Abstract
Introduction:
Long-term smokeless tobacco (ST) use is known to increase the risk for oropharyngeal cancer, heart attack, and stroke. Extant literature on cigarette smokers suggests that smoking reduction increases smoking abstinence among smokers not interested in quitting. Similarly, a reduction strategy may reduce ST exposure and increase ST abstinence rates among ST users not interested in quitting.
Methods:
We conducted a pilot study to obtain preliminary data on the use of 12 weeks of varenicline as a tobacco reduction strategy among ST users not interested in quitting.
Results:
We enrolled 20 male ST users with a mean age of 42.8 ± 11.7 years who used an average of 3.9 ± 1.7 cans/pouches per week for 18.6 ± 8.6 years. At end of treatment (12 weeks), 60% (12/20) of subjects reduced their ST use by ≥50% and 15% (3/20) were biochemically confirmed abstinent from tobacco. At end of study (6 months), 50% (10/20) reduced by ≥50% of baseline use and 10% (2/20) were biochemically confirmed abstinent from tobacco. Varenicline reduced ST satisfaction, reward, and craving. Among subjects able to reduce ST, all subjects reported that reduction increased motivation and confidence in being able to maintain reduction and quit. The most common side effects were sleep disturbance (25%) and nausea (15%).
Discussion:
Varenicline may be effective in reducing ST use and achieving ST abstinence among ST users with no plans to quit but who are interested in reducing their ST use.
Introduction
In 2008, an estimated 8.7 million U.S. adults (3.5%) used smokeless tobacco (ST; Substance Abuse and Mental Health Services Administration, 2009). Long-term use is associated with death from heart disease, stroke, and cancer (Henley, Thun, Connell, & Calle, 2005). Cigarette manufacturers have entered the ST market and ST is being proposed as a harm reduction strategy for cigarette smokers (McNeill, 2004; National Institute of Health, 2006). The impact of these factors on the prevalence of ST use remains unclear but suggests an urgency for developing techniques to reduce risk posed by ST use, either through quitting or reduction.
Among cigarette smokers, tobacco reduction strategies are effective in decreasing smoking and increasing abstinence rates among smokers not interested in quitting (Falba, Jofre-Bonet, Busch, Duchovny, & Sindelar, 2004; Stead & Lancaster, 2007; Wang et al., 2008). Only two published studies have examined ST reduction using ST brand switching (Hatsukami et al., 2007) and tobacco-free snuff (Hatsukami et al., 2008). Significant reductions in nicotine and toxicant exposure were observed with both approaches.
Varenicline is a partial agonist/antagonist binding at the α4β2 nicotinic acetylcholine receptors resulting in (a) receptor stimulation releasing dopamine resulting in “reward” and (b) receptor antagonism attenuating rewarding effects of nicotine from tobacco. Pharmacokinetic studies demonstrate that among smokers not instructed to reduce smoking while taking varenicline, varenicline was associated with a 60%–80% reduction in the mean number of cigarettes smoked within 2–4 days of initiation accompanied by a reduction in plasma nicotine and cotinine concentrations (Faessel et al., 2006). Varenicline’s unique mechanism of action suggests a possible role for the use of varenicline for tobacco reduction among tobacco users willing to reduce use but not quit ST. No published studies exist assessing varenicline for ST reduction. To explore this line of research, we conducted a pilot study to obtain preliminary data on the use of varenicline as a tobacco reduction strategy in an open-label study enrolling 20 ST users.
Methods
Subject recruitment
We conducted the study at the Mayo Clinic in Rochester, MN. The Institutional Review Board approved the protocol prior to recruitment. Enrollment took place between May and July 2009. Subjects were eligible for inclusion if they were ≥18 years of age, reported ST as their primary tobacco of use (i.e., occasional use of other forms of tobacco was not exclusionary) and used it daily for ≥6 months, and were interested in reducing ST but had no plans to quit in the next 30 days. Subjects were excluded if they were currently using treatments for ST use (past 30 days); had an acute coronary syndrome in the past 6 months; had a history of kidney disease; had, as defined by the Columbia-Suicide Severity Rating Scale (C-SSRS; Posner, 2007; Posner, Oquendo, Gould, Stanley, & Davies, 2007), current suicidal thoughts or had a lifetime history of a suicidal attempt; or had a history of bipolar disorder, psychosis, or schizophrenia.
Study procedures
Potential subjects underwent screening and eligible subjects completed informed consent and enrolled. Baseline demographics and tobacco use history were collected. Patients completed the Fagerström Test for Nicotine Dependence—Smokeless Tobacco (Ebbert, Patten, & Schroeder, 2006), the Center for Epidemiological Studies—Depressed Mood Scale (CES-D; Radloff, 1977), and the C-SSRS. Subjects also completed the Smokeless Tobacco Evaluation Questionnaire (STEQ) based on the Cigarette Evaluation Questionnaire (mCEQ) modified for ST users. The mCEQ is a 12-item scale assessing the degree to which subjects experience the reinforcing effects of smoking (Cappelleri et al., 2007). The scale has five domains: smoking satisfaction, psychological reward, enjoyment of respiratory tract sensations, craving reduction, and aversion. The STEQ was collected on subjects who continued to use any amount of ST since their last visit.
Subjects recorded their baseline ST use for 1 week prior to starting varenicline. Subjects received varenicline at a dose of 0.5 mg once daily for 3 days, which was increased to 0.5 mg twice daily for Days 4–7 and then to a maintenance dose of 1 mg twice daily for 12 weeks of treatment. Subjects were instructed to continue their usual ST dose during the first 7 days of varenicline therapy. On Day 8 of varenicline, subjects were instructed to decrease their baseline rate of ST use by 50% during the course of the day. No other specific counseling or suggestions were provided as to how to reduce ST.
Subjects completed the C-SSRS at Weeks 6 and 12 and the CES-D at Weeks 4, 8, 12, and 14. Biochemical confirmation of self-reported abstinence was obtained at end of treatment (Week 12) and end of study (6 months postrandomization). If subjects reported tobacco abstinence, a urine cotinine of <50 ng/ml was used to confirm abstinence (Benowitz et al., 2002).
Subjects also completed an end-of-study questionnaire assessing how reduction increased their confidence and motivation for future abstinence or reduction. Scores ranged from 0 (completely unhelpful) to 5 (extremely helpful).
Statistical analyses
Data were summarized using mean ± SD for continuous variables and frequency percentages for categorical variables. The primary outcomes for this study were the percentage of subjects who achieved a ≥50% reduction in ST use (cans or pouches/week) at 12 weeks and 6 months. Percentage reduction was calculated assuming that subjects who discontinued study participation were using ST at the baseline rate. Subjects discontinuing study were assumed to be using ST for the abstinence outcome. Scores on the CES-D were calculated and compared with baseline using the paired t-test. STEQ data were scored using subscales based on methods previously outlined (Cappelleri et al., 2007). These data were analyzed using mixed effects models (SAS PROC MIXED) using an AR(1) covariance structure accounting for the repeated measurements within subjects over time. For subscales in which significant changes over time were detected, supplemental analyses were performed to assess differences from baseline. In all cases, two-tailed tests were performed, with p ≤ .05 used to denote statistical significance.
Results
Subjects
Thirty-two potential subjects passed screening, 22 consented, and 20 enrolled. Enrolled subjects were all male who used an average of four cans/pouches per week (Table 1). One subject used gutkha, a type of ST used in India. At baseline, three subjects reported never having previously reduced their ST use, 60% (12/20) reported having reduced ST use out of concern for their health, and 20% (4/20) reported having reduced use to save money. Fifty percent (10/20) endorsed having previously reduced their ST in preparation for quitting, 40% (8/20) endorsed that reducing ST increased their confidence for eventually quitting, and 80% endorsed that ST reduction was an important technique in preparation for eventual tobacco abstinence. Of the 20 subjects enrolled, 6 (30%) discontinued study participation prior to the end of the 12-week medication phase. The reasons for discontinuation included adverse events (n = 2), consent withdrawn (n = 1), scheduling difficulty (n = 1), and loss to follow-up (n = 2).
Table 1.
Baseline characteristics (N = 20)a of smokeless tobacco (ST) users enrolled in a pilot study of varenicline for ST reduction
| Characteristic | |
| Age, years | 42.8 ± 11.7 |
| Range | 24–65 |
| Male, n (%) | 20 (100) |
| Caucasian, n (%) | 17 (85) |
| Marital status, n (%) | |
| Married/living as married | 17 (85) |
| Never married | 1 (5) |
| Separated/divorced | 2 (10) |
| Highest level of education, n (%) | |
| <High school graduate | 1 (5) |
| High school graduate | 3 (15) |
| Some college | 5 (25) |
| College graduate | 11 (55) |
| Current type of smokeless tobacco used, n (%) | |
| Snuff | 18 (90) |
| Chewing tobacco | 1 (5) |
| Gutkha | 1 (5) |
| Average age started using smokeless tobacco, years | 23.0 ± 11.6 |
| Range | 6–56 |
| Smokeless tobacco used per week, cans/pouchesb | 3.9 ± 1.7 |
| Range | 2–7 |
| Years of regular smokeless tobacco use, years | 18.6 ± 8.6 |
| Range | 6–40 |
| Current use of other tobacco productsc, n (%) | 1 (5) |
| Other users of tobacco in household, n (%) | 4 (20) |
| Number of closest friends who use ST | |
| None | 8 (40) |
| 1 | 5 (25) |
| 2 | 6 (30) |
| 3 | 1 (5) |
| Number of serious stop attempts (≥24 hr), n (%) | |
| 0 | 4 (20) |
| 1–2 | 3 (15) |
| 3–4 | 7 (35) |
| 5+ | 6 (30) |
| Longest time off tobacco, n (%) | |
| <24 hr | 3 (16) |
| 1–7 days | 3 (16) |
| 2–8 weeks | 7 (37) |
| 9 weeks to 6 months | 1 (5) |
| >6 months | 5 (26) |
| Fagerström Test for Nicotine Dependence–Smokeless Tobacco | 5.9 ± 1.7 |
| Range | 2–8 |
| Confidence in being tobacco free in 1 year | |
| Not at all confident | 0 (0) |
| Not very confident | 0 (0) |
| Somewhat confident | 11 (55) |
| Very confident | 6 (30) |
| Completely confident | 3 (15) |
Note. aData are presented as mean ± SD, range, or n (%) as indicated.
Summary excludes one subject who used gutkha.
In addition to smokeless tobacco, one subject reported smoking cigarettes (2 cigarettes/day).
Reduction and abstinence
At 12 weeks (end of treatment), 60% (12/20) of subjects reduced their ST use by ≥50% of baseline. The mean ± SD percentage reduction was 53.6% ± 42.0% with a median of 58% (interquartile range [IQR] 0%–96%).
At 6 months, 50% (10/20) of subjects reduced their ST use by ≥50% of baseline. The mean percentage reduction was 44.5% ± 43.7% with a median of 42% (IQR 0%–85%).
The biochemically confirmed tobacco abstinence rate at 12 weeks was 15% (3/20). Of four subjects self-reporting all tobacco abstinence at 6 months, one was biochemically disconfirmed and one did not provide a sample, thus the biochemically confirmed tobacco abstinence rate at 6 months was 10% (2/20).
Of the 16 subjects (80%) reducing their ST use during the trial, all subjects reported that reduction increased their motivation and confidence in being able to quit as well as their confidence in being able to maintain ST reduction “somewhat” to “a great deal.” Among the subjects who were able to quit, all of them reported that reduction was “helpful” or “extremely helpful” in helping them quit.
Smokeless Tobacco Evaluation Questionnaire
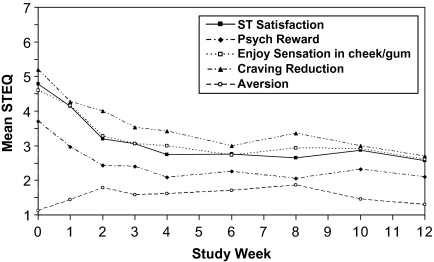
Subscales of ST satisfaction, psychological reward, and craving reduction were significantly decreased from baseline starting at Week 1 and remained decreased thereafter (Figure 1). Enjoyment of cheek/gum sensations was significantly decreased starting at Week 2 and remained decreased thereafter. The aversion subscale was not found to change significantly over time (p = .456).
Figure 1.
Subscales of the Smokeless Tobacco Evaluation Questionnaire among subjects reporting any ST use since previous visit.
Adverse events
One patient with a history of suicidal ideation but no prior suicide attempts experienced recurrence of symptoms after starting varenicline. Suicidal ideation occurred on Day 17 of therapy. At this time, he had reduced his ST use from 2.5 cans/week to 1 can/week. The patient was seen 3 days after he stopped the medications and his symptoms had resolved. Other observed side effects adjudicated to be possibly, probably, or definitely related to study medication included sleep disturbance (25%), nausea (15%), vivid dreams (10%), abdominal pain (10%), dyspepsia (10%), constipation (5%), diarrhea (5%), fatigue (5%), palpitations (5%), rash (5%), and shakiness (5%). Depressive symptoms as assessed by the CES-D did not differ significantly from baseline at any follow-up visit (all p ≥ .35). No changes were observed on the C-SSRS.
Discussion
Varenicline was associated with a reduction in ST use among ST users who had no intention of quitting in the next 30 days. Consistent with the mechanism of varenicline, ST users reported significantly decreased reinforcing effects of ST. ST users reported that reduction increased their confidence in maintaining reduction and quitting.
We observed lower abstinence rates than in previous ST reduction trials. In a larger trial (N = 66) investigating the effect of switching ST users not interested in quitting but willing to reduce to ST products with less nicotine, subjects were randomized to controlled ST use or to ad lib ST use. At 12 weeks, 17.1% of subjects in the controlled ST use group achieved biochemically confirmed 7-day point prevalence abstinence compared with 25.8% in the ad lib group (Hatsukami et al., 2007). In another study (N = 106) investigating the effects of tobacco-free snuff or no snuff among ST users not interested in quitting but willing to reduce, 34.6% of subjects in the tobacco-free snuff group reduced their ST by 50% at Week 12 compared with 25.9% in the control group (Hatsukami et al., 2008). Subjects in the second trial received counseling on behavioral methods for reduction, while we provided no counseling. Also, subjects in these trials were younger with fewer years of regular ST use than in our trial, which may have an effect on the observed differences in abstinence rates.
We noted that one half of our subjects reported having previously reduced their ST in preparation for quitting and 80% endorsed that this was an important technique in preparation for quitting. Although this likely reflects the type of ST user we recruited, this observation may provide insight into the general population of ST users. Research in smokers suggests that those planning to reduce are doing so as part of a quit attempt with the most common goal of a 50% reduction over a month (Hughes, Callas, & Peters, 2007). In combination with data suggesting that reduction decreases tobacco use and increases abstinence rates among smokers (Stead & Lancaster, 2007), our data suggests exciting new potential avenues of research among ST users not interested in quitting.
Subject recruitment was challenging because potential subjects eligible by telephone frequently reported their intention to quit when they attended the screening visit. These subjects endorsed a vague intent to quit but without a firm quit date. This observation seemed to confirm observations made by previous investigators who concluded that “many reducers report intention to quit because it is the socially desirable response … but have no real plans to quit” (Hughes et al., 2007). We excluded individuals who maintained new intentions to quit and included only those with no intention in the next 30 days.
Our study has significant limitations. First, the medication intervention was open label without a control group. Second, we did not collect biochemical measures to determine nicotine or toxicant exposure reduction or elimination, but reductions in these markers have been observed in previous trials of ST reduction (Hatsukami et al., 2007, 2008). Finally, we used the previously validated mCEQ and modified it for ST users but have not validated this tool. Other than changing “smoking” and “cigarettes” with “chewing” and “chew,” the only major adaptation was modifying “did you enjoy the sensations in your throat and chest” to “did you enjoy the sensations in your cheek and gum.”
We observed that varenicline may facilitate reduction among ST users willing to reduce who have not set a quit date. Both the efficacy and the clinical utility of this approach need to be explored.
Funding
This work was supported, in part, by the National Cancer Institute (grant number CA132621). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Declaration of Interests
None declared.
Acknowledgments
We would like to thank the subjects who participated in this research and the staff of the Mayo Clinic Nicotine Research Program without whom this project would not have been possible.
References
- Benowitz NL, Ahijevych K, Hall S, Hansson A, Henningfield J, Hurt RD, et al. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addictive Behaviors. 2007;32:912–923. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Patten CA, Schroeder DR. The Fagerstrom Test for Nicotine Dependence-Smokeless Tobacco (FTND-ST) Addictive Behaviors. 2006;31:1716–1721. doi: 10.1016/j.addbeh.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faessel HM, Gibbs MA, Clark DJ, Rohrbacher K, Stolar M, Burstein AH. Multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy smokers. Journal of Clinical Pharmacology. 2006;46:1439–1448. doi: 10.1177/0091270006292624. [DOI] [PubMed] [Google Scholar]
- Falba T, Jofre-Bonet M, Busch S, Duchovny N, Sindelar J. Reduction of quantity smoked predicts future cessation among older smokers. Addiction. 2004;99:93–102. doi: 10.1111/j.1360-0443.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Ebbert JO, Anderson A, Lin H, Le C, Hecht SS. Smokeless tobacco brand switching: A means to reduce toxicant exposure? Drug and Alcohol Dependence. 2007;87:217–224. doi: 10.1016/j.drugalcdep.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Ebbert JO, Edmonds A, Li C, Lin H, Le C, et al. Smokeless tobacco reduction: Preliminary study of tobacco-free snuff versus no snuff. Nicotine & Tobacco Research. 2008;10:77–85. doi: 10.1080/14622200701704897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes & Control. 2005;16:347–358. doi: 10.1007/s10552-004-5519-6. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Callas PW, Peters EN. Interest in gradual cessation. Nicotine & Tobacco Research. 2007;9:671–675. doi: 10.1080/14622200701365293. [DOI] [PubMed] [Google Scholar]
- McNeill A. Harm reduction. BMJ. 2004;328:885–887. doi: 10.1136/bmj.328.7444.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Health. National Institutes of Health State-of-the-Science Conference Statement: Tobacco use: Prevention, cessation, and control. Annals of Internal Medicine. 2006;145:839–844. doi: 10.7326/0003-4819-145-11-200612050-00141. [DOI] [PubMed] [Google Scholar]
- Posner K. Suicidality issues in clinical trials: Columbia Suicide Adverse Event Identification in FDA Safety Analyses. 2007. Retrieved from http://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4306s1-01-CU-Posner.ppt. [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. American Journal of Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database of Systematic Reviews(3) 2007 doi: 10.1002/14651858.CD005231.pub2. CD005231. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Health: National findings. Rockville, MD: Office of Applied Studies; 2009. NSDUH Series H-36, HHS Publication No. SMA 09-4434. [Google Scholar]
- Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: A systematic review of effectiveness and economic analysis. Health Technology Assessment. 2008;12(2):iii–iv. doi: 10.3310/hta12020. ix-xi, 1–135. [DOI] [PubMed] [Google Scholar]