can identify high-risk groups who would benefit from screening programs and preventive strategies to reduce mortality from myeloma.
TABLE 1.
Comparison of the International Myeloma Working Group's Diagnostic Criteria for MGUS and Multiple Myeloma
MGUS is one of the most prevalent premalignant disorders in the world among people aged 50 years or older. The high prevalence in the general population has made the incidental diagnosis of MGUS with numerous other diseases a common clinical occurrence. In these instances, it is impossible to determine if a causal relationship exists without accurate expected rates of MGUS in the given patient population. Moreover, accurate estimates of prevalence in various risk groups allow us to determine potential etiologic factors.
This article is a systematic review of the published literature on the prevalence and descriptive epidemiology of MGUS. Because of the chronic nature of MGUS, prevalence estimates are most appropriate to assess health burden, and our review will mainly focus on these measures. Studies that assess the incidence of MGUS are also highlighted because incidence data can provide insight into risk factors and etiologies of disease. We address the influence of methodological factors on prevalence estimates of MGUS, including estimates of MGUS by age, sex, and race. Finally, we summarize potential associations between the prevalence and incidence of MGUS and immune status, family history, and occupational/environmental risk factors.
METHODS
Data Sources and Searches
This review was conducted from January 6, 2009, through January 15, 2010. A systematic literature search of PubMed was completed to identify all studies investigating the prevalence and incidence of MGUS. The following MeSH search headings were used: monoclonal gammopathy, benign and prevalence; monoclonal gammopathy, benign and incidence; paraproteinemia and prevalence; and paraproteinemia and incidence. Articles were limited to those written in English and published by January 2009.
Study Selection
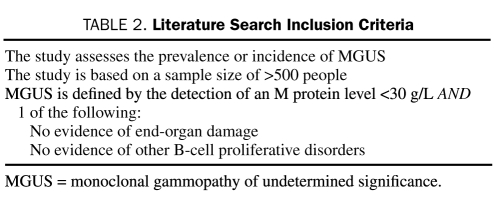
Abstracts and available full texts of articles resulting from the MeSH search were reviewed manually by one investigator (R.K.W.) to determine which articles met the inclusion criteria listed in Table 2. This process was then repeated to verify results. The preliminary search yielded 460 articles, 14 of which met the inclusion criteria for this review.
TABLE 2.
Literature Search Inclusion Criteria
Additionally, a separate literature search was carried out to explore associations between the prevalence or incidence of MGUS and the following: immune status, family history of monoclonal gammopathies, and occupational or environmental risk factors. These studies were included in this review to emphasize the multiple factors that can influence the prevalence of MGUS.
Data Extraction, Synthesis, and Quality Assessment
One investigator (R.K.W.) extracted information pertaining to the characteristics of each study population, types of screening or diagnostic tests used, and prevalence or incidence estimates of MGUS. Extracted information and data were then rereviewed. First, all 14 studies were verified to ensure that MGUS was defined using either the IMWG criteria11,12 or the standard definition of MGUS used in clinical practice and in the most recent large epidemiological studies that assessed the natural history and prevalence of MGUS.7,13 The latter definition differs from the IMWG criteria in that a bone marrow biopsy is considered an optional component of the definition. Few studies of MGUS meet the IMWG criteria of MGUS because it is not practical to incorporate routine bone marrow biopsies in normal, asymptomatic individuals in large-scale studies. Next, we assessed the quality of each study to address the key questions of this review. For the key question of crude overall prevalence of MGUS in the adult population, we extracted data from all studies that captured prevalence estimates, but at the data synthesis stage preference was given to studies that were population-based, used modern electrophoretic methods, or provided data on patients aged 40 years or older, which is the target population of interest clinically. Prevalence estimates from studies restricted to a specific racial or ethnic group and from those that lacked a lower age limit were also extracted but were not considered relevant for this key question in the data synthesis stage. To address key questions pertaining to prevalence of MGUS by age and sex, only studies that reported a breakdown of prevalence by these features were included. For these questions, we excluded studies that were hospital-based, did not use modern electrophoretic methods, and intentionally oversampled for certain ethnic groups. For sex-specific rates, we excluded studies in which the rate for adults aged 40 years or older could not be computed from the data in the relevant articles. For key questions pertaining to race- and ethnicity-based prevalence estimates, the same criteria were used with the exception that studies that oversampled for certain racial groups were included, provided that data pertaining to that specific racial group could be extracted from the article.
RESULTS
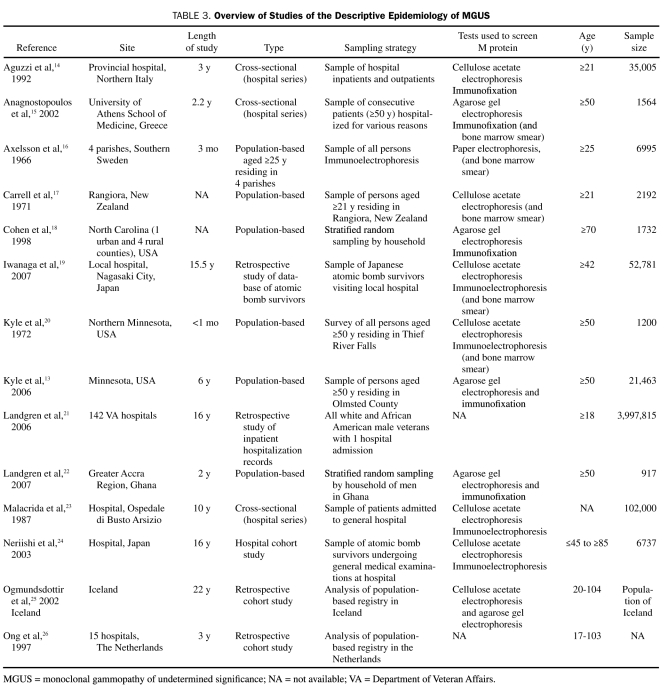
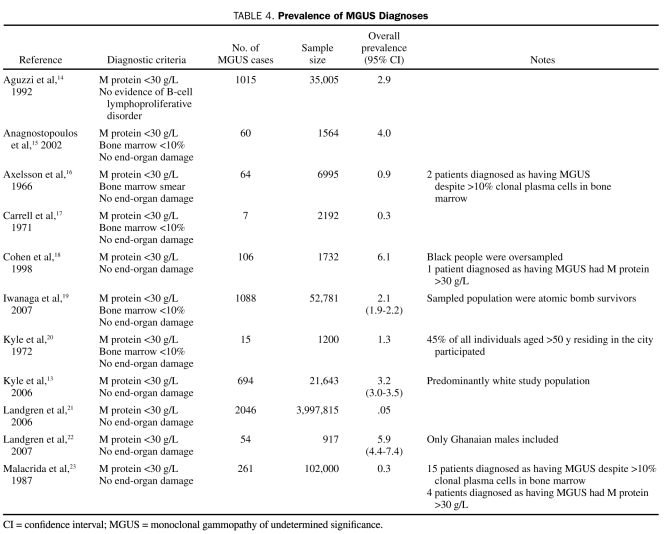
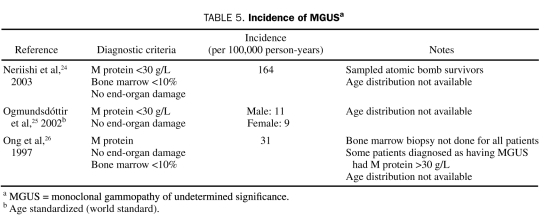
Of the 14 studies pertaining to the epidemiology of MGUS (Table 3), 11 (79%) included prevalence measures (Table 4). Of these, 6 (55%) were population-based, 3 (27%) were studies of hospital populations, and 2 (18%) were retrospective. The remaining 3 studies, 2 of which were retrospective, registry-based studies and one of which was a hospital series study, provided estimates of the incidence of MGUS (Table 5).
TABLE 3.
Overview of Studies of the Descriptive Epidemiology of MGUS
TABLE 4.
Prevalence of MGUS Diagnoses
TABLE 5.
Incidence of MGUSa
Prevalence of MGUS
The prevalence measures of MGUS from 11 different studies ranged from 0.05% to 6.1% (Table 4). The demographic composition (in terms of age, race, and sex) of the study cohorts varied by study. Of the studies reviewed, Cohen et al18 in the United States reported the highest prevalence of MGUS (6.1%). In Greece, Anagnostopoulos et al15 reported the next highest prevalence value (4.0%) in a cohort of approximately 1600 consecutive hospital patients. The third and fourth highest prevalence estimates of MGUS were reported in population-based studies by Landgren et al22 and Kyle et al.13 Of interest is the fact that all 3 studies used the most sensitive methods available to detect M protein (initial agarose gel electrophoresis and immunofixation for those with abnormal findings on electrophoresis). Additionally, the populations assessed in all 3 studies were at least 50 years of age. In contrast, Malacrida et al,23 Carrell et al,17 and Landgren et al21 reported the 3 lowest prevalence estimates of MGUS (0.3%, 0.3%, and 0.05%, respectively). All 3 studies included young participants in their analysis. Additionally, cellulose acetate, a less sensitive test for M protein, was used by Malacrida et al23 and Carrell et al.17 Finally, in Italy, Aguzzi et al14 used a hospital cohort of 35,005 individuals to estimate the prevalence of MGUS.
Only 5 studies used all criteria established by IMWG (including bone marrow biopsy) to diagnose MGUS in their respective study populations. Carrell et al17 evaluated 82% of the adult population in Rangiora, New Zealand, and diagnosed 0.3% of study participants as having MGUS using all criteria. In southern Sweden, Axelsson et al16 sampled 70% of all persons older then 25 years living in 4 parishes. Only 0.9% of the study population was found to have MGUS. Kyle et al20 surveyed those older than 50 years in one town and reported the prevalence of MGUS to be 1.3%. In addition to these studies, the aforementioned work by Anagnostopoulos et al15 and a large-scale study by Iwanaga et al19 also adhered to IMWG diagnostic criteria.
Two studies assessed the prevalence of MGUS in specific races. In a population-based study of Ghanaian men, Landgren et al22 found the prevalence of MGUS to be 5.9%. Iwanaga et al19 evaluated 52,802 Japanese atomic bomb survivors for MGUS and reported a prevalence of 2.1%.
Incidence
Data in the studies to determine accurate incidence rates for MGUS are limited. Incidence rates for MGUS ranged from 9 to 164 per 100,000 person-years in 3 studies (Table 5). The highest incidence rate was reported in a study of approximately 7000 atomic bomb survivors in Japan. Significantly lower incidence rates were found in retrospective registry-based studies by Ogmundsdóttir et al25 and Ong et al.26
Age
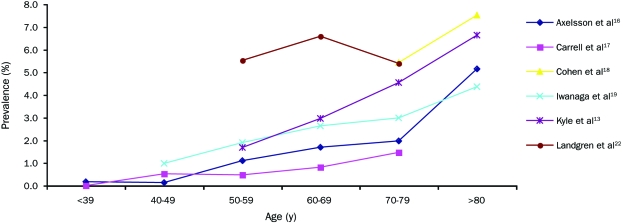
Six studies each reported prevalence data across several age groups (Figure 1). The largest of these studies by Kyle et al13 found the prevalence of MGUS to be 4-fold higher in those older than 80 years (6.6%) compared with those aged 50 to 59 years (1.7%) in a sample of approximately 22,000 people. The remaining 5 prevalence studies were not included in this figure either because they did not assess prevalence across differing age groups or because they were hospital-based investigations.
FIGURE 1.
Prevalence of monoclonal gammopathy of undetermined significance by age. (This illustration is in color online only.)
Sex
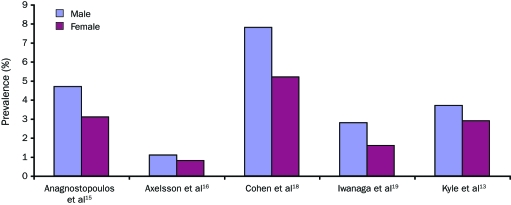
The prevalence of MGUS has been reported to be as much as 2-fold higher in males than in females.27 Figure 2 depicts 5 studies that contrasted the prevalence of MGUS between males and females. All studies included in this review consistently reported an increased prevalence of MGUS in males compared with females. The ratio of males affected by MGUS to females affected within each study ranged from 1.3 to 1.8.
FIGURE 2.
Prevalence of monoclonal gammopathy of undetermined significance in males vs females. (This illustration is in color online only.)
Race
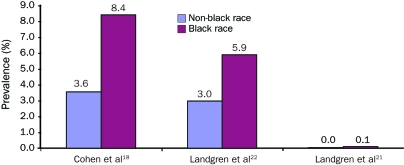
Figure 3 highlights apparent race-based differences in the prevalence of MGUS in blacks and whites. A community-based study of the elderly conducted by Cohen et al18 noted that prevalence of MGUS among blacks was approximately 2-fold higher than among whites. Landgren et al21 confirmed this finding in a large-scale study of inpatients from US Veterans Affairs hospitals, in which the frequency of MGUS was 3-fold higher in blacks than whites. This study also found no difference in rate of transformation to myeloma in blacks compared with whites, suggesting that the increased risk of myeloma in blacks was solely due to the increased prevalence of the precursor condition MGUS. Investigations that assess incidence of myeloma in African whites would aid in further delineating factors that influence risk of progression. Another large, population-based screening study by Landgren et al22 in Ghana found that the prevalence of MGUS in Ghanaian men was twice that of the white population in Minnesota. The studies by Landgren et al21,22 identified a similar prevalence of MGUS in Americans of African origin and in black African patients, suggesting that the increased risk of MGUS in African Americans may be based on racial predisposition rather than dietary or other environmental influences.
FIGURE 3.
Prevalence of monoclonal gammopathy of undetermined significance in nonblack vs black races. (This illustration is in color online only.)
The prevalence of MGUS in other racial/ethnic groups has not been well studied. There are limited data that the prevalence of MGUS is lower in the Japanese than in whites28 and that the prevalence in the admixed Mexican population is one quarter of that in white Americans.29
Immune Status
An increased incidence of MGUS is documented in immunosuppressed and/or immunocompromised patients. An association between MGUS and human immunodeficiency virus (HIV)–seropositive (immunocompromised) patients has been reported, but this association has not been well defined. Patients infected by HIV have a risk of MGUS that ranges from 9% to 45%, substantially higher than that of HIV-seronegative patients of the same age.30-35 Several studies also suggest that monoclonal gammopathy occurs at a younger age in those infected with HIV than in the general population.30,32,36,37 The incidence of MGUS is also higher in patients receiving forms of immunosuppressive therapy. For example, the incidence of MGUS is significantly greater in patients after renal transplant.38
Occupational and Environmental Risk Factors
Evidence pertaining to the influence of environmental factors on risk of MGUS has been inconsistent overall. However, a higher risk of MGUS has been demonstrated in farmers, industry workers, and those with a history of occupational or environmental exposure to toxins.39 Occupational exposure to asbestos, aromatic hydrocarbons, fertilizers, mineral oils and petroleum, paints and related products, pesticides, and radiation is also significantly associated with an increased risk of MGUS.40,41 A link also exists between heavy tobacco smoking and various hematologic conditions, including MGUS.42,43
Familial Clustering
Several small-scale reports suggest that, in some cases, familial predisposition to MGUS may be increased.44,45 Familial aggregation of multiple myeloma, the malignant stage that follows MGUS, has been recognized for years.46 Recently, a large population-based study in Sweden demonstrated a 2-fold increased risk of multiple myeloma among first-degree relatives of patients with multiple myeloma.47 In the same study, there was evidence of an increased risk of MGUS among relatives of patients with multiple myeloma. Furthermore, a prospective study found that the relative risk of MGUS in first-degree relatives of an MGUS proband was 3-fold higher than in the general population.48
Data Synthesis and Summary of the Evidence
Crude Prevalence. The studies assessed (Table 3) provide a wide range of prevalence estimates of MGUS (0.05%-6.1%). This variation among studies is most likely due to differences in the age, sex, and racial distribution of study participants as well as study design, laboratory techniques used, and diagnostic criteria. It is difficult to compare crude prevalence estimates between these studies because of dissimilarities related to the aforementioned factors. In order to determine the benchmark reference rate for crude prevalence of MGUS, we excluded hospital-based studies, those that did not use modern electrophoretic methods, those that tested only specific ethnic groups or oversampled for certain ethnic groups, and those that included young patients (aged <40 years). Only one of the 11 studies satisfied all of the above criteria (Kyle et al13). Therefore, using the population-based study of a predominantly white population by Kyle et al,13 the crude prevalence rate in those older than 50 years can be narrowed down to 3.2% of the general population. This estimate is affected by many factors, such as age, race, sex, and family history (a discussion of these follows), and can therefore be refined further if such information is available.
Prevalence of MGUS by Age and by Race/Ethnicity. The studies reviewed demonstrate unequivocally that the prevalence of MGUS consistently increases with age.13,16-19,22 The median age of diagnosis is 70 years, and less than 2% of patients with MGUS are younger than 40 years.13 Six of the investigations included in this review provided age-stratified breakdowns of prevalence (Figure 1). Of these, we excluded hospital-based studies, those that did not use modern electrophoretic methods, and those that intentionally oversampled for certain ethnic groups in order to determine the age-specific prevalence rate for MGUS.
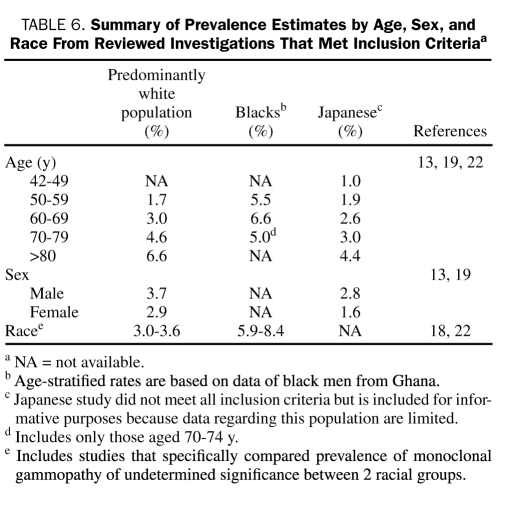
Three investigations were conducted independently in Sweden,16 New Zealand,17 and the United States13 in primarily white populations. Only the study in the United States used modern electrophoretic methods. Based on this study, Table 6 provides age-specific estimates of MGUS prevalence that likely reflect the actual prevalence of MGUS in predominantly white populations because this was a population-based study in a geographic area with a very low percentage of nonwhites. Anagnostopoulos et al15 demonstrated an age-related increase in prevalence of MGUS, but this study was a cross-sectional hospital series in design. Consequently, although the age gradient may be accurate, actual age-specific prevalence estimates for each age group are probably inflated because participants were derived from a pool of generally less healthy persons and are not representative of the general population. Thus, this study was not used in Table 6 for age-related prevalence estimates.
TABLE 6.
Summary of Prevalence Estimates by Age, Sex, and Race From Reviewed Investigations That Met Inclusion Criteriaa
There is a striking disparity in the prevalence of MGUS between blacks and whites.18 Table 6 also provides age-specific estimates of prevalence in Ghanaian males.22 The Ghanaian study used stratified random sampling to determine prevalence in an entirely black male population. Because this study was population-based and used modern electrophoretic methods, the estimates probably reflect the actual prevalence of MGUS in specific age groups in adult black men from western Africa.
The study in Japanese adults19 used the older cellulose actetate method and therefore does not meet the criteria required to be used for calculation of age-specific rates. However, this study is included in Table 6 because it provides age-stratified prevalence estimates of MGUS in a racial group for which no other data exist. This study investigated approximately 53,000 atomic bomb survivors; consequently, the estimates provided in this study for those of Japanese ancestry may be overestimated due to the link between radiation exposure and risk of MGUS. Furthermore, prevalence could be underestimated because an older, less sensitive electrophoretic method was used in this investigation.40,41
Table 6 also includes data from investigations that compared the prevalence of MGUS in different racial groups and met the criteria previously specified in the Methods section.18,22
Effect of Sex and Sex-Specific Prevalence Rates. The prevalence of MGUS differs significantly between men and women. Five studies characterized the prevalence of MGUS in males and females (Figure 2). Despite diminished comparability of male and female prevalence estimates between these investigations, a higher prevalence of MGUS is seen in men compared with women in each of the 6 respective studies. On average, the prevalence of MGUS is 1.5 times higher in men than in women.13,15,16,18,20,21,39,49 Differences in age composition of cohorts between studies influence sex-specific prevalence estimates because the condition is very rare in those younger than 40 years. For example, the population-based study by Axelsson et al16 reported a prevalence of 1.1% in men and 0.8% in women aged 25 to 99 years. In contrast, investigations that only included older patients (>50 years) reported much higher prevalence estimates in men and women.13,15,18,19
Of the 5 studies that characterized sex-specific prevalence rates in this review, only one that was conducted in the United States13 met the criteria to determine the sex-specific prevalence of MGUS (Table 6). These data likely reflect the actual prevalence of MGUS by sex in predominantly white populations because this study, as noted earlier, was in a geographic area with a very low percentage of nonwhites.
The other study we include in Table 6 for information provides sex-specific prevalence rates in Japan.19 The caveats pertaining to the inclusion of this study in Table 6 have already been discussed.
Other Factors Influencing Prevalence. On the basis of our supplementary literature review of the evidence, immune status, occupational and environmental risk factors, and hereditary factors influence the risk of developing MGUS. MGUS occurs more frequently in those who are immunocompromised, such as patients who are infected with HIV or who have undergone a transplant.30,31,33,35,38 Exposure to toxins, including asbestos, fertilizers, and pesticides, appears to increase the likelihood of developing MGUS.40,41 Finally, first-degree relatives of those affected by MGUS are at a 3-fold higher risk of having the disease, raising the possibility of shared genetic or environmental factors.48
DISCUSSION
MGUS falls under an overarching group of plasma cell disorders known as monoclonal gammopathies (or paraproteinemias). This broad category of conditions includes MGUS, multiple myeloma, Waldenström macroglobulinemia, and primary amyloidosis.50 To date, this is the first systematic review of the descriptive epidemiology of MGUS.
The true frequency of MGUS can most accurately be determined through population-based studies that test persons in a geographically defined area during a specified period. Unfortunately, large studies of this type pertaining to MGUS have been uncommon. Most epidemiological studies of MGUS acquire data from inpatient clinics, outpatient clinics, population registries, and the general population. Thus, these studies use diverse methods of case ascertainment to estimate the prevalence and incidence of MGUS. Even in population-based studies, given the age-, sex-, and race-based distinctions in the occurrence of MGUS, prevalence estimates can only be generalized to populations sharing similar demographic characteristics. For example, the population-based study by Kyle et al13 offers prevalence estimates for those older than 50 years. Because MGUS is rare in young people, this study captures the risk of MGUS within the age demographic most likely to be affected by MGUS (>50 years).
For the best estimates of prevalence, we favor population-based screening studies (all participants from a general population or participants selected by stratified random sampling) in which all participants have an equal opportunity of being screened.13,16-18,20,22 On the basis of the most recent estimates,13 we conclude that the prevalence of MGUS estimated using serum protein electrophoresis as the initial screening test in persons older than 50 years is 3% to 4% and that the overall risk of MGUS in blacks from a similar age group is approximately twice as high. If serum immunofixation and free light chain assay are used as initial screening tests, the rates will likely be higher because of the higher sensitivity of these tests compared with electrophoresis.
We encountered several important limitations. Although uniform, established criteria to identify MGUS exist, divergent laboratory methods have made it difficult to ascertain the true prevalence of MGUS. Initially, serum M protein was estimated by electrophoretic methods. Over time, electrophoretic techniques have improved, and tests such as protein immunofixation and the serum free light chain assay have greatly increased the sensitivity of detecting M proteins in the serum.25 The differences in sensitivities of laboratory tests used to identify M protein may influence studies' estimates of the prevalence of MGUS.13 Furthermore, the second diagnostic criterion of MGUS requires the proportion of clonal plasma cells to be less than 10% in bone marrow. However, a number of studies establish a diagnosis of MGUS solely on the basis of an M protein less than 30 g/L (and no end-organ damage) without performing a bone marrow biopsy to verify that the clonal plasma cell proliferation is indeed less than 10%. This is attributed to the cost and inconvenience to the patient of the procedure. Additionally, the detection of greater than 10% of clonal plasma cells in bone marrow is rare when levels of M proteins are low (<30 g/L).11
A number of criteria aid in characterizing the external validity of prevalence estimates of MGUS included in this review. In particular, study design can influence epidemiological estimates. The investigations conducted by Aguzzi et al14 and Malacrida et al23 in Italy and Landgren et al21 in the United States all used large sample sizes (ranging from 35,000 to approximately 4,000,000) to determine the prevalence of MGUS. However, these studies assessed prevalence in hospital cohorts. Consequently, these estimates cannot be extrapolated to the general population because they were based on a pool of generally less healthy individuals. Furthermore, in one instance, MGUS cases were identified through the discharge diagnosis, rather than screening, which likely led to underascertainment of MGUS cases.21
The findings of this study raise several important mechanistic questions that need further research. First, although the data are convincing for the effect of age on prevalence of MGUS, the underlying mechanisms are poorly understood. The increase in prevalence with age suggests that the occurrence of monoclonal gammopathies may reflect loss of control of limited response to antigenic stimulation with age and may involve age-related loss of immune surveillance measures. This is supported by the fact that immunosuppression related to drug therapy or disease (ie, HIV infection) also increases the risk of MGUS. Second, it is still unclear from a pathogenesis standpoint why men appear to be at a higher risk for developing MGUS. Third, the basis for racial disparities in prevalence of MGUS is unclear. It is likely that this increased risk is secondary to the increased prevalence of premalignant MGUS in blacks rather than to an increased rate of transformation of MGUS to myeloma. The finding that the increased prevalence extends to blacks in Africa suggests that genetic factors may underlie these racial differences.22 However, no mechanistic studies have addressed the reasons for the racial disparities in the occurrence of MGUS.
CONCLUSION
MGUS is one of the most common premalignant disorders in the general population. It is clear that epidemiological investigations will aid in the identification of groups at a high risk of MGUS and thus at a high risk of the subsequent development of multiple myeloma. Such insight will be beneficial for early screening and intervention in high-risk demographics. Future epidemiological investigations must standardize the criteria used for the diagnosis of MGUS, establish universal methods to evaluate these criteria, and be population-based to ensure that prevalence estimates of MGUS are accurate and can be generalized to specific populations. Studies should also pursue etiologic research avenues highlighted by these prevalence studies by exploring mechanisms underlying differences in prevalence by age, sex, race, degree of immunosuppression, occupational exposure, and family history.
Acknowledgments
We extend our gratitude to Victoria A. Dickens, MA, Wael Salem, MPhil, and Fima Macheret, BS, for their thoughtful insight.
Footnotes
This study was supported in part by grants CA107476 and CA62242 from the National Cancer Institute, Bethesda, MD.
An earlier version of this article appeared Online First.
REFERENCES
- 1.Rajkumar SV. MGUS and smoldering multiple myeloma: update on pathogenesis, natural history, and management. Hematology Am Soc Hematol Educ Program. 2005:340-345 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962-2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860-1873 [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516-2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bida JP, Kyle RA, Therneau TM, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc. 2009;84:685-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ., III Long-term follow-up of 241 patients with monoclonal gammopathy of undetermined significance: the original Mayo Clinic series 25 years later. Mayo Clin Proc. 2004;79:859-866 [DOI] [PubMed] [Google Scholar]
- 7.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564-569 [DOI] [PubMed] [Google Scholar]
- 8.Blade J, Rosinol L, Cibeira MT, de Larrea CF. Pathogenesis and progression of monoclonal gammopathy of undetermined significance. Leukemia. 2008;22:1651-1657 [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Br J Haematol. 2006;134:573-589 [DOI] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812-817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749-757 [PubMed] [Google Scholar]
- 12.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362-1369 [DOI] [PubMed] [Google Scholar]
- 14.Aguzzi F, Bergami MR, Gasparro C, Bellotti V, Merlini G. Occurrence of monoclonal components in general practice: clinical implications. Eur J Haematol. 1992;48:192-195 [DOI] [PubMed] [Google Scholar]
- 15.Anagnostopoulos A, Evangelopoulou A, Sotou D, Gika D, Mitsibounas D, Dimopoulos MA. Incidence and evolution of monoclonal gammopathy of undetermined significance (MGUS) in Greece. Ann Hematol. 2002;81:357-361 [DOI] [PubMed] [Google Scholar]
- 16.Axelsson U, Bachmann R, Hallen J. Frequency of pathological proteins (M-components) in 6,995 sera from an adult population. Acta Med Scand. 1966;179:235-247 [DOI] [PubMed] [Google Scholar]
- 17.Carrell RW, Colls BM, Murray JT. The significance of monoclonal gammopathy in a normal population. Aust N Z J Med. 1971;1:398-401 [DOI] [PubMed] [Google Scholar]
- 18.Cohen HJ, Crawford J, Rao MK, Pieper CF, Currie MS. Racial differences in the prevalence of monoclonal gammopathy in a community-based sample of the elderly. Am J Med. 1998;104:439-444 [DOI] [PubMed] [Google Scholar]
- 19.Iwanaga M, Tagawa M, Tsukasaki K, Kamihira S, Tomonaga M. Prevalence of monoclonal gammopathy of undetermined significance: study of 52,802 persons in Nagasaki City, Japan. Mayo Clin Proc. 2007;82:1474-1479 [DOI] [PubMed] [Google Scholar]
- 20.Kyle RA, Finkelstein S, Elveback LR, Kurland LT. Incidence of monoclonal proteins in a Minnesota community with a cluster of multiple myeloma. Blood. 1972;40:719-724 [PubMed] [Google Scholar]
- 21.Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landgren O, Katzmann JA, Hsing AW, et al. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin Proc. 2007;82:1468-1473 [DOI] [PubMed] [Google Scholar]
- 23.Malacrida V, De Francesco D, Banfi G, Porta FA, Riches PG. Laboratory investigation of monoclonal gammopathy during 10 years of screening in a general hospital. J Clin Pathol. 1987;40:793-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neriishi K, Nakashima E, Suzuki G. Monoclonal gammopathy of undetermined significance in atomic bomb survivors: incidence and transformation to multiple myeloma. Br J Haematol. 2003;121:405-410 [DOI] [PubMed] [Google Scholar]
- 25.Ogmundsdóttir HM, Haraldsdóttir V, M Jóhannesson G, et al. Monoclonal gammopathy in Iceland: a population-based registry and follow-up. Br J Haematol. 2002;118:166-173 [DOI] [PubMed] [Google Scholar]
- 26.Ong F, Hermans J, Noordijk EM, et al. A population-based registry on paraproteinaemia in The Netherlands: Comprehensive Cancer Centre West, Leiden, The Netherlands. Br J Haematol. 1997;99:914-920 [DOI] [PubMed] [Google Scholar]
- 27.Kyle RA, Rajkumar SV. Monoclonal gammopathies of undetermined significance. Best Pract Res Clin Haematol. 2005;18:689-707 [DOI] [PubMed] [Google Scholar]
- 28.Bowden M, Crawford J, Cohen HJ, Noyama O. A comparative study of monoclonal gammopathies and immunoglobulin levels in Japanese and United States elderly. J Am Geriatr Soc. 1993;41:11-14 [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Delgado GJ, Gomez Rangel JD. Monoclonal gammopathy of undetermined significance (MGUS) in Mexican mestizos: one institution's experience [in Spanish]. Gac Med Mex. 2004;140(4):375-379 [PubMed] [Google Scholar]
- 30.Dezube BJ, Aboulafia DM, Pantanowitz L. Plasma cell disorders in HIV-infected patients: from benign gammopathy to multiple myeloma. AIDS Read. 2004;14:372-374, 377-379 [PubMed] [Google Scholar]
- 31.Papadopoulos NM, Lane HC, Costello R, et al. Oligoclonal immunoglobulins in patients with the acquired immunodeficiency syndrome. Clin Immunol ImmunoPathol. 1985;35:43-46 [DOI] [PubMed] [Google Scholar]
- 32.Briault S, Courtois-Capella M, Duarte F, Aucouturier P, Preud'Homme JL. Isotypy of serum monoclonal immunoglobulins in human immunodeficiency virus-infected adults. Clin Exp Immunol. 1988;74:182-184 [PMC free article] [PubMed] [Google Scholar]
- 33.Heriot K, Hallquist AE, Tomar RH. Paraproteinemia in patients with acquired immunodeficiency syndrome (AIDS) or lymphadenopathy syndrome (LAS). Clin Chem. 1985;31:1224-1226 [PubMed] [Google Scholar]
- 34.Crapper RM, Deam DR, Mackay IR. Paraproteinemias in homosexual men with HIV infection: lack of association with abnormal clinical or immunologic findings. Am J Clin Pathol. 1987;88:348-351 [DOI] [PubMed] [Google Scholar]
- 35.Kouns DM, Marty AM, Sharpe RW. Oligoclonal bands in serum protein electrophoretograms of individuals with human immunodeficiency virus antibodies [letter]. JAMA. 1986;256:2343 [PubMed] [Google Scholar]
- 36.Amara S, Dezube BJ, Cooley TP, Pantanowitz L, Aboulafia DM. HIV-associated monoclonal gammopathy: a retrospective analysis of 25 patients. Clin Infect Dis. 2006;43:1198-1205 [DOI] [PubMed] [Google Scholar]
- 37.Fiorino AS, Atac B. Paraproteinemia, plasmacytoma, myeloma and HIV infection. Leukemia. 1997;11:2150-2156 [DOI] [PubMed] [Google Scholar]
- 38.Passweg J, Thiel G, Bock HA. Monoclonal gammopathy after intense induction immunosuppression in renal transplant patients. Nephrol Dial Transplant. 1996;11:2461-2465 [DOI] [PubMed] [Google Scholar]
- 39.Saleun JP, Vicariot M, Deroff P, Morin JF. Monoclonal gammopathies in the adult population of Finistere, France. J Clin Pathol. 1982;35:63-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasqualetti P, Casale R, Colantonio D, Collacciani A. Occupational risk for hematological malignancies. Am J Hematol. 1991;38:147-149 [DOI] [PubMed] [Google Scholar]
- 41.Pasqualetti P, Collacciani A, Casale R. Risk of monoclonal gammopathy of undetermined significance: a case-referent study. Am J Hematol. 1996;52:217-220 [DOI] [PubMed] [Google Scholar]
- 42.Pasqualetti P, Festuccia V, Acitelli P, Collacciani A, Giusti A, Casale R. Tobacco smoking and risk of haematological malignancies in adults: a case-control study. Br J Haematol. 1997;97:659-662 [DOI] [PubMed] [Google Scholar]
- 43.Landgren O, Kyle RA, Hoppin JA, et al. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood. 2009;113:6386-6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bizzaro N, Pasini P. Familial occurrence of multiple myeloma and monoclonal gammopathy of undetermined significance in 5 siblings. Haematologica. 1990;75:58-63 [PubMed] [Google Scholar]
- 45.Lynch HT, Ferrara K, Barlogie B, et al. Familial myeloma. N Engl J Med. 2008;359:152-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch HT, Sanger WG, Pirruccello S, Quinn-Laquer B, Weisenburger DD. Familial multiple myeloma: a family study and review of the literature. J Natl Cancer Inst. 2001;93:1479-1483 [DOI] [PubMed] [Google Scholar]
- 47.Landgren O, Linet MS, McMaster ML, Gridley G, Hemminki K, Goldin LR. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: a population-based case-control study. Int J Cancer. 2006;118:3095-3098 [DOI] [PubMed] [Google Scholar]
- 48.Vachon CM, Kyle RA, Therneau TM, et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood. 2009;114:785-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogt RF, Marti GE. Overview of monoclonal gammopathies of undetermined significance. Br J Haematol. 2007;139:687-689 [DOI] [PubMed] [Google Scholar]
- 50.Kyle RA, Rajkumar SV. Epidemiology of the plasma-cell disorders. Best Pract Res Clin Haematol. 2007;20:637-664 [DOI] [PubMed] [Google Scholar]