Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant plasma cell disorder present in more than 3% of the general population aged 50 years and older.1,2 MGUS is important to clinicians because it is associated with a 1% per year risk of progression to multiple myeloma (MM) or related malignancy.3 It is also important because it is found incidentally during the work-up of a variety of symptoms and disorders, and has confirmed and reported associations with numerous diseases commonly encountered in clinical practice, such as osteoporosis, peripheral neuropathy, and venous thrombosis.4 In addition, because MGUS is easily detected on blood tests and can be monitored noninvasively, it represents a readily accessible model to study the conversion of premalignancy to malignancy.5
In the past several years, new concepts and advances have emerged concerning the diagnosis, classification, risk stratification, and management of MGUS. This commentary highlights discoveries that provide valuable insight into the process of malignant transformation, as well as management strategies for dealing with a common premalignancy in which preventive strategies are thwarted by low rates of progression.6
Clinical Disease Definitions
Since MGUS was first described more than 30 years ago, the definition of the entity has evolved.7 Currently, 3 distinct clinical types of MGUS are identified: non-IgM (IgG or IgA) MGUS, IgM MGUS, and light chain MGUS (Table 1). Each clinical subtype is characterized by unique intermediate stages and progression events. For example, the more advanced premalignant stage of plasma cell proliferation in non-IgM MGUS is termed smoldering multiple myeloma (SMM) and is characterized by a much higher risk of progression to MM: 10% per year risk of progression for SMM vs 1% per year risk collectively for all forms of MGUS.8 The IgM type of MGUS is associated with a predisposition mainly to Waldenström macroglobulinemia and infrequently to IgM MM.9,10 Recently, a new disease entity termed light chain MGUS has been defined; it represents the premalignant precursor of a subtype of MM called light chain MM that accounts for nearly 20% of all new cases of MM.11 The equivalent of SMM and smoldering Waldenström macroglobulinemia in the spectrum of light chain monoclonal gammopathies is called idiopathic Bence Jones proteinuria (Table 1).12,13
TABLE 1.
Disease Definitions for the Monoclonal Gammopathies: MGUS and Related Disordersa
What insight do these definitions offer in terms of the process to be used in establishing disease definitions? First, in each of these disease categories, one or more large cohorts of patients meeting a specified disease definition were assembled. Next, the natural history of each disease cohort was determined using sound epidemiological methods.3,8,9,11,12,14-16 As a result, we clearly know how to diagnose each of these entities accurately, and we also know the outcome of patients meeting the specific disease definition to assist with management and counseling. This approach to developing disease definitions is preferable to arbitrary criteria, in which the characteristics and outcome of patients meeting such criteria are unknown.
The specific criteria listed in Table 1 are of major importance in patient care and are based on the epidemiological and clinical studies that used clear criteria to define each entity.3,8,9,11,12,14-16 As a result of these large studies, we now know the prevalence, risk of progression, and natural history of non-IgM MGUS, IgM MGUS, and light chain MGUS. These studies illustrate that, although these disorders represent clonal proliferation of plasma cells, they do not behave like malignancies, and patients with these diagnoses should be reassured rather than being labeled as having a cancer. For example, patients with less than 10% infiltration of the marrow by lymphoplasmacytic cells have an overall survival that is as good as the general population at large, and should therefore not be labeled as having a lymphoma or Waldenström macroglobulinemia merely because the bone marrow pathology shows clonal proliferation of lymphoid cells.15 As our diagnostic methods improve and become increasingly sensitive, the line between malignancy and premalignancy will continue to blur. As our understanding of disease progression improves, it will become increasingly important to recognize that well-designed epidemiological studies and clinicopathologic disease definitions will be required to separate patients who need treatment such as chemotherapy or stem cell transplant for cancer like myeloma17 from those who need no therapy and need reassurance.5
Pathogenesis and Cytogenetic Classification
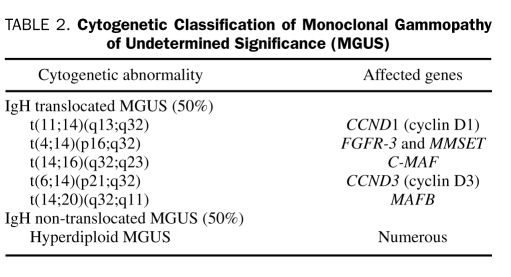
Race and ethnicity play a role in the pathogenesis of MGUS. African Americans, and blacks from Africa, have a 2- to 3-fold higher incidence of MGUS compared with whites.18,19 In contrast, the risk is lower in Asians from Japan20 and in Mexicans.21 Advancing age,1 male sex, family history,22 immunosuppression, and exposure to certain pesticides23 all increase the risk of MGUS. Understanding the mechanisms that underlie these risk factors will probably provide clues to the etiology of MGUS. The first step in the pathogenesis is likely an abnormal response to antigenic stimulation, mediated possibly by aberrant expression of toll-like receptors and overexpression of interleukin (IL) 6 receptors and IL-1β24,25 This then results in the development of primary cytogenetic abnormalities, either hyperdiploidy or immunoglobulin heavy chain (IgH) translocations (Table 2). The progression of MGUS to myeloma is likely secondary to a random second hit, the nature of which is unknown. Ras and p53 mutations, p16 methylation, myc abnormalities, and induction of angiogenesis are all associated with progression. In addition, there is increased osteoblast RANKL (receptor activator of nuclear factor κB ligand) expression and reduction in the level of its decoy receptor, osteoprotegerin, which results in osteoclast activation and increased bone resorption and turnover.26 This is accompanied by increased levels of IL-3, IL-7, and dickkopf 1 that simultaneously inhibit osteoblast differentiation, leading to the characteristic pure lytic lesions typical of myeloma.27-29
TABLE 2.
Cytogenetic Classification of Monoclonal Gammopathy of Undetermined Significance (MGUS)
In approximately 50% of MGUS cases, the primary pathogenetic event is likely hyperdiploidy, and in the remaining 50% the pathogenetic event is a translocation event at the IgH locus on chromosome 14q32.30 These pathogenetic events result in at least 6 different cytogenetic subtypes of MGUS and myeloma (Table 2).31,32 The excess risk of MGUS in blacks is likely due to a higher predisposition to hyperdiploid MGUS on the basis of recent studies that suggest that the outcome of myeloma in blacks with lenalidomide-based therapy is better compared with that in whites.33 These observations are important because they highlight the increasing complexity that has accompanied many of the advances in cancer research. As more detailed genetic analysis is performed, it becomes apparent that what phenotypically was considered a single malignancy is actually several cytogenetically different diseases, each of which may have a different pathogenetic mechanism, and a different natural history and response to therapy.5
Risk Stratification
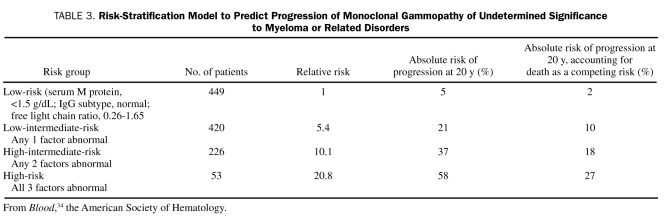
An abnormal serum free light chain ratio (ie, the ratio of free immunoglobulin κ to λ light chains in the serum), non-IgG MGUS, and a high serum M protein level (≥1.5 g/dL) are 3 major risk factors for the progression of MGUS to myeloma (Table 3).34 The risk-stratification model is helpful for patient counseling and management. The key concept applicable to other premalignancies is that, as the sensitivity of diagnostic tests increases, it is critical to develop risk-stratification models to distinguish patients with premalignancy of clinical relevance from those whose abnormalities do not result in a sufficiently higher risk compared with that in healthy people.
TABLE 3.
Risk-Stratification Model to Predict Progression of Monoclonal Gammopathy of Undetermined Significance to Myeloma or Related Disorders
Management
We recently demonstrated the major problem with managing asymptomatic premalignant disorders like MGUS, which have a low but definite risk of progression to malignancy.35 Preventive therapy cannot be justified without safe treatment options and evidence of benefit from phase 3 clinical trials. On the other hand, close follow-up without treatment can seldom identify progression before serious complications can occur. In these circumstances, risk stratification is needed to help guide optimal management and identification of biomarkers that signal malignant transformation before the onset of serious symptoms. Risk-stratification studies of MGUS indicate that follow-up is unnecessary for low-risk patients, whereas follow-up strategies, prevention trials, and continued research on biomarkers such as malignant immunophenotype and plasma cell proliferative rate for early detection of malignant transformation are needed for high-risk patients.35
REFERENCES
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362-1369 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Durie BGM, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564-569 [DOI] [PubMed] [Google Scholar]
- 4.Bida JP, Kyle RA, Therneau TM, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc. 2009;84(8):685-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkumar SV. Prevention of progression in monoclonal gammopathy of undetermined significance. Clin Cancer Res. 2009;15(18):5606-5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1β-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84(2):114-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590 [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759-3764 [DOI] [PubMed] [Google Scholar]
- 10.Schuster S, Rajkumar SV, Dispenzieri A, et al. IgM multiple myeloma: disease definition, prognosis, and differentiation from Waldenstrom's macroglobulinemia [published online ahead of print July 28, 2010]. Am J Hematol. doi 10.1002/ajh.21845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721-1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyle R, Therneau T, Dispenzieri A, et al. Idiopathic Bence Jones proteinuria: clinical course and prognosis [abstract 3493]. Blood (ASH Annual Meeting Abstracts). 2006;108(11):3493 [Google Scholar]
- 13.Kyle RA, Maldonado JE, Bayrd ED. Idiopathic Bence Jones proteinuria–a distinct entity? Am J Med. 1973;55(2):222-226 [DOI] [PubMed] [Google Scholar]
- 14.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1,027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33 [DOI] [PubMed] [Google Scholar]
- 15.Gobbi PG, Baldini L, Broglia C, et al. Prognostic validation of the international classification of immunoglobulin M gammopathies: a survival advantage for patients with immunoglobulin M monoclonal gammopathy of undetermined significance? Clin Cancer Res. 2005;11(5):1786-1790 [DOI] [PubMed] [Google Scholar]
- 16.Baldini L, Goldaniga M, Guffanti A, et al. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenstrom's macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. J Clin Oncol. 2005;23(21):4662-4668 [DOI] [PubMed] [Google Scholar]
- 17.Gertz MA, Ansell SM, Dingli D, et al. Autologous stem cell transplant in 716 patients with multiple myeloma: low treatment-related mortality, feasibility of outpatient transplant, and effect of a multidisciplinary quality initiative. Mayo Clin Proc. 2008;83(10):1131-1138 [DOI] [PubMed] [Google Scholar]
- 18.Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107(3):904-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landgren O, Katzmann JA, Hsing AW, et al. Prevalence of monoclonal mammopathy of undetermined significance among men in Ghana. Mayo Clin Proc. 2007;82(12):1468-1473 [DOI] [PubMed] [Google Scholar]
- 20.Iwanaga M, Tagawa M, Tsukasaki K, Kamihira S, Tomonaga M. Prevalence of monoclonal gammopathy of undetermined significance: study of 52,802 persons in Nagasaki City, Japan. Mayo Clin Proc. 2007;82(12):1474-1479 [DOI] [PubMed] [Google Scholar]
- 21.Ruiz-Delgado GJ, Ruiz-Arguelles GJ. Genetic predisposition for monoclonal gammopathy of undetermined significance. Mayo Clin Proc. 2008;83(5):601-602 [DOI] [PubMed] [Google Scholar]
- 22.Vachon CM, Kyle RA, Therneau TM, et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood. 2009;114(4):785-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landgren O, Kyle RA, Hoppin JA, et al. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance (MGUS) in the Agricultural Health Study. Blood. 2009;113(25):6386-6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jego G, Bataille R, Geffroy-Luseau A, Descamps G, Pellat-Deceunynck C. Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia. 2006;20(6):1130-1137 [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA. Targeting the pathogenic role of interleukin 1β in the progression of smoldering/indolent myeloma to active disease. Mayo Clin Proc. 2009;84(2):105-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435-441 [DOI] [PubMed] [Google Scholar]
- 27.Drake MT. Bone disease in multiple myeloma. Oncology (Williston Park). 2009;23(14, suppl 5):28-32 [PubMed] [Google Scholar]
- 28.Drake MT, Rajkumar SV. Effects of bortezomib on bone disease in multiple myeloma. Am J Hematol. 2009;84(1):1-2 [DOI] [PubMed] [Google Scholar]
- 29.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483-2494 [DOI] [PubMed] [Google Scholar]
- 30.Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. Oncogene. 2001;20(40):5611-5622 [DOI] [PubMed] [Google Scholar]
- 31.Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84(12):1095-1110Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajkumar SV. Treatment of myeloma: cure vs control. Mayo Clin Proc. 2008;83(10):1142-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance (MGUS). Blood. 2005;106:812-817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianchi G, Kyle RA, Colby CL, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance (MGUS) on early diagnosis and prevention of myeloma-related complications [published online ahead of print May 21, 2010]. Blood. doi 10.1182/blood-2010-04-277566 [DOI] [PMC free article] [PubMed] [Google Scholar]