Abstract
The immune system responds to tuberculosis (TB) infection by the formation of granulomas. However, subsequent immune-mediated destruction of lung tissue is a cause of significant morbidity and contributes to disease transmission. Lactoferrin, an iron-binding glycoprotein, has demonstrated immunomodulatory properties which decrease tissue destruction and promote TH1 immune responses, both of which are essential for controlling TB infection. The cord factor trehalose 6,6’-dimycolate (TDM) model of granuloma formation mimics many aspects of TB infection, with similar histopathology accompanied by proinflammatory cytokine production. C57BL/6 mice were intravenously injected with TDM. A subset of mice was given 1 mg bovine lactoferrin 24 hours post-TDM challenge. Lung tissue was analyzed for histological response and production of proinflammatory mediators. C57BL/6 mice demonstrated granuloma formation that correlated with increased production of IL-1β, IL-6, TNF-α, IL-12p40, IFN-γ, and IL-10 protein. Mice treated with lactoferrin post-challenge had significantly fewer and smaller granulomas compared to those given TDM alone. Proinflammatory and TH1 cytokines essential to the control of mycobacterial infections, such as TNF-α and IFN-γ, were not significantly different in mice treated with lactoferrin. Furthermore, the anti-inflammatory cytokines IL-10 and TGF-β were increased. A potential mechanism for decreased tissue damage seen in the lactoferrin treated mice is proposed. Because of its influence to modulate immune responses, lactoferrin may be a useful adjunct in the treatment of granulomatous inflammation occurring during mycobacterial infection.
Keywords: TDM, Cord Factor, Lactoferrin, Granuloma, Inflammation, Lung, Tuberculosis
Introduction
TB is responsible for approximately 1.8 million deaths each year, making it the leading bacterial cause of death worldwide (1). Additionally, nearly one-third of the world is latently infected with Mycobacterium tuberculosis (MTB) (1). With the exception of the potential role of the fluoroquinolones, there have been no new anti-TB drugs created since rifampin was introduced in the 1960s (2). Thus, agents with anti-mycobacterial activity or a beneficial effect when used in combination with the current anti-TB drugs are desperately needed.
The immunocompetant host responds to MTB infection by the formation of granulomas, which consist of central infected macrophages that may differentiate into Langhan’s giant cells, foamy macrophages, and epithelioid cells (3). A fibrous cuff develops around the infected macrophages, which serves to sequester and prevent dissemination of the bacteria. However, granuloma formation also causes physical partitioning of infected cells from peripheral lymphocytes capable of killing the infected cells (3). Furthermore, it is thought that the TB bacilli enter a non-replicating latency state within the granuloma; such bacilli are resistant to the action of antimicrobials (4, 5). The majority of granulomas do not result in active disease. However, certain granulomas develop an increase in foamy macrophages that are hypothesized to cause caseation, the hallmark of active disease (3, 6). The result is necrosis and liquification of the granuloma, forming a cavity that places infectious bacilli in the airways. The damage to the lung causes a productive cough, producing infectious aerosols that may infect a new host. Thus, modulation of the granulomatous pathology will likely facilitate improved response to antimicrobials, limit transmission of the disease, and decrease morbidity.
New evidence has identified lactoferrin as a regulator of the immune response to a variety of infectious and injurious stimuli. Lactoferrin is a member of the transferrin family and is found in mucosal secretions as well as in neutrophil granules (7). Receptors for lactoferrin are found on many immune system cells, including dendritic cells, macrophages, and T-cells (8–10). Specific effects of lactoferrin include the activation of macrophages, increasing polymorphonuclear cell phagocytosis, enhancement of killer cell activity, and promotion of B- and T-cell maturation (11–15). Of considerable interest, lactoferrin modulates macrophage production of cytokines in the context of their environment (16–18). For example, lactoferrin added to macrophages that were sub-optimally stimulated with LPS increased proinflammatory cytokine production (19). However, lactoferrin decreased cytokine production in macrophages fully stimulated with LPS. Critical to the studies proposed here, lactoferrin has been shown to protect against immune-mediated tissue damage. Mice treated with lactoferrin had increased survival and decreased gut tissue destruction after LPS injection (7, 20). Additionally, lactoferrin added to the BCG vaccine resulted in increased protection against an aerosol TB challenge and decreased lung damage (21).
Cord factor TDM is the most abundant glycolipid produced on the surface of MTB. TDM plays multiple roles in the pathogenesis of MTB (22), including the formation of caseation in the lung post-infection (23). TDM emulsion injected intravenously into mice induces lung immunopathology that mimics many aspects of MTB infection, including granuloma formation and the production of proinflammatory cytokines. The TDM model of granuloma formation has been used to elucidate the immunological factors involved in the granulomatous response (24, 25). TDM can induce activated foreign body type granulomas in naïve mice (26, 27), and hypersensitivity (immune) granulomas in appropriately sensitized mice (23, 28, 29). Therefore, this model system is ideal for exploring the potential of immunomodulators to alter granuloma structure, with perhaps specific utility to extrapolate findings to immune related pathology identified during clinical manifestation of tuberculosis disease.
Methods
Animals
Four-week old, female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animal studies were approved by the University of Texas Health Science Center Institutional Review Board (protocol number HSC-AWP 07-093), performed under approved and accepted ethical guidelines. Up to eight mice were used per group, per time points indicated.
Lactoferrin and MTB
Bovine-derived lactoferrin (>95% purity, <20% iron saturated) was generously provided by PharmaReview Corporation (Houston, TX). Lactoferrin was dissolved in PBS, and stored at −80°C. The lactoferrin used in experimentation was identical in composition as that used in published investigations into immune mediation of DTH development (19, 30, 31).
Mycobacterium tuberculosis
Erdman (TMC 107, American Type Cell Culture) was grown to log phase in 7H9 broth (Remel, Lenesa, KS) with 10% supplement at 37°C in an orbital shaker. MTB was diluted in 1× PBS to 3 × 108 bacteria per ml by using McFarland standards (Sigma, St. Louis, MO). The bacterial concentration was confirmed by plating serial dilutions on 7H11 plates (Remel), and enumerated after incubation for 3–4 weeks at 37°C.
Isolation of bone marrow-derived macrophages (BMM), challenge with TDM-coated microspheres, and infection with mycobacteria
BMMs from C57BL/6 mice were prepared as per previously described procedures (31). Femurs were flushed with McCoy’s medium (Sigma) and 5 × 105 cells were added to 24-well tissue culture plates (Corning, Corning, NY). Cells were grown in McCoy’s medium containing 10% fetal bovine serum (FBS) (Sigma), antibiotics (50 µg/ml gentamycin and 100 µg/ml penicillin G) (Sigma), and 10 ng/ml recombinant murine granulocyte/macrophage colony stimulating factor (GM-CSF) (Cell Sciences, Canton, MA). Cells were incubated at 37°C with 5% CO2 for seven days with two media changes containing GM-CSF. The cells were washed with 1× PBS and the media changed to Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS, 0.01% L-arginine (Sigma), 0.01% HEPES (Sigma) prior to stimulation with TDM-coated beads or infection with MTB.
TDM- and BSA-coated microspheres were prepared as previously described (32). Beads were briefly sonicated before use, and added to cells at a ratio of 10 beads per cell in the presence or absence of 100 µg/ml lactoferrin. Alternatively, BMM were infected with MTB Erdman at a multiplicity of infection (MOI) of 1:1 with or without lactoferrin (100 µg/ml). For all conditions, supernatants were collected after 24 and 72 hours and assessed for cytokine production by ELISA (see below).
In vivo administration of TDM, tissue processing, and lung histopathology
A water-in-oil emulsion of TDM was prepared as previously described (25, 27). Briefly, mice were injected intravenously with 50 µl of the emulsion. The emulsion was prepared by dissolving 25 µg per dose of purified TDM (Sigma) in 9:1 hexane/ethanol followed by evaporation of the solvent. Two percent Drakeol (Penreco, Dickinson, TX) was added and homogenized. Finally, 0.2% Tween 80 (Mallinckrodt, Hazelwood, MO) in PBS was added to give the final volume and admixed. An emulsion control without TDM was also prepared. Subsets of mice were administered either 1 mg of lactoferrin or 1 mg of bovine apo-transferrin (Sigma) in 100 µl of PBS intravenously 24 hours after administration of TDM.
Mice were sacrificed at days 0, 4, and 7 post-TDM challenge. Lung tissues were immediately removed, weighed, and processed for histological studies or cytokine analysis. Approximately 30 mg of lung tissue was homogenized and placed into 2 ml of DMEM and incubated for 4 hours at 37 °C. The supernatants were removed and stored at −20 °C for cytokine ELISA analysis. For histological analysis, the left lung was fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin. An entire 5 µm section of at least 6 mice per group was digitized with a 2× objective. An analysis was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Total granulomas were enumerated over the whole section of the lung. The average granuloma size was determined by randomly selecting 10 granulomas in 3 microscopic fields for each mouse.
ELISA analysis of lung cytokines
The levels of TNF-α, IL-6, IL-1β, IL-12p40, IFN-γ, IL-10, and TGF-β in the lung homogenate supernatants were determined by a sandwich ELISA as per the manufacturer’s instructions (R&D Systems, Minneapolis, MN). The mean of duplicate wells was determined based on a standard curve generated for each assay using manufacturer supplied recombinant molecules. The lower limit for detection sensitivity was 15–32 pg/ml, according to the manufacturer.
Statistics
The data are presented as the mean ± 1 SD. Two-way ANOVA was used to compare the differences between groups using GraphPad Prism (GraphPad Software, La Jolla, CA). A p-value of < 0.05 was considered as being statistically significant.
Results
Lactoferrin modulation of inflammatory response to TDM in bone marrow-derived macrophages
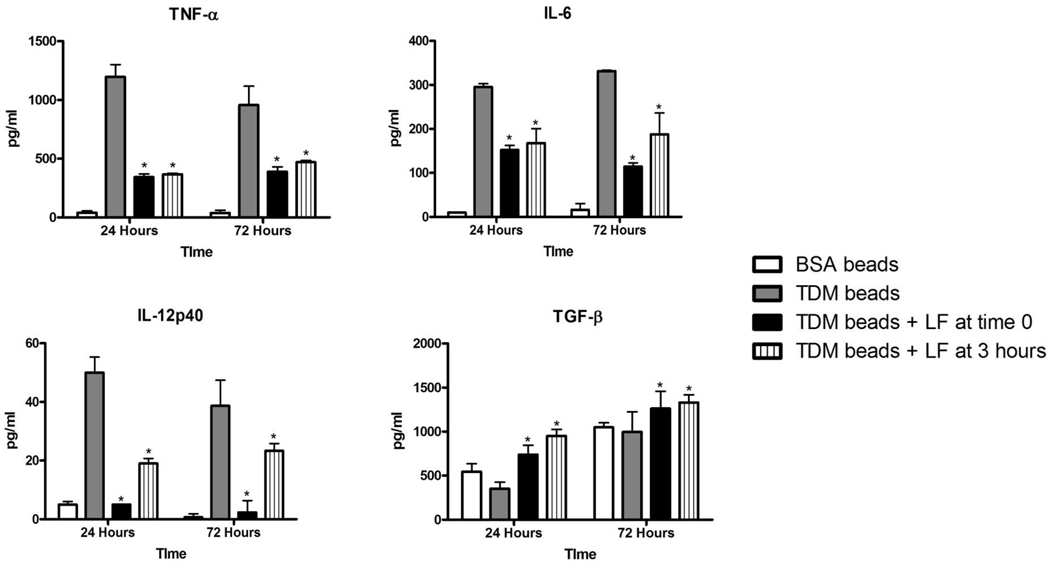
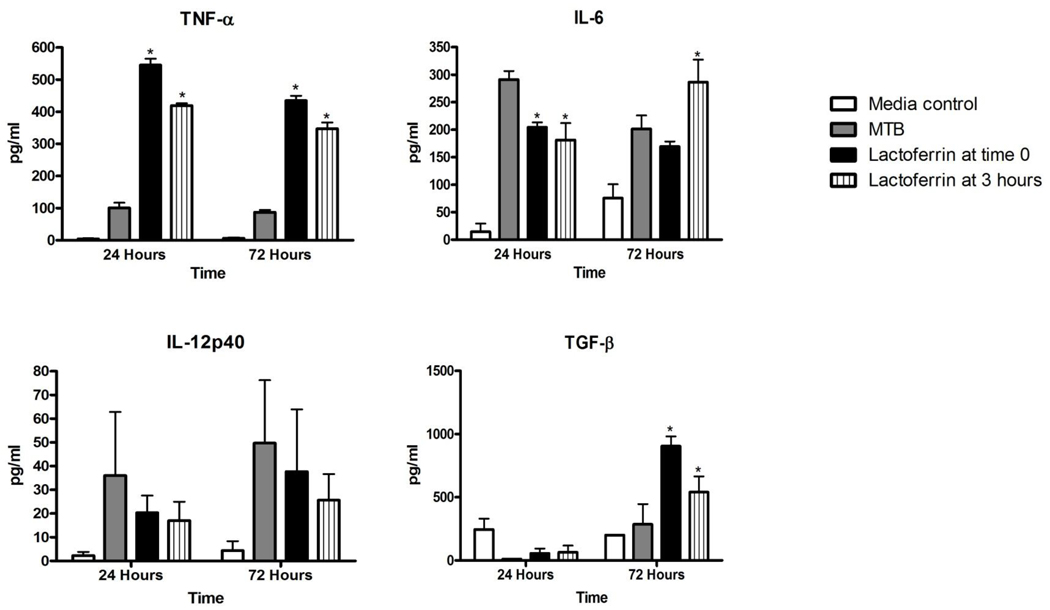
TDM is a potent inducer of proinflammatory cytokines from macrophages (3, 27, 33). The production of TNF-α, IL-6, and IL-12p40 were significantly elevated in BMM stimulated with TDM-coated beads, compared to macrophages administered BSA-coated beads (Figure 1). In contrast, the anti-inflammatory cytokine TGF-β decreased with TDM-bead stimulation. Addition of 100 µg/ml bovine lactoferrin, either at the same time as TDM bead stimulation or three hours after, resulted in significantly decreased production of the pro-inflammatory cytokines TNF-α, IL-6, and IL-12p40, but increased production of TGF-β. Lactoferrin alone increased TGF-β when cells were incubated with control BSA-coated beads, as has been previously reported (30).
Figure 1.
Lactoferrin modulation of TNF-α, IL-6, IL-12p40, and TGF-β production by BMM in response to TDM-coated beads. Cells were treated with 3 µm BSA- or TDM-coated beads alone at a ratio of 10:1, or with 100 µg/ml lactoferrin (LF) at the same time as bead stimulation, or with 100 µg/ml lactoferrin 3 hours after bead addition. Values were measured by ELISA and expressed as mean pg/ml with standard deviation (SD). * p<0.05 compared to treatment with TDM-coated beads alone.
Lactoferrin modulation of TDM-induced histopathology
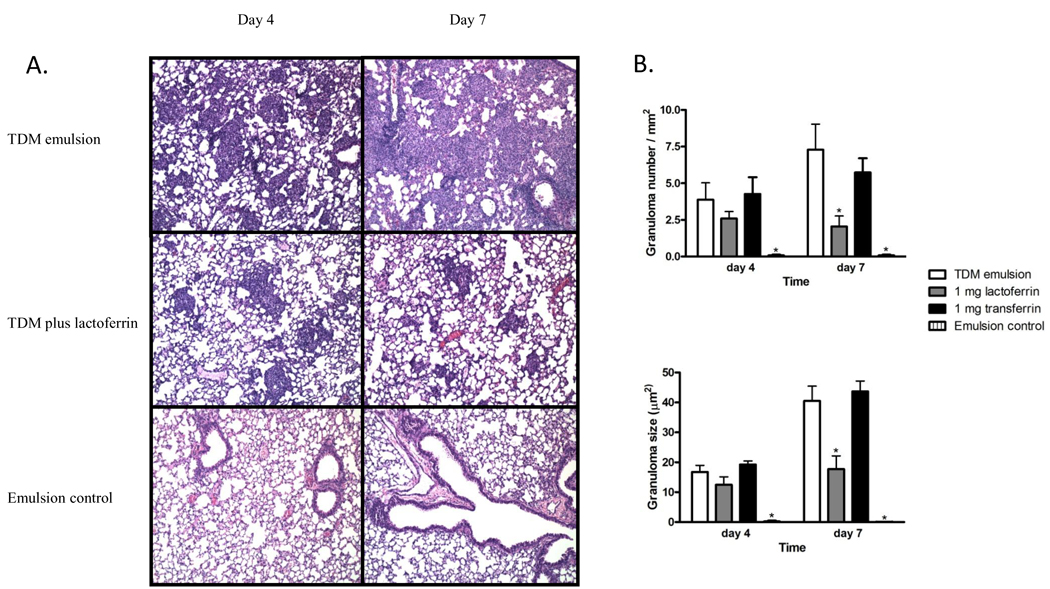
C57BL/6 mice challenged with TDM formed distinct lung granulomas after a single intravenous dose of TDM. Small, focal, monocytic clusters were apparent 4 days post-TDM challenge (Figure 2A). The granulomas increased in size and number by day 7 (Figure 2B), accompanied by additional pathological manifestations, which included lymphocytic perivascular cuffing. Mice administered an emulsion control failed to form granulomas and exhibited normal lung histology with only minor indications of inflammation. Mice challenged with TDM and treated subsequently with 1 mg of intravenous bovine lactoferrin had fewer and smaller granulomas post-TDM administration, with the granulomatous response markedly diminished by day 7. This response was not due to the iron binding capability of lactoferrin; granuloma formation was not altered in mice given transferrin, an iron-binding control, when given after TDM challenge.
Figure 2.
Granulomatous response to TDM in lactoferrin treated mice. A) Mice were challenged with 25 µg TDM prepared in a water-in-oil emulsion. One mg of lactoferrin or transferrin (not shown) was administered 24 hours post-TDM challenge. Hematoxylin and eosin staining, magnification 100×. B) Number of lung granulomas per mm2 and the size of the granulomas (µm2). Data are expressed as the mean ± SD. * p<0.05 with comparisons made to the mice administered the TDM emulsion.
Cytokine protein profiles in TDM-challenged mice treated with lactoferrin
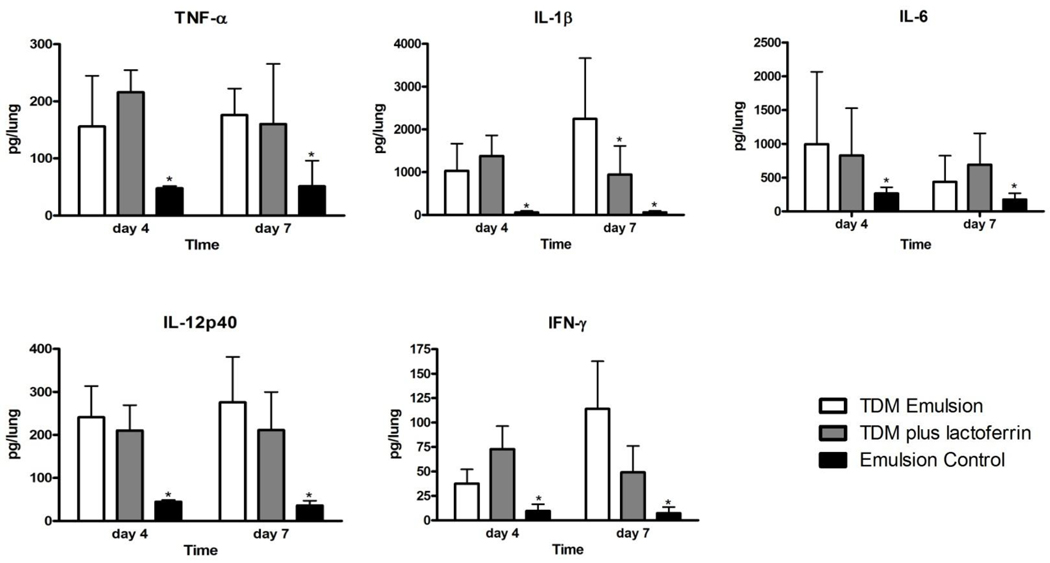
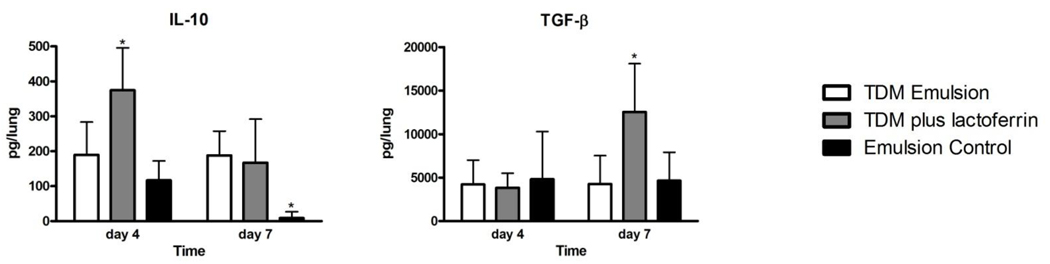
Lung cytokines produced were evaluated in mice challenged with TDM (Figures 3 and 4). Mice administered the TDM emulsion had significantly elevated levels of TNF-α, IL-1β, IL-6, IL-12p40, and IFN-γ on both days 4 and 7 compared to mice given an emulsion control (Figure 3). Mice treated with 1 mg of bovine lactoferrin 24 hours after challenge with TDM had similar levels of most pro-inflammatory cytokines. Of note, the levels of TNF- α and IFN-γ were not significantly different in the lactoferrin treated mice. The mice administered lactoferrin had decreased IL-1β at day 7. In contrast, the levels of the anti-inflammatory cytokines IL-10 and TGF-β were increased at day 4 and day 7, respectively, in the lactoferrin treated mice (Figure 4). Mice administered transferrin 24 hours after mice were administered TDM had similar levels of cytokines compared to mice given a TDM emulsion alone (data not shown).
Figure 3.
Production of pro-inflammatory mediators in mice challenged with TDM and treated with lactoferrin. The levels of TNF-α, IL-1β, IL-6, IL-12p40, and IFN-γ in lung tissue were determined by ELISA. Data are shown as the mean with standard deviation. N = 8 mice per group, per time point. *p<0.05 with comparison to the TDM emulsion treated mice.
Figure 4.
Lactoferrin induced production of anti-inflammatory cytokines in TDM challenged mice. The levels of IL-10 and TGF-β were quantified in lung tissue homogenates by ELISA. Data are expressed as the mean with standard deviation. N = 8 mice per group, per time points indicated. *p<0.05, comparisons made to the TDM-treated mice.
Relationship of model to mycobacterial infection
Certain cytokines such as TNF-α have been shown to be critical for the control of MTB infection (34–36). A concern involved the potential decrease in TNF-α production from macrophages stimulated with TDM-coated beads and administered lactoferrin might generate conditions favorable for MTB proliferation inside host cells. Therefore, C57BL/6 BMM were infected with MTB at a MOI of 1:1, with or without lactoferrin. Cytokine production in response to MTB infection is shown in Figure 5. TNF-α production was significantly increased in macrophages given lactoferrin at the same time as bacterial seeding or three hours later. IL-6 was significantly decreased, and there was no alteration in production of IL-12p40. Furthermore, the anti-inflammatory cytokine TGF-β was significantly increased at 72 hours post-infection. These results suggest that there is a potential for lactoferrin to decrease the cytokines that mediate tissue destruction while maintaining those that essential for development of responses correlating with protection against mycobacteria.
Figure 5.
Lactoferrin modulation of BMM cytokine production in response to MTB infection. BMM were infected with MTB Erdman using a MOI of 1:1. 100 µg/ml of bovine lactoferrin was added at the same time as infection or 3 hours after infection. The levels of TNF-α, IL-6, IL-12p40, and TGF-β were measured in the filtered supernatants 24 and 72 hours after infection by ELISA. Data are shown as the mean and SD. *p<0.05, comparisons are to cells infected with MTB alone.
Discussion
MTB is a highly successful pathogen, accounting for 1.8 million deaths annually and infects one-third of the world’s population (1). The incidence of this disease continues to increase, likely because of the increased occurrence of multi-drug resistant strains and co-infection with HIV (37). The treatment of TB is lengthy, complex, and requires drugs with significant side-effects that often result in poor patient compliance. The WHO’s directly observed therapy short-course (DOTS) program is highly effective, yet places a significant financial burden on developing countries with a high incidence of TB. Thus, new therapeutics with the potential to shorten treatment are desperately needed. Immunomodulatory agents, such as lactoferrin, may represent a novel approach to the treatment of TB in that they have the ability to overcome the issue of drug resistance. In the case of lactoferrin, its use may also decrease immune-related pathology that may enhance drug penetration into tissue, and increase the development of protective immune response to potentially kill latent bacilli (4, 37).
TDM is the most immunostimulatory and granulomagenic lipid produced by MTB. Administration of TDM leads to an extensive recruitment of inflammatory cells accompanied by the cytokines and chemokines similar to those induced during natural TB infection (3, 23). Our results demonstrate that mice given lactoferrin after TDM challenge had decreased lung pathology at the peak of the granulomatous response. Modulation of the granuloma has potential implications for the treatment of both latent and active TB. MTB within granulomas adapt by conversion to a dormancy phenotype that includes decreased replication and changes in biochemical pathways (4, 5, 38). Antibiotics require actively growing bacilli for effectiveness and are thus less capable of eliminating dormant MTB sequestered within granulomas. Furthermore, decreasing immunopathology in the lung may enhance antibiotic penetration within infected tissue, thus promoting faster response to antimycobacterials (37). The combination of immunomodulators with TB antibiotics has yielded promising results in human clinical trials. One clinical trial examined the addition of etanercept, a soluble TNF-α receptor, to antimicrobial chemotherapy in HIV patients with TB and found significantly decreased time to sputum culture conversion with trends toward improved chest x-rays in the patients treated with the combination of etanercept and anti-TB agents (39). A second trial examined the use of high-dose prednisolone with TB antibiotics in patients with HIV and TB (40). The rate of sputum culture conversion was significantly increased in patients supplemented with prednisolone, compared to anti-TB chemotherapy alone. Lactoferrin as an adjuvant immunomodulator to TB therapy has a distinct advantage over other immunomodulators available because it is not immunosuppressive and has an excellent safety profile in numerous animal models and human clinical trials (21, 41–44)
A possible mechanism for the decreased lung pathology observed is the increase production of IL-10 and TGF-β, which have demonstrated critical roles in reducing pathology due to cell-mediated immunity (45). It is theoretically possible that TH2 cytokines may decrease immunity to TB (4). However, it is noteworthy that the classical cytokines critical for the control of TB, TNF-α and IFN-γ (46), were not significantly altered by lactoferrin treatment. IFN-γ is essential for controlling mycobacterial burden, the importance of which is highlighted by the severe mycobacterial infections experienced by patients who lack components of IFN-γ signaling (47). TNF-α is essential for granuloma formation and maintenance; absence of TNF-α results in severe necrotic pathology and reactivation of latent disease (38, 46, 48), as has been demonstrated for both infection and lipid models of pathology (25, 34, 49) The ability of lactoferrin to mount an anti-inflammatory response while maintaining pro-inflammatory cytokine production is not unique to these studies (7). For example, oral administration of lactoferrin to rats in a colitis model increased the anti-inflammatory cytokines IL-10 and IL-4 (50), as well as modulated immune related pathology in murine LPS models of sepsis (20, 51). In contrast, lactoferrin enhanced the delayed-type hypersensitivity response to BCG, ovalbumin, and sheep red blood cells (11, 52, 53). The ability of lactoferrin to exert anti-inflammatory and pro-inflammatory response makes it a candidate immunomodulator for a variety of infectious and inflammatory diseases.
We found that lactoferrin-treated macrophages infected with MTB had an increase in TNF-α production. This is in contrast to the global decrease in proinflammatory cytokines observed when the bone marrow-derived macrophages where given TDM-coated beads and treated with lactoferrin. This is likely due to the fact that MTB has a number of immunostimulatory molecules in addition to TDM, including ligands for the toll-like receptors, C-type lectins, and NOD-like receptors (54). Of interest, preliminary experiments found no direct effect of lactoferrin to modulate growth of organisms in vitro. Indeed, lactoferrin has an effect on growth of MTB in macrophages, but only if the macrophages are pre-activated or co-activated prior to lactoferrin treatment (unpublished data, Welsh and Actor). The data presented here utilizing infected macrophages highlight the need to examine the impact of lactoferrin in models of MTB infection. Studies in our laboratory are currently underway exploring the effect of lactoferrin on the pathology and bacterial burden in mice infected with MTB, as well as determining the mechanism of lactoferrin modulation of regulatory cytokine production
The TDM model of granuloma formation mimics many aspects of the immunopathology observed during aerosol infections in mice. This model system is therefore ideal for screening immunomodulators that are expected to alter the lung pathology observed in TB. These studies indicate that lactoferrin decreases lung pathology in a model of TB granuloma formation while maintaining a protective cytokine profile. Lactoferrin may therefore have clinical advantages as a useful adjuvant in the therapeutic treatment of TB.
Acknowledgements
We would like to thank the Quantitative Genomic Core Laboratory at the UTHealth Science Center for assistance with analysis of data and generation of primer-probe sets for use in the quantification of gene expression. This work was supported in part by NIH-NIAID grant R42-AI051050-04. This work was accomplished in partial fulfillment of the requirements for the M.D.-Ph.D. degree from The University of Texas Graduate School of Biomedical Sciences at Houston, TX. This work was presented in part at the joint meeting for the Central Society for Clinical Research, the American Physician Scientists Association and the American Society for Clinical Investigation (Chicago, IL, April, 2010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. WHO report 2009. Global tuberculosis control. Geneva: World Health Organization; Epidemiology, strategy, financing. 2009
- 2.Gillespie SH, Kennedy N. Fluoroquinolones: a new treatment for tuberculosis? Int J Tuberc Lung Dis. 1998;2(4):265–271. [PubMed] [Google Scholar]
- 3.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nature Immunology. 2009;10(9):943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churchyard GJ, Kaplan G, Fallows D, Wallis RS, Onyebujoh P, Rook GA. Advances in immunotherapy for tuberculosis treatment. Clin Chest Med. 2009;30(4):769–782. doi: 10.1016/j.ccm.2009.08.009. ix. [DOI] [PubMed] [Google Scholar]
- 5.Wayne LG, Sohaskey CD. Nonreplicating persistence of mycobacterium tuberculosis. Annu Rev Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 6.Hunter RL, Armitige L, Jagannath C, Actor JK. TB research at UT-Houston--a review of cord factor: new approaches to drugs, vaccines and the pathogenesis of tuberculosis. Tuberculosis (Edinb) 2009;89 Suppl 1:S18–S25. doi: 10.1016/S1472-9792(09)70007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des. 2009;15(17):1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birgens HS. The interaction of lactoferrin with human monocytes. Dan Med Bull. 1991;38(3):244–252. [PubMed] [Google Scholar]
- 9.Hammarstrom ML, Mincheva-Nilsson L, Hammarstrom S. Functional lactoferrin receptors on activated human lymphocytes. Adv Exp Med Biol. 1995;371A:47–53. doi: 10.1007/978-1-4615-1941-6_9. [DOI] [PubMed] [Google Scholar]
- 10.Iyer S, Yip TT, Hutchens TW, Lonnerdal B. Lactoferrin-receptor interaction. Effect of surface exposed histidine residues. Adv Exp Med Biol. 1994;357:245–252. [PubMed] [Google Scholar]
- 11.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol. 2002;2:475–486. doi: 10.1016/s1567-5769(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 12.Damiens E, Mazurier J, el Yazidi I, Masson M, Duthille I, Spik G, et al. Effects of lactoferrin on NK cell cytotoxicity again haematopoietic and epithelial tumour cells. Biochim Biophys Acta. 1998;1402:277–287. doi: 10.1016/s0167-4889(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 13.Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 2005;62(22):2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shau H, Kim A, Golub SH. Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. J Leukoc Biol. 1992;51(4):343–349. [PubMed] [Google Scholar]
- 15.Zimecki M, Mazurier J, Spik G, Kapp JA. Human lactoferrin induces phenotypic and functional changes in murine splenic B cells. Immunology. 1995;86(1):122–127. [PMC free article] [PubMed] [Google Scholar]
- 16.Sorimachi K, Akimoto K, Hattori Y, Ieiri T, Niwa A. Activation of macrophages by lactoferrin: secretion of TNF-alpha, IL-8 and NO. Biochem Mol Biol Int. 1997;43(1):79–87. doi: 10.1080/15216549700203841. [DOI] [PubMed] [Google Scholar]
- 17.Wilk KM, Hwang SA, Actor JK. Lactoferrin modulation of antigen-presenting-cell response to BCG infection. Postepy Hig Med Dosw. 2007;61:277–282. (Online). [PMC free article] [PubMed] [Google Scholar]
- 18.Kruzel ML, Actor JK, Boldogh I, Zimecki M. Lactoferrin in health and disease. Postepy Hig Med Dosw. 2007;61:261–267. (Online). [PubMed] [Google Scholar]
- 19.Hwang SA, Wilk KM, Bangale YA, Kruzel ML, Actor JK. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med Microbiol Immunol. 2007;196(3):171–180. doi: 10.1007/s00430-007-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruzel ML, Harari Y, Chen CY, Castro GA. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation. 2000;24(1):33–44. doi: 10.1023/a:1006935908960. [DOI] [PubMed] [Google Scholar]
- 21.Hwang SA, Wilk K, Kruzel ML, Actor JK. A novel recombinant human lactoferrin augments the BCG vaccine and protects alveolar integrity upon infection with Mycobacterium tuberculosis in mice. Vaccine. 2009;27(23):3026–3034. doi: 10.1016/j.vaccine.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006;36(4):371–386. [PubMed] [Google Scholar]
- 23.Hunter RL, Olsen M, Jagannath C, Actor JK. Trehalose 6,6'-dimycolate and lipid in the pathogenesis of caseating granulomas of tuberculosis in mice. Am J Pathol. 2006;168(4):1249–1261. doi: 10.2353/ajpath.2006.050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott AN, Guidry TV, Welsh KJ, Thomas AM, Kling MA, Hunter RL, et al. 11beta-hydroxysteroid dehydrogenases are regulated during the pulmonary granulomatous response to the mycobacterial glycolipid trehalose-6,6'-dimycolate. Neuroimmunomodulation. 2009;16(3):147–154. doi: 10.1159/000204227. [DOI] [PubMed] [Google Scholar]
- 25.Welsh KJ, Abbott AN, Hwang SA, Indrigo J, Armitige LY, Blackburn MR, et al. A role for tumour necrosis factor-alpha, complement C5 and interleukin-6 in the initiation and development of the mycobacterial cord factor trehalose 6,6'-dimycolate induced granulomatous response. Microbiology. 2008;154(Pt 6):1813–1824. doi: 10.1099/mic.0.2008/016923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bekierkunst A, Yarkoni E. Granulomatous hypersensitivity to trehalose-6,6'-dimycolate (cord factor) in mice infected with BCG. Infect Immun. 1973;7(4):631–638. doi: 10.1128/iai.7.4.631-638.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez RL, Roman J, Roser S, Little C, Olsen M, Indrigo J, et al. Cytokine message and protein expression during lung granuloma formation and resolution induced by the mycobacterial cord factor trehalose-6,6'-dimycolate. J Interferon Cytokine Res. 2000;20(9):795–804. doi: 10.1089/10799900050151067. [DOI] [PubMed] [Google Scholar]
- 28.Yamagami H, Matsumoto T, Fujiwara N, Arakawa T, Kaneda K, Yano I, et al. Trehalose 6,6'-dimycolate (cord factor) of Mycobacterium tuberculosis induces foreign-body- and hypersensitivity-type granulomas in mice. Infect Immun. 2001;69(2):810–815. doi: 10.1128/IAI.69.2.810-815.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidry TV, Hunter RL, Jr, Actor JK. Mycobacterial glycolipid trehalose 6,6'-dimycolate-induced hypersensitive granulomas: contribution of CD4+ lymphocytes. Microbiology. 2007;153(Pt 10):3360–3369. doi: 10.1099/mic.0.2007/010850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang SA, Actor JK. Lactoferrin modulation of BCG-infected dendritic cell functions. Int Immunol. 2009;21(10):1185–1197. doi: 10.1093/intimm/dxp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang SA, Kruzel ML, Actor JK. Influence of bovine lactoferrin on expression of presentation molecules on BCG-infected bone marrow derived macrophages. Biochimie. 2009;91(1):76–85. doi: 10.1016/j.biochi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kan-Sutton C, Jagannath C, Hunter RL., Jr Trehalose 6,6'-dimycolate on the surface of Mycobacterium tuberculosis modulates surface marker expression for antigen presentation and costimulation in murine macrophages. Microbes Infect. 2009;11(1):40–48. doi: 10.1016/j.micinf.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Indrigo J, Hunter RL, Jr, Actor JK. Influence of trehalose 6,6'-dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiology. 2002;148(Pt 7):1991–1998. doi: 10.1099/00221287-148-7-1991. [DOI] [PubMed] [Google Scholar]
- 34.Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41 Suppl 3:S189–S193. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- 35.Kaneko H, Yamada H, Mizuno S, Udagawa T, Kazumi Y, Sekikawa K, et al. Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab Invest. 1999;79(4):379–386. [PubMed] [Google Scholar]
- 36.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 37.Roy E, Lowrie DB, Jolles SR. Current strategies in TB immunotherapy. Curr Mol Med. 2007;7(4):373–386. doi: 10.2174/156652407780831557. [DOI] [PubMed] [Google Scholar]
- 38.Paige C, Bishai WR. Penitentiary or penthouse condo: the tuberculous granuloma from the microbe's point of view. Cell Microbiol. 2010;12(3):301–309. doi: 10.1111/j.1462-5822.2009.01424.x. [DOI] [PubMed] [Google Scholar]
- 39.Wallis RS, Kyambadde P, Johnson JL, Horter L, Kittle R, Pohle M, et al. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. Aids. 2004;18(2):257–264. doi: 10.1097/00002030-200401230-00015. [DOI] [PubMed] [Google Scholar]
- 40.Mayanja-Kizza H, Jones-Lopez E, Okwera A, Wallis RS, Ellner JJ, Mugerwa RD, et al. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. J Infect Dis. 2005;191(6):856–865. doi: 10.1086/427995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. Jama. 2009;302(13):1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 42.Tamano S, Sekine K, Takase M, Yamauchi K, Iigo M, Tsuda H. Lack of chronic oral toxicity of chemopreventive bovine lactoferrin in F344/DuCrj rats. Asian Pac J Cancer Prev. 2008;9(2):313–316. [PubMed] [Google Scholar]
- 43.Ueno H, Sato T, Yamamoto S, Tanaka K, Ohkawa S, Takagi H, et al. Randomized, double-blind, placebo-controlled trial of bovine lactoferrin in patients with chronic hepatitis C. Cancer Sci. 2006;97(10):1105–1110. doi: 10.1111/j.1349-7006.2006.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manzoni P, Decembrino L, Stolfi I, Pugni L, Rinaldi M, Cattani S, et al. Lactoferrin and prevention of late-onset sepsis in the preterm neonate in the NICU. Early Hum Dev. Feb 5; doi: 10.1016/j.earlhumdev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28(4):468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Co DO, Hogan LH, Kim SI, Sandor M. Mycobacterial granulomas: keys to a long-lasting host-pathogen relationship. Clin Immunol. 2004;113(2):130–136. doi: 10.1016/j.clim.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med. 1996;335(26):1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 48.Chan J, Flynn J. The immunological aspects of latency in tuberculosis. Clin Immunol. 2004;110(1):2–12. doi: 10.1016/s1521-6616(03)00210-9. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Juarrero M, Hattle JM, Izzo A, Junqueira-Kipnis AP, Shim TS, Trapnell BC, et al. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77(6):914–922. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- 50.Togawa J, Nagase H, Tanaka K, Inamori M, Umezawa T, Nakajima A, et al. Lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. Am J Physiol Gastrointest Liver Physiol. 283(1):G187–G195. doi: 10.1152/ajpgi.00331.2001. 200. [DOI] [PubMed] [Google Scholar]
- 51.Kruzel ML, Harari Y, Chen CY, Castro GA. The gut. A key metabolic organ protected by lactoferrin during experimental systemic inflammation in mice. Adv Exp Med Biol. 1998;443:167–173. [PubMed] [Google Scholar]
- 52.Kocieba M, Zimecki M, Kruzel M, Actor J. The adjuvant activity of lactoferrin in the generation of DTH to ovalbumin can be inhibited by bovine serum albumin bearing alpha-D-mannopyranosyl residues. Cell Mol Biol Lett. 2002;7(4):1131–1136. [PubMed] [Google Scholar]
- 53.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol Lett. 2000;74(3):183–188. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 54.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206(13):2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]