Abstract
Objective
To establish the test-retest reliability of linear and nonlinear measures, including intra- and inter- session reliability, when used to analyze the center of pressure (COP) time series during the development of infant sitting postural control in infants with or at risk for cerebral palsy (CP).
Design
Longitudinal study.
Setting
University hospital laboratory.
Participants
Infants with or at risk for CP (N=18; mean age at entry in the study ± SD, 13.1.7 ± 3.6mo).
Interventions
Not applicable.
Main Outcome Measures
Infant sitting COP data was recorded for 3 trials at each session (2 sessions for each month within 1 week) for 4 consecutive months. The linear COP parameters of root mean square and range of sway for both the anterior-posterior and the medial-lateral directions, and sway path, were calculated. In addition, the nonlinear parameters of approximate entropy, Lyapunov exponent (LyE), and correlation dimension for both directions were also calculated. Intra-session and inter-session reliability was computed by the intraclass correlation coefficient (ICC).
Results
Regarding nonlinear measures, LyE showed high intra-session and inter-session ICC values in comparison to all other parameters evaluated. Intra-session and inter-session reliability increased overall in the last 2 months of the data collections and as sitting posture improved.
Conclusions
Our results suggested that the methodology presented is reliable way of examining the development of sitting postural control in infants with or at risk for CP, and the reliability results generally parallels values found in sitting postural behavior in typical infants. Therefore, this methodology may be helpful in examining efficacy of therapy protocols directed at advancing sitting postural control in infants with motor developmental delays.
Keywords: Cerebral palsy, Developmental disabilities, Nonlinear dynamics, Posture, Rehabilitation, Reproducibility of results
Cerebral palsy (CP) is defined as a nonprogressive disorder of posture and movement, which is caused by damage to the motor control centers of the developing brain, and can occur pre-, peri- and post-natally1. Children with CP have several fundamental limitations in postural control of static and dynamic tasks, such as sitting, standing and walking2. In particular, a delay in achieving the first milestone of postural control, which is independent sitting, is one early sign that a child’s development is not following a normal course3. Disruptions in sitting postural control significantly affect the development of a child, and can limit the ability to develop eventual independent movement4–6.
A diagnosis of CP is often delayed until the child is over 2 years of age. Initial identification of a developmental problem during early infancy is difficult since current clinical testing methods are not highly specific or sensitive, and some early neurological symptoms may be transient and resolve spontaneously7. On the other hand, early intervention is considered essential to take advantage of the plasticity of the developing infant’s nervous system for optimal development8. Thus, there is a need to identify a quantifiable method that will assess the developing mechanisms of sitting postural control in children with early postural control problems, describe and identify the types of problems to target in early intervention, and help to determine early intervention efficacy.
Postural control can be described using a simple paradigm of sitting and standing on a force platform to measure the center of pressure (COP) to quantify body sway. The organization of posture has been described repeatedly in the literature by the COP9. COP data have been used in investigations of postural control during standing in healthy adults during a dual task paradigm10 and Parkinson’s disease patients11, as well as in healthy young children12 and children with cerebral palsy13. The reliability of this methodology has been examined thoroughly during standing for both healthy and unhealthy populations. Intraclass correlation coefficient (ICC), which is a statistical method of evaluating reproducibility of results, revealed that COP measures in general produced poor to fair reliability (0.3 to 0.75) under static and dynamic balance tasks14–17.
Furthermore, in the past few years new concepts and methods for studying postural control have been introduced. Currently, COP data have been evaluated not only with conventional linear measures, which provide an “average” picture and lose the temporal aspect of sitting, but also with nonlinear measures, which describe the temporal organization of the postural sway pattern of sitting18. Nonlinear measures can provide new insights in the ways that the nervous system controls the complexity of dynamic balance19, 20. Moreover, nonlinear measures unveil different features of the COP data. For example, range and the length of path traced by the COP, which are traditional linear measures, evaluate the quantity of movement variations of the COP during a specific task independently of their order in the distribution. On the other hand, Lyapunov Exponent (LyE) and Approximate Entropy (ApEn), which are nonlinear measures, they are able to capture the temporal component of the movement variation in COP regarding how motor behavior emerges in time. Temporal organization or “structure” can be measures by the extent to which values of COP data emerge in a predictable way19–22. The usage of these measures has increased recently because they allow the quantification of constructs such as regularity, complexity, and stability20. Thus, nonlinear analyses of the COP data as sitting develops can provide a window into the neurological status of the infant with CP, and allow insight into the multifaceted strategies these infants utilize to organize movement and posture.
Recently, the COP methodology has also been utilized to investigate sitting postural control19,20,23,24. However, the reliability of COP measures for the evaluation of infant sitting postural control has been identified only for typically developing infants25. Specifically, Kyvelidou et al.25 found that COP measures for the evaluation of infant sitting postural control is a fairly reliable methodology. They examined both linear and nonlinear measures of COP during the development of sitting posture in typically developing infants. They found that both types of measures presented inter-session and intra-session ICC values ranging from poor to good reproducibility, with the last two months of data collection presenting consistently fair to good ICC values25. However, the reliability of this methodology for infants with cerebral palsy is currently unknown.
Therefore, the purpose of this study was to establish the reliability of linear and nonlinear measures, including intra- and inter- session reliability, when used to analyze the COP data during the development of sitting postural control in infant with or at risk of CP. Based on the previous reliability data on typical development of infant sitting25, we hypothesized that the nonlinear tools will be more reliable in assessing development of infant sitting postural control and that reliability measures will increase with development. The identification of the reliability of linear and nonlinear tools from COP data is necessary in order to validate the reliability of the procedure, so that it can then used in the future to assess efficacy of treatment and increments of change over time in children with or at risk for CP. Once this procedure is established, comparisons of the sitting behavior of infants with typical development and infants with cerebral palsy can be made, and be certain that our results are not measurement artifacts but true differences.
Methods
Participants
For the present study we recruited 18 infants with or at risk for CP (mean age at entry in the study ± SD, 13.1.7 ± 3.6mo; gender, 10 males 8 females). The infants were referred from local early intervention programs. The infants were followed from the age where they could exhibit at least 10 sec of independent sitting and for four months after that time. Infants were recruited from employee announcements at the campus of the university. The parents of the infants provided informed consent that was approved by the university human research ethics committee before data collection initiation. The inclusion criteria for entry into the study for the infants with or at risk for CP as well as the exclusion criteria are presented in Table 1. Furthermore, the Gross Motor Function Classification Scale (GMFCS) level as well as the diagnosis that the infants with or at risk for CP received after two years of age is presented in Table 2.
Table 1.
Inclusion and exclusion criteria of the study
| Inclusion Criteria |
| Age from five months to two years |
| Score less than 1.5 SD below the mean for their corrected age on the peabody Gross Motor Scales |
| Sitting skills |
|
|
Exclusion Criteria |
| Age over two years |
| Score greater than 1.5 SD below the mean for their corrected age on Peabody Gross Motor Scale |
| Diagnosed visual impairment |
| Diagnosed hip dislocation or subluxation greater than 50% |
Table 2.
Gross Motor Function Classification Scale scores for all infants.
| Subject | Diagnosis at 2 years old | Severity | GMFCS |
|---|---|---|---|
| C01 | Hypotonic, overall delays | Moderate | 3 |
| C02 | Developmental delay | Mild* | |
| C03 | Premature (28 weeks), BPD | Mild* | |
| C04 | Athetoid CP | Moderate | 2 |
| C05 | Mixed Quadriplegic CP | Moderate | 3 |
| C06 | Spastic Quadriplegic CP | Severe | 4 |
| C07 | Right Hemiplegic CP | Mild | 1 |
| C08 | Noonan’s Syndrome | Mild* | |
| C09 | Spastic Hemiplegic CP | Moderate | 3 |
| C10 | Spastic Quadriplegic CP | Severe | 4 |
| C11 | Hypotonic, motor delay | Moderate | 2 |
| C12 | Hypotonic, motor delay | Mild | 1 |
| C13 | Spastic Diplegia | Moderate | 2 |
| C14 | Motor delay, hearing impaired | Mild | 1 |
| C15 | Premature, motor delay | Mild* | |
| C16 | Premature, left hemiplegia | Mild | 1 |
| C17 | Premature, motor delay | Mild* | |
| C18 | Hypotonia, motor delay | Mild | 1 |
Diagnosis of CP excluded; children considered to have developmental delay and not CP
BPD - Brochial Pulmonary Dysplasia
GMFCS - Gross Motor Function Classification Scale
Experimental design
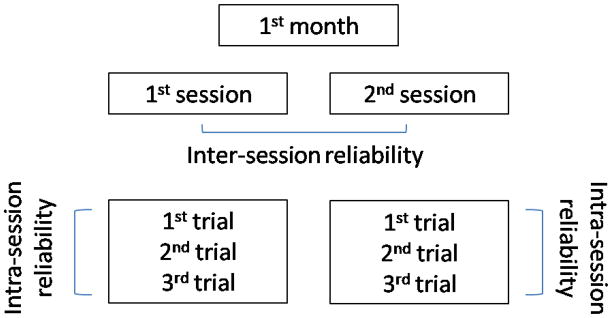
Each infant participated in nine sessions. The first session and was used to perform the Peabody Gross Motor Scale26 which is a standardized clinical test37. In addition, the child was tested to determine adequate prop sitting skills to begin the study, and to familiarize the family with the procedures used in the study. The other eight sessions were dispersed over a time period of four months. To assure that inter-session measures captured the infants at the same stage of sitting development, the infants were tested twice in one week at each of the four months of the study. Three trials per session were used to determine intra-session reliability (Figure 1). The repeat testing within one week of each month’s testing was utilized for the estimation of the inter-session reliability (Figure 1).
Figure 1.
Schematic representation of inter and intra-session reliability. This procedure was repeated for each month of data collections.
Protocol
For all sessions, the infants and the parents were given time to get used to the laboratory environment. Subsequently, they sat on the force platform with their parent in front of them for the data collection. The sessions lasted approximately 30 minutes to one hour. After the force platform was covered with an absorbent pad, which was securely adhered with tape, infants were positioned by their parent on the top of the force platform. The infant was in the sitting position in the middle of the plate when calm (Figure 2). For safety reasons, the investigator and the parent remained at one side and in front of the infant respectively during all data collection. When the child was ready, and was not held by the examiner, COP data were collected continuously while the child attempted to maintain the sitting position control without falling. Once we had collected three trials that were acceptable for our criteria (see below), or until the infants were indicating that they were done, data collections were completed.
Figure 2.

Position of infant during data collection.
From the videotape record we selected three acceptable trials (8.3 seconds each) based on the following criteria: a) infant did not move the arms (not reaching, holding an object, or flapping their arms), b) infant did not vocalize or cry, c) infant was not in the process of falling, d) trunk was not inclined more than 45 degrees to either side, e) not being touched, f) the arm position (propping or not propping) of the infants was noted during the entire trial and only trials that have the infant using a consistent base of support was used.
For the collection of the COP data, infants sat on an AMTI force platforma (Model OR6-7-1000), interfaced to a computer system running Vicon data acquisition softwareb. The force platform simultaneously measures three force components Fx, Fy, and Fz and three moment components Mx, My, and Mz. The forces and moments are measured by strain gauges attached to load cells at the four corners of the platform. The force plate has a 4450 N (1000 lb) capacity for Fz and a 2225 N (500 lb) capacity for Fx and Fy. The Fz channel has a natural frequency of 480 Hz and Fx and Fy have a natural frequency of 300 Hz. COP data in both the anterior-posterior (AP) and the medial-lateral (ML) directions were acquired through the Vicon software at 240 Hz, in order to be above a factor of ten higher than the highest frequency contained in the signal. No filtering was performed on the data because such a procedure can affect the nonlinear results. Furthermore, video of each trial was collected using two Panasonic recordersc (Model 5100 HS) interfaced with a Panasonic Digital AV Mixerc (Model WJ-MX30). The cameras were positioned to record a sagittal and a frontal view of the subject. Segments of acceptable (described below) data were analyzed using custom MatLab softwared. The COP data selected allowed for the examination of 2000 data points (8.3 sec times 240 Hz) for each COP direction for each trial. This number is considered adequate for nonlinear analysis27,28.
Data analysis
Customized MatLab software was utilized to calculate the linear measures from the COP data from the selected trials, using the methodology of Prieto et al.29 and included root-mean-square (RMS), maximum minus minimum (range) and length of the path traced by the COP (sway path) for the AP and the ML directions. These parameters are all independent of the effect of biomechanical factors such as weight30, which may changed rapidly during infancy. These linear measures characterized the amount of variability present in the data18.
Furthermore, three nonlinear measures of variability were calculated from the selected trials: the approximate entropy (ApEn), the largest Lyapunov exponent (LyE), and the correlation dimension for both the AP and the ML directions. Calculation of the nonlinear measures of the variability present in postural sway was performed as presented by Harbourne and Stergiou19. Chaos Data Analyzer Professional software31 was used to calculate the Lyapunov Exponent and the Correlation Dimension. In order to precisely compute these measures, the embedded dimension must be chosen with extreme care. We estimated the embedded dimension by performing the Global False Nearest Neighbor (GFNN) analysis32, with the Tools for Dynamics software. The embedded dimension is a depiction of the number of dimensions needed to unfold the attractor of a dynamical system in state space33. For the analysis of all COP traces, the same embedding dimension (6) was used even if they had a dimension lower than six. Lastly, for the calculation of the ApEn custom written MATLAB code was used based on the Pincus34 algorithms.
Statistical Analysis
Intra-session and inter-session reliability was quantified by the intraclass correlation coefficient35 (ICC). Specifically, a one-way ANOVA model with a random subject effect was used to estimate the intra-session reliability based on data from the first visit of the month for each child (ICC[1,1] in the notation of Shrout and Fleiss35). To estimate the inter-session reliability, the averages of the three measurements during each session are analyzed using a one-way ANOVA model with a random subject effect similar to the model for intra-session reliability. In the results section ICC findings are reported based on Rosner36. Specifically, an ICC of less than 0.4 indicates poor reproducibility while an ICC between 0.4 and 0.75 indicates fair to good reproducibility. Lastly, an ICC over 0.75 indicates excellent reproducibility.
Results
Linear Parameters
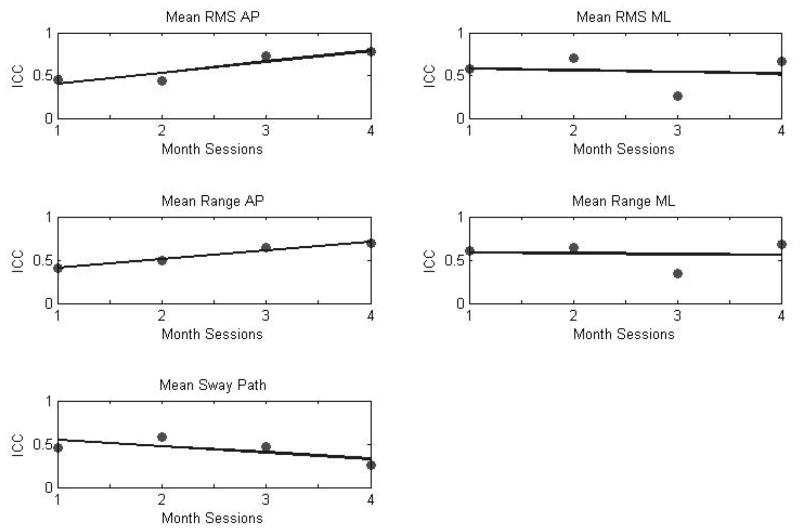
Inter-session ICCs for the linear parameters were between 0.25 and 0.78 (Table 3). The RMS in the AP direction presented the highest ICC value. All linear parameters presented ICC values ranging from poor to fair to excellent reproducibility. The highest mean ICC value across months was observed for RMS in AP direction. However, the last month of data collections presented consistently fair to good ICCs with the exception of the sway path parameter (Figure 3). RMS and mean range in AP direction showed consistently increasing values in ICCs across months of sitting postural development. However, sway path presented consistently decreasing values in ICCs across months of sitting postural development.
Table 3.
Inter-session (within a week per month) reliability, as expressed with the Intra-class correlation coefficient (ICC), for all linear parameters.
| Variables | ICC’s | ||||
|---|---|---|---|---|---|
| 1st Month | 2nd Month | 3rd Month | 4th Month | Mean | |
| RMS AP | 0.44 | 0.44 | 0.72 | 0.78 | 0.59 |
| RMS ML | 0.58 | 0.70 | 0.25 | 0.67 | 0.55 |
| Range AP | 0.40 | 0.49 | 0.65 | 0.69 | 0.56 |
| Range ML | 0.61 | 0.64 | 0.35 | 0.68 | 0.57 |
| Sway Path | 0.46 | 0.57 | 0.46 | 0.25 | 0.43 |
Figure 3.
Inter-session reliability (ICC) for linear parameters of COP across months. Most linear parameters ICCs are averaging around 0.5 and there is an increasing trend as the infant develops. This is not true for Mean Sway Path where ICC are presenting a decreasing trend across development.
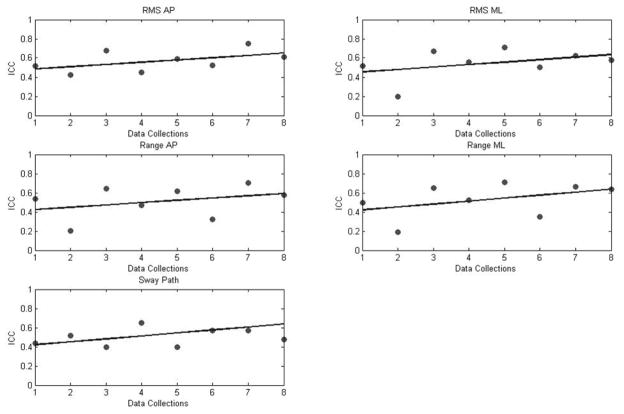
Intra-session ICCs for linear parameters were between 0.19 and 0.75 (Table 4). RMS in the AP direction presented the highest ICC value, which suggests excellent reproducibility. All linear parameters presented ICC values ranging from poor to fair to excellent reproducibility. The highest mean ICC value across months was observed for RMS in AP direction. However, the last three data collections, which are included in the third and fourth month sessions, presented consistently fair to good ICCs (Table 4, Figure 4). We can observe that RMS, range and sway path presented consistently increasing values in ICC’s across data collections. The above findings are in agreement with the inter-session reliability with the exception of sway path.
Table 4.
Intra-session (within each session) reliability, as expressed with the Intra-class correlation coefficient (ICC), for all linear parameters.
| Variables | ICC’s | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sessions | 1st Month | 2nd Month | 3rd Month | 4th Month | |||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | Mean | |
| RMS AP | 0.51 | 0.42 | 0.68 | 0.45 | 0.59 | 0.53 | 0.75 | 0.61 | 0.57 |
| RMS ML | 0.52 | 0.20 | 0.67 | 0.55 | 0.71 | 0.50 | 0.62 | 0.57 | 0.54 |
| Range AP | 0.53 | 0.20 | 0.64 | 0.47 | 0.62 | 0.32 | 0.70 | 0.58 | 0.51 |
| Range ML | 0.50 | 0.19 | 0.65 | 0.52 | 0.71 | 0.35 | 0.66 | 0.64 | 0.53 |
| Sway Path | 0.44 | 0.52 | 0.37 | 0.65 | 0.40 | 0.57 | 0.57 | 0.48 | 0.50 |
Figure 4.
Intra-session reliability (ICC) for linear parameters of COP across data collection sessions. All linear parameters ICCs are averaging around 0.5 and there is an increasing trend as the infant develops
Nonlinear Parameters
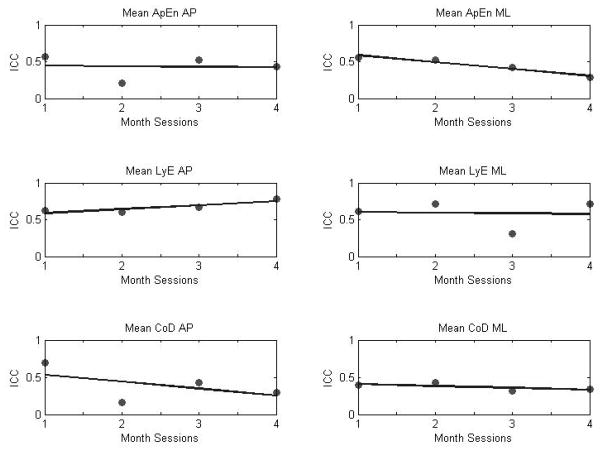
Inter-session ICCs for nonlinear parameters were between 0.16 and 0.78 (Table 5). LyE in the AP direction presented the highest ICC value, which suggests excellent reproducibility. All nonlinear parameters presented ICC values ranging from poor to fair to excellent reproducibility. The highest mean ICC value across months was observed for LyE in AP direction. However, the last two months of data collections presented alternating fair to good reproducibility (Table 4, Figure 5).
Table 5.
Inter-session (within a week per month) reliability, as expressed with the Intra-class correlation coefficient (ICC), for all nonlinear parameters
| Variables | ICC’s | ||||
|---|---|---|---|---|---|
| 1st Month | 2nd Month | 3rd Month | 4th Month | Mean | |
| ApEn AP | 0.57 | 0.21 | 0.52 | 0.44 | 0.43 |
| ApEn ML | 0.56 | 0.53 | 0.42 | 0.28 | 0.45 |
| LyE AP | 0.62 | 0.60 | 0.67 | 0.78 | 0.67 |
| LyE ML | 0.61 | 0.72 | 0.31 | 0.72 | 0.59 |
| CoD AP | 0.69 | 0.15 | 0.43 | 0.29 | 0.39 |
| CoD ML | 0.39 | 0.43 | 0.31 | 0.34 | 0.37 |
Figure 5.
Inter-session reliability (ICC) for nonlinear parameters of COP across months. All nonlinear parameters ICCs are averaging lower than 0.5 except of LyE in both directions.
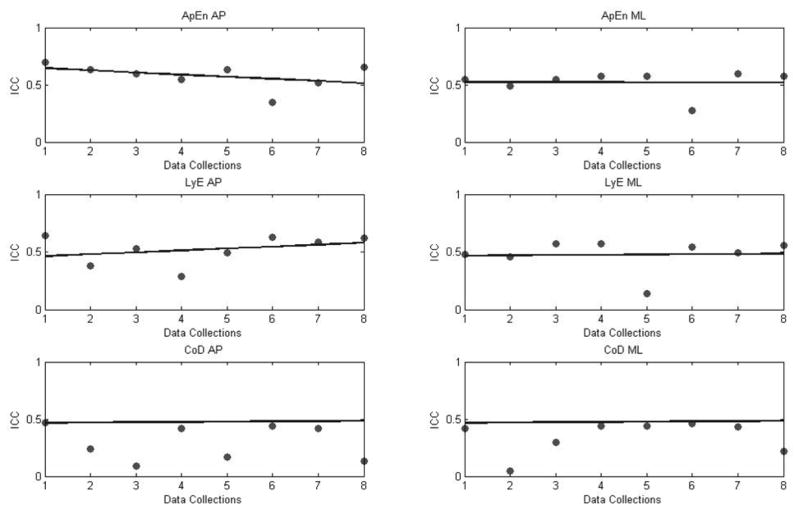
Intra-session ICCs for nonlinear parameters were between 0.05 and 0.70 (Table 6). Overall, nonlinear parameters presented ICC values ranging from poor to fair to good reproducibility. The highest mean ICC value across months was observed by ApEn in the AP direction. Furthermore, with the exception of correlation dimension all other nonlinear parameters present fair to good reproducibility across data collections (Figure 6).
Table 6.
Intra-session (within each session) reliability, as expressed with the Intra-class correlation coefficient (ICC), for all nonlinear parameters.
| Variables | ICC’s | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sessions | 1st Month | 2nd Month | 3rd Month | 4th Month | |||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | Mean | |
| ApEn AP | 0.70 | 0.63 | 0.60 | 0.54 | 0.63 | 0.35 | 0.52 | 0.65 | 0.58 |
| ApEn ML | 0.54 | 0.49 | 0.55 | 0.57 | 0.57 | 0.27 | 0.59 | 0.57 | 0.52 |
| LyE AP | 0.64 | 0.38 | 0.53 | 0.29 | 0.49 | 0.63 | 0.58 | 0.62 | 0.52 |
| LyE ML | 0.48 | 0.45 | 0.57 | 0.57 | 0.13 | 0.54 | 0.49 | 0.56 | 0.47 |
| CoD AP | 0.47 | 0.24 | 0.09 | 0.42 | 0.17 | 0.44 | 0.42 | 0.13 | 0.30 |
| CoD ML | 0.42 | 0.05 | 0.31 | 0.44 | 0.44 | 0.46 | 0.43 | 0.22 | 0.35 |
Figure 6.
Intra-session reliability (ICC) for nonlinear parameters of COP across data collection sessions. All nonlinear parameters ICCs are averaging around 0.5 except of correlation dimension in both directions.
Discussion
The goal of the present study was to establish the reliability of linear and nonlinear measures, including intra- and inter- session reliability, when utilized to examine the COP data during the development of sitting postural control in infants with or at risk for CP. Based on our previous study20, we hypothesized that the linear and nonlinear measures will present different reliability values because they are quantifying different features of the COP data.
Reliability assessment of all linear parameters during sitting posture in infants with or at risk for CP presented inter- and intra- session ICC values ranging from poor, to good, to excellent reproducibility. Similarly to our previous study in the development of sitting postural control in typically developing infants20, the last two months of data collections presented consistently fair to good ICCs. In contrast the sway path parameter presented decreased values of inter-session reliability across development, while the intra- session ICCs were increased across development. Similarly, reliability assessment of all nonlinear parameters during sitting posture in infants with or at risk for CP presented inter- and intra- session ICC values ranging from poor to good reproducibility. However, the last two months of data collections did not present increased ICC values but were consistently fair to good across development with the exception of correlation dimension in both anterior-posterior and medial-lateral directions. Overall, RMS and LyE presented the highest ICC values compared to all other parameters examined, while the rest of the linear and nonlinear parameters presented acceptable values with the exception of correlation dimension which showed low reproducibility.
Reliability of linear parameters during sitting posture in infants with or at risk for CP paralleled the results of a reliability study of typical infants during the development of sitting25. Specifically, RMS in both directions showed fair to good ICC inter- (0.59 in AP and 0.55 in ML) and intra- session (0.57 in AP and 0.54 in ML) values in infants with or at risk for CP while typical infants showed also fair to good ICC values inter- (0.44 in AP and 0.41 in ML) and intra- session (0.51 in AP and 0.49 in ML) 25. Similar results were observed in range and sway path in the infants with or at risk for CP and typical infants. Furthermore, standing posture studies in healthy adults14 and elderly individuals15, 37 showed similar reliability findings with sitting posture in infants with or at risk for CP. Particularly, the nonlinear measure RMS in AP and ML directions presented fair to good intra-session reproducibility (0.58) during a standing task of healthy elderly individuals37. Moreover, intra-session ICC values for the range of COP during standing in healthy adults were fair to good for both the AP and ML directions16. However, inter-session reproducibility of linear measure during a standing task of healthy adults presented fair to poor reliability 14. In addition, children without disabilities exhibited similar ICC values of linear parameters during standing balance tasks to those infants with or at risk for CP during the development of sitting16. Intra-session reliability of the Smart Balance Master System, which examines standing posture under different sensory conditions, presented ICC values with a wide range between 0 and 0.7916. Lastly, inter-session reliability of Smart Balance Master System ranged between 0.08 to 0.6816. Therefore, our present findings are parallel to those reported in the literature from standing posture studies.
With regards to the reproducibility of the nonlinear measures during sitting posture in infants with or at risk for CP presented here, we observed fairly similar results as the reliability data from sitting postural control of typically developing infants25. In typical infants, ApEn presented the highest ICC values, while in infants with CP or at risk for CP, LyE presented the highest ICC values. correlation dimension presented poor to moderate ICC values in both groups of infants. In a recent study, a different nonlinear measure, fractal dimension, presented most of the times higher intra-session reliability than linear measures from COP data during standing in young healthy people, and overall fair to good to excellent reliability values 38. Analogous to the findings of the present study, ApEn, which is a measure of complexity in the time series, demonstrated fair to good intra-session (>0.50) reproducibility of COP during development of sitting in infants with or at risk for CP.
It is important to note that intra- and inter- session reliability of sitting posture in infants with or at risk for CP improved on the last two months of data collections, especially with the linear measures. Similarly, younger children showed lower ICC values than older children when their COP sway index was investigated during a standing task.
Study Limitations
It should be mentioned that inter-subject variability may have influenced our results. Possibly, when infants with CP or at risk for CP entered the study, their sitting behavior was not at the same level. For example, some infants may have entered the study while being able to prop sit, while other infants may did not use the help of their hands at the onset of the study. Presumably, this may be one reason why we observed differences in the sitting behavior in the first two months of sitting development. The usage of stages of sitting instead of months could be used as an alternative to describe sitting postural development. Moreover, the rapid physiological, neuromuscular and psychological changes that infants undergo early on may be the reason why inter-session reliability did not show consistently excellent reproducibility. Therefore, multiple repeated testing distributed across the months of sitting development may allow us to describe more accurately sitting postural control in both typically developing infants and infants with or at risk of CP, since infants are going through a period of rapid growth and change along many interwoven line
CONCLUSIONS
We determined that linear and nonlinear description of COP data is a reliable method for assessing the development of sitting postural control in infants with or at risk of CP. Our results from our linear and nonlinear parameters were similar to those reported in the literature from sitting and standing posture studies. Regarding the linear tools, RMS presented the highest intra- and inter- session ICC values among all other parameters. Regarding the nonlinear tools, LyE presented the highest intra- and inter- session ICC values among all other parameters. In contrast, correlation dimension presented the lowest intra- and inter- session ICC values in comparison to all other parameters examined. Therefore, the presented methodology is not only a reliable tool for the evaluation of sitting postural control using linear and nonlinear tools of COP data, but also a tool to quantifying small amounts of change in the variability patterns of COP data during the development of sitting postural control in infants with or at risk for CP. The present study is extremely important because we can use the presented methodology to assess efficacy of treatment and increments of change over time in children with or at risk for CP. Once this procedure is established we can compare infants with typical development and infants with cerebral palsy and be certain that our results are not measurement artifacts but true differences.. The next step is to determine the validity of these measures in explaining differences in these parameters between infants with typical development and infants with neuromotor disorders. Changes in developing postural control due to learning, maturation and intervention for children with neuromotor disorders can then be examined using measures that better quantify small increments of improving or decreasing motor control. Furthermore, in our future research we plan to explore how COP measures relate with other functional tasks during infant sitting.
Clinical Implications
Infant assessment is notoriously unreliable, with the results being that most testing requires either a scale with many items to obtain a reliable overall picture of the function or behavior of interest, or examination over time to determine problems needing intervention. Because of the variability in the reliability of the many measures described in this paper, it is likely that a scale using a composite of the variables will better represent the postural behavior of the child reliably.
Acknowledgments
Supported by National Institutes of Health (grant no. K25HD047194), National Institute of Disability and Rehabilitation Research (grant no. H133G040118), the Nebraska Research Initiative, and the Bukey Fellowship and MacDonald Fellowship from University of Nebraska Medical Center.
List of Abbreviations
- AP
anterior/posterior
- ApEn
approximate entropy
- COP
center of pressure
- CP
cerebral palsy
- ICC
intra class correlation coefficient
- LyE
Lyapunov exponent
- ML
medial/lateral
- RMS
root mean square
Footnotes
Presented in part at the Combined Section Meeting of the American Physical Therapy Association, February 9, 2009, Las Vegas, NV.
Model OR6-7-1000; Advanced Mechanical Technology Inc, 176 Waltham St, Watertown, MA 02472-4800.
Vicon-Los Angeles, 5419 McConnell Ave, Los Angeles, CA 90066.
Panasonic Corp of North America, One Panasonic Way, Secausus, NJ 07094.
The MathWorks, 3 Apple Hill Dr, Nantick, MA 01760-2098.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hughes I, Newton R. Genetic aspects of cerebral palsy. Dev Med Child Neurol. 1992;34:80–86. doi: 10.1111/j.1469-8749.1992.tb08568.x. [DOI] [PubMed] [Google Scholar]
- 2.Woollacott MH, Shumway-Cook A. Postural dysfunction during standing and walking in children with cerebral palsy: what are the underlying problems and what new therapies might improve balance? Neural Plast. 2005;12:211–9. doi: 10.1155/NP.2005.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell SK. The child’s development of functional movement. In: Campbell SK, Vander Linden DW, Palisano RJ, editors. Physical therapy for children. 3. Missouri: St. Louis; 2006. pp. 33–76. [Google Scholar]
- 4.van der Heide JC, Hadders-Algra M. Postural muscle dyscoordination in children with cerebral palsy. Neural Plast. 2005;12:197–203. doi: 10.1155/NP.2005.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogren E, Hadders-Algra M, Forssberg H. Postural control in sitting children with cerebral palsy. Neurosci Biobehav Rev. 1998;22:591–596. doi: 10.1016/s0149-7634(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 6.Hadders-Algra M, van der Fits IB, Stremmelaar EF, Touwen BC. Development of postural adjustments during reaching in infants with CP. Dev Med Child Neurol. 1999;41:766–776. doi: 10.1017/s001216229900153x. [DOI] [PubMed] [Google Scholar]
- 7.Campbell SK. The infant at risk for developmental disability. In: Campbell SK, editor. Decision Making in Pediatric Neurologic Physical Therapy. Churchhill Livingstone; Philadelphia: 1999. pp. 260–332. [Google Scholar]
- 8.Deffeyes JE, Kochi N, Harbourne RT, Kyvelidou A, Stuberg WA, Stergiou N. Nonlinear Detrended Fluctuation Analysis of Sitting Center-of-Pressure Data as an Early Measure of Motor Development Pathology in Infants. Nonlinear Dynamics Psychol Life Sci. 2009;3:351–68. [PubMed] [Google Scholar]
- 9.Massion J. Movement, posture, and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 10.Donker FS, Roerdink M, Greven AJ, Beek PJ. Regularity of center-of-pressure trajectories depends on the amount of attention invested in postural control. Exp Brain Res. 2007;181:1–11. doi: 10.1007/s00221-007-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J Neurol Neurosurg Psyc hiatry. 2002;73:267–74. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riach CL, Hayes KC. Maturation of postural sway in young children. Dev Med Child Neurol. 1987;29:650–8. doi: 10.1111/j.1469-8749.1987.tb08507.x. [DOI] [PubMed] [Google Scholar]
- 13.Cherng RJ, Su FC, Chen JJ, Kuan TS. Performance of static standing balance in children with spastic diplegic cerebral palsy under altered sensory environments. Am J Phys Med Rehabil. 2007;78:336–43. doi: 10.1097/00002060-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Brouwer B, Culham EG, Liston RAL, Grant T. Normal variability of postural measure: implications for the reliability of relative balance performance outcomes. Scand J Rehab Med. 1998;30:131–7. doi: 10.1080/003655098444048. [DOI] [PubMed] [Google Scholar]
- 15.Lafond L, Corriveau H, He′bert R, Prince MF. Intrasession Reliability of Center of Pressure Measures of Postural Steadiness in Healthy Elderly People. Arch Phys Med Rehabil. 2004;85:896–901. doi: 10.1016/j.apmr.2003.08.089. [DOI] [PubMed] [Google Scholar]
- 16.Liao H, Mao P, Hwang A. Test-retest reliability of balance tests in children with cerebral palsy. Dev Med Child Neurol. 2001;43:180–6. [PubMed] [Google Scholar]
- 17.Baker CP, Newstead AH, Mossberg KA, Nicodemus CL. Reliability of static standing balance in nondisabled children: comparison of two methods of measurement. Pediatr Rehabil. 1998;2:15–20. doi: 10.3109/17518429809078611. [DOI] [PubMed] [Google Scholar]
- 18.Stergiou N, Harbourne RT, Cavanaugh JT. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30:120–9. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- 19.Harbourne RT, Stergiou N. Nonlinear analysis of the development of sitting postural control. Dev Psychobiol. 2003;42:368–77. doi: 10.1002/dev.10110. [DOI] [PubMed] [Google Scholar]
- 20.Harbourne RT, Deffeyes JE, Kyvelidou A, Stergiou N. Complexity of postural control in infants: linear and nonlinear features revealed by principal component analysis. Nonlinear Dynamics Psychol Life Sci. 2009;13:123–44. [PubMed] [Google Scholar]
- 21.Sosnoff JJ, Newell KM. Are age-related increases in force variability due to decrements in strength? Exp Brain Res. 2006;174:86–94. doi: 10.1007/s00221-006-0422-x. [DOI] [PubMed] [Google Scholar]
- 22.Harbourne TH, Stergiou N. Movement Variability and the Use of Nonlinear Tools: Principles to Guide Physical Therapist Practice. Phys Ther. 2009;89:267–282. doi: 10.2522/ptj.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertenthal BI, Rose JL, Bai DL. Perception-action coupling in the development of visual control of posture. J Exp Psychol. 1997;23:1631–1643. doi: 10.1037//0096-1523.23.6.1631. [DOI] [PubMed] [Google Scholar]
- 24.Boker SM, Schreiber T, Pompe B, Bertenthal BI. Nonlinear analysis of perceptual-motor coupling in the development of postural control. In: Kantz H, Kurths J, Mayer-Kress G, editors. Nonlinear Techniques in Physiological Time Series Analysis. Heidelberg, Germany: Springer; 1998. [Google Scholar]
- 25.Kyvelidou A, Harbourne RT, Stuberg WA, Sun J, Stergiou N. Reliability of center of pressure measures for assessing the development of sitting postural control. Arch Phys Med Rehabil. 2009;90:1176–1184. doi: 10.1016/j.apmr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell D, Rosenbaum P, Gowland C, Hardy S, Lane M, Plews N, McGavin H, Cadman D, Jarvis S. Gross Motor Function Measure. McMaster University Pub; Ontario, Canada: 1993. [Google Scholar]
- 27.Grassberger P, Procaccia I. Measuring the strangeness of strange attractors. Physica D. 1983;9:189–208. [Google Scholar]
- 28.Pincus SM, Gladstone IM, Ehrenkranz RA. A regularity statistic for medical data analysis. Journal of Clinical Monitoring. 1991;7:335–345. doi: 10.1007/BF01619355. [DOI] [PubMed] [Google Scholar]
- 29.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans Biomed Eng. 1996;43:956–66. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 30.Chiari L, Rocchi L, Capello A. Stabilometric parameters are affected by anthropometry and foot placement. Clin Biomech. 2002;17:666–677. doi: 10.1016/s0268-0033(02)00107-9. [DOI] [PubMed] [Google Scholar]
- 31.Sprott JC, Rowlands G. Chaos datas analyzer: the professional version. Raleigh, NC: Physics Academic Software; 1998. [Google Scholar]
- 32.Stergiou N, Buzzi UH, Kurz MJ, Heidel J. Nonlinear Tools in Human Movement. In: Stergiou N, editor. Innovative Analyses for Human Movement. Champaign, IL: Human Kinetics Publishers; 2004. pp. 63–90. [Google Scholar]
- 33.Mitra S, Riley MA, Turvey MT. Chaos in human rhythmic movement. J Mot Behav. 1997;29:195–198. doi: 10.1080/00222899709600834. [DOI] [PubMed] [Google Scholar]
- 34.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrout PE, Fleiss JL. Intraclass Correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 36.Rosner B. Fundamentals of biostatistics. 5. Duxbury Thomsom Learning; 2000. p. 563. [Google Scholar]
- 37.Hughes MA, Duncan PW, Rose DK, Chandler JM, Studenski SA. The relationship of postural sway to sensorimotor function, functional performance, and disability in the elderly. Arch Phys Med Rehabil. 1996;77:567–72. doi: 10.1016/s0003-9993(96)90296-8. [DOI] [PubMed] [Google Scholar]
- 38.Doyle TL, Newton RU, Burnett AF. Reliability of traditional and fractal dimension measures of quiet stance center of pressure in young, healthy people. Arc Phys Med Rehabil. 2005;86:2034–40. doi: 10.1016/j.apmr.2005.05.014. [DOI] [PubMed] [Google Scholar]