Abstract
Purpose
Based on preclinical and clinical abuse liability assessments, fentanyl and other potent μ opioid agonists (e.g. hydromorphone and morphine) should be the most misused opioids if accessibility in the real world were not an issue. Since the latter is seldom true, we postulated that there would be a significant mismatch between actual and predicted rates of misuse.
Methods
We recruited 1,818 prescription-opioid dependent patients entering drug treatment programs to complete an anonymous survey, covering drug use and health related issues.
Results
Hydrocodone and oxycodone products were the drugs of choice in 75% of patients, whereas potent μ opioid agonists (fentanyl, hydromorphone and morphine), with the greatest predicted abuse potential, were very rarely chosen (<5% each). Most unexpectedly, the rank order of the actual drug of choice and the preferred drug in an ideal world were highly correlated. The reason most commonly given for the failure to endorse fentanyl, for example, as an actual or preferred drug, was fear of toxicity and overdose. We found few differences in drug use patterns between a subset of high-risk, impaired health care professionals (N=196) and all other patients other than source of drug, (forged prescriptions and doctors more common and dealers much less common in the HC sample).
Conclusions
These results indicate that it should not be assumed- particularly for new drug formulations- that a high potential for abuse will result in actual abuse; and, most importantly, that the hesitancy to use potent opioids because of fears of abuse may be misguided.
Keywords: fentanyl, μ opioid agonists, abuse, pain management, opioid exposure
Background
Fentanyl is a synthetic opioid that has much greater affinity for the mu opioid receptor than any currently marketed opioid (1,2), except for its even more potent, but seldomly used, analogues- sufentanyl and alfentanyl. As such, it is a highly potent and efficacious analgesic for individuals in severe chronic pain (3,4). Unfortunately, like all opioid drugs, fentanyl's affinity for the mu opioid receptor not only imparts analgesic activity, but also strong positive reinforcing properties (i.e. euphoria), which translates into substantial abuse potential. Based on preclinical (e.g., discriminative stimulus properties and self-administration studies in animals [5–7]) and clinical abuse liability assessments, (e.g., “liking” scores in recreational drug users relative to other opioids [8–11]), it has without equivocation been found that Fentanyl should be amongst the most highly abused opioid analgesics in the “real world” outside the structured setting of laboratory-based abuse liability assessments. However, the potential for abuse does not necessarily translate into actual abuse, since many factors other than a drug's intrinsic properties determine whether it will be abused, such as: availability; cost; adverse side effects; and, particularly for fentanyl, difficulty in titrating dose to produce maximum euphoria without overdosing.
The actual rate of fentanyl abuse/misuse relative to other opioids in the population of substance misusers in this country has not been firmly established, but some reports indicate that the abuse is quite modest (12–15) whereas others (8–9, 16–18), including documents on the DEA website and certainly numerous articles in the popular press, suggest that fentanyl abuse is rapidly growing and that it is one of the most abused, diverted, and dangerous drugs in the illicit drug market.
In this study, we sought to examine whether the frequency of the non-therapeutic use of fentanyl and other opioids to get “high”, in the real world, matched their predicted abuse potential from abuse liability assessments. To do so, we recruited 1,818 patients entering drug treatment programs around the country, 196 of whom identified themselves as impaired health care professionals, a group thought to be at great risk for abuse of Fentanyl and other potent opioids. (19–22).
Methods
As reported previously, the treatment centers were balanced geographically [15, 26–27] with a good representation of large urban, suburban and rural treatment centers. Each of the treatment specialists (N=70) was asked to recruit as many patients/clients as possible who had a diagnosis of prescription opioid analgesic abuse or dependence using the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) [28]. Inclusion criteria were very broad: first, those people who met DSM-IV criteria for substance abuse whose primary drug was a prescription opioid (i.e., not heroin); and, second, use of prescription opioid drugs to get high at least once in the past 30 days prior to treatment. The only exclusion criteria were those individuals who did not meet these criteria and any individual who listed heroin as their primary drug. Overall, 85% of the patients approached by the treatment counselors completed surveys and submitted them. The patients were asked to complete a detailed survey instrument, covering: demographics; licit and illicit patterns of drug use; diagnostic criteria for alcohol and opioid abuse or dependence (DSM-IV criteria; e.g., loss of control of drinking or drugging, disruption of everyday activities as a consequence of use, family and friend complaints about abuse, withdrawal, craving, and so forth); the Fagerström test for Nicotine Dependence [29]; general health status using the SF-36v2 Health Survey; chronic non-withdrawal bodily pain and its intensity (scale of 1–10 with 1 being none and 10 the worst possible pain); and, whether they were currently being treated for a psychiatric condition.
Patient/subject confidentiality
Completed survey instruments were identified solely by a unique case number and were sent directly to Washington University School of Medicine. The treatment specialists did not see the detailed responses of their patients/clients. The protocol was approved by the Washington University Institutional Review Board (IRB).
Statistical analysis
Since most of our data were binary or categorical, logistic regression tests were used to determine odds ratios (OR) with HC the independent variable (yes =1; no =0). Odds Ratios (OR) were established for each of the 40 plus dependent variables. For those tests that were shown to be statistically significant (p<0.05), gender was introduced to the model as a moderator to see if HC had any significant interaction with gender. When the dependent variables were binary or categorical, we did multiple logistic regression tests with two independent variables (health care, gender). When the dependent variables were continuous, such as age, chronic pain level etc., we did two way ANOVA tests using SAS GLM procedures with interaction added to the model.
SF-36 V2 Health Survey
With the release of SF-36v2, norms were updated using data from the 1998 National Survey of Functional Health Status (NSFHS) and norm-based scoring (NBS) algorithms were introduced [30]. Using this model, a score of 50 represents the average health status of all adults in this country. A score greater than 50 indicates better than normal health, whereas a score below 50 represents poorer health.
Results
Population demographics
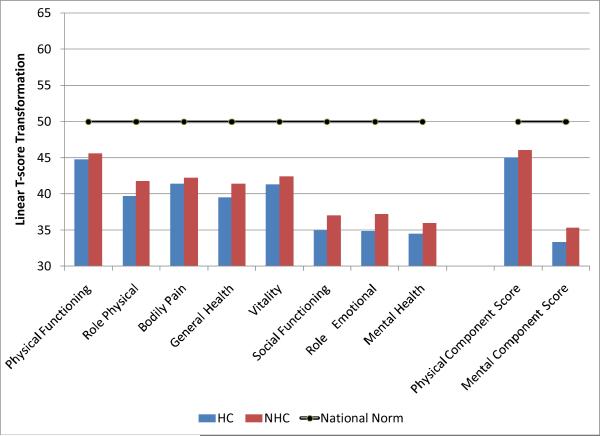
Table 1 summarizes the demographics of our patients. We had a tot al of 1,818 primarily Caucasian (82–84%) clients who completed the questionnaire: 196 Health Care professionals (HC) and 1,622 Non Health Care professionals (NHC). The HC were significantly older (t=3.02, p <.01) and decidedly more female than the NHC (OR 2.76, p<.01). In the NHC group, 49% of the total clients were female, whereas in the HC group over 72% were. We found no other gender differences on any of the multiple dependent variables and, hence, gender is ignored in all other presentations of the results. The SF-36 health survey showed (Figure 1) that both HC and NHC had moderately poor physical health component scores, but very serious mental health issues (depression and anxiety the most common) with a mental health component score of 32–35. Moreover, as shown in Table 1, nearly 90% satisfied DSM-IV criteria for opioid dependence; 60–70% in both groups were nicotine dependent; nearly 40% satisfied DSM-IV criteria for alcohol dependence; and approximately 55% had a diagnosed psychiatric condition. Pain was also a factor in 85–90% of both groups with a ranking of pain intensity at 4.9 on a 10-point scale for both HC and NHC groups. A long history of substance abuse problems was evident given that both groups had been enrolled in 3.5–4 treatment programs prior to the current episode at which time the questionnaire was completed.
Table 1.
Demographics, health status and substance abuse issues in HC (N=196) and NHC (N-1622)
| HC n=196 | NHC n=1622 | |
|---|---|---|
| Age | 37.37 ± 0.82* | 34.83 ± 0.28 |
| Gender | ||
| Male | 27.66%* | 51.31% |
| Female | 72.34%* | 48.69% |
| Ethnicity | ||
| White | 84.46% | 82.48% |
| African-American | 4.66% | 5.47% |
| Hispanic | 8.29%* | 3.91% |
| Other | 2.59% | 8.14% |
| DSM-IV Rx Dependence | 88.42% | 89.85% |
| Times Entered Tx | 3.95 ± 0.73 | 3.34 ± 0.20 |
| Pain Score | 4.93 ± 0.20 | 4.89 ± 0.08 |
| Psychiatric Condition | 57.65% | 55.82% |
| Nicotine Dependence | 59.85%* | 68.79% |
| Alcohol Abuse | 39.88% | 39.46% |
Significantly different than NHC
Figure 1.
The average score of HC and NHC on the SF-36v2 (see methods). All of the differences from the national norm (shown as a black line) are significant, with mental health scores much poorer in opioid dependent individuals regardless of their occupation than the population norm.
Opioid abuse
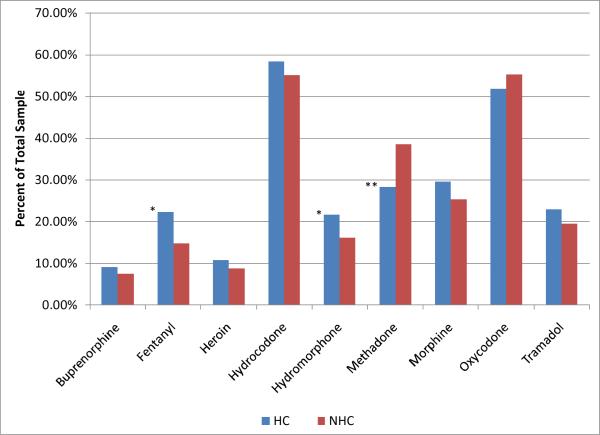
Figure 2 shows the use of opioids to get high, expressed as a percent of the total sample in the HC and NHC groups, in the 30 days prior to treatment. Hydrocodone and oxycodone products (mainly ER-oxycodone) were by far the most widely misused drugs. With the exception of buprenorphine and heroin that were used rarely to get high in these prescription opioid preferring groups, a significant fraction of the population in both groups reported the use of nearly all opioid drugs at least once in the past 30 days. Although group differences in drug preferences were relatively small, there were significant differences between groups: the HC were significantly more likely to use fentanyl (OR 1.65, p< .01),, but significantly less likely to use methadone (OR 0.63, p< .01) than their NHC counterparts.
Figure 2.
Licit and illicit opioids used in the last 30 days to get high in HC and NHC. Percentages listed are the precent of each sample who identified that they used the drug listed to get high. *Significantly higher (P<.01) than NHC; ** significantly lower (P<.01) than NHC.
Primary Drug
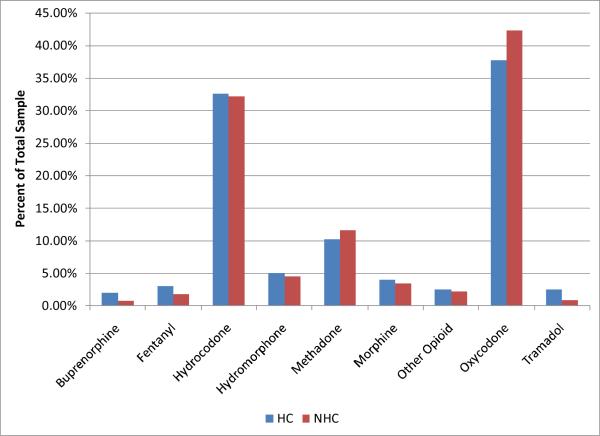
In terms of primary drug, ER oxycodone was the overwhelming choice followed closely by hydrocodone products (Figure 3). All other drugs were selected much less frequently. Particularly striking was that the drugs with the most predicted abuse potential- fentanyl, hydromorphone and morphine– were very rarely endorsed as the primary drug in either HC and NHC groups.
Figure 3.
Primary Drugs (e.g., the drug used most often) in HC and NHC. Percentages listed are the precent of each sample who identified that they used the drug listed to get high as their primary drug. There were no significant differences found between groups.
Price and availability
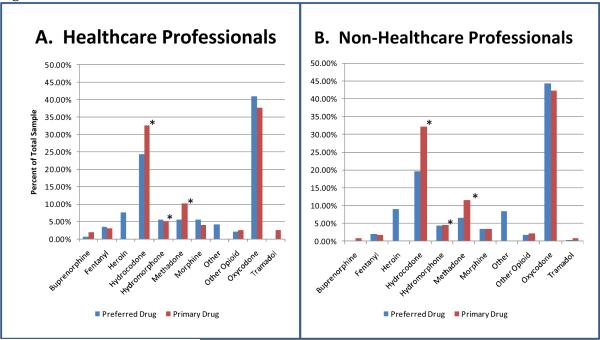
We asked: “If price and availability were not a factor which drug would you use most frequently?” The results are shown in Figure 4. The most striking aspect of these data is the rare endorsement of fentanyl, hydromorphone and morphine as either the actual or preferred primary drug in both the HC and NHC groups. In this connection, the only prescription opioids that were used to a much greater extent in the real world than the preferred drug were hydrocodone and methadone. In response to a directed question about the reason that fentanyl was so rarely endorsed as a primary drug, two thirds of HC and NHC individuals indicated that toxicity and overdose deaths were defining factors.
Figure 4.
Percent of the HC (4A) and NHC (4B) who selected the drugs listed as their actual primary drug and their preferred drug if price and availability were not an issue. Primary drug was significantly (P<.05) higher than preferred drug. The “other” category reflects the use of non-opioids, primarily benzodiazepines and cocaine (crack).
Controlling for Prior Experience
Since experience with a drug's psychoactive effects would be a prerequisite for it to be selected as the actual or preferred drug, we calculated the percentage of opioid dependent patients who had experience taking each opioid- ever or within the last 30 days- who then listed that drug as their preferred drug. Table 2 shows that over 50% of those exposed to oxycodone indicated that it was now their drug of choice with heroin and hydrocodone the next most prevalent. Very small percentages (<10%) of those who had experience with tramadol, buprenorphine, and fentanyl indicated that these drugs were now their actual or preferred drug of choice.
Table 2.
The percentage of HC and NHC individuals who used the listed opioids to produce euphoria and subsequently chose the drug as their preferred drug if availability were not a problem.
| HC | NHC | |
|---|---|---|
| Oxycodone | 51.46% | 52.59% |
| Heroin | 22.50% | 24.44% |
| Hydrocodone | 27.42% | 21.05% |
| Hydromorphone | 13.56% | 10.94% |
| Methadone | 12.50% | 11.25% |
| Morphine | 9.88% | 5.90% |
| Fentanyl | 7.84% | 6.58% |
| Buprenorphine | 3.45% | .094% |
| Tramadol | 0.00% | 0.82% |
Source of the drug
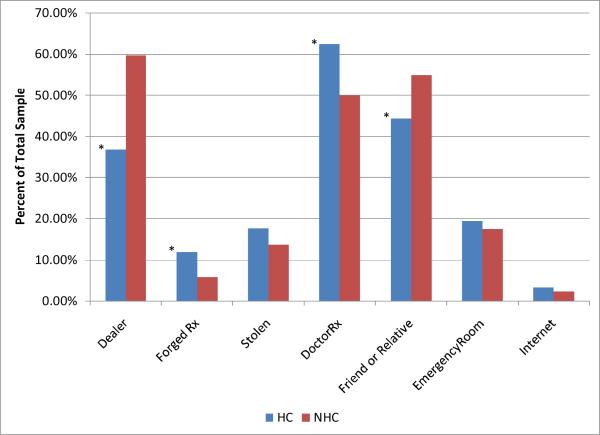
Figure 5 shows the source of the primary drug used by NHC and HC to get high. The HC group was much more likely to use a doctors' prescriptions (OR = 1.67, P < .01) and forged prescriptions (OR = 2.19, P < .01) and much less likely to use a dealer (OR =0.39, P <.001) or friends/families (OR=0.657, P< .01) than their NHC counterparts.
Figure 5.
Source of Primary Drugs in HC and NHC listed as percent of total in each sample. * Significantly different (P<.01) than in the NHC sample.
Route of administration
Swallowing the drug whole was the most common route of administration, followed by chewing and snorting that were almost equally popular. Since the IV route of administration represents the most serious public health concern, we asked respondents if IV route was their preferred route of administration for their primary drug. Only 12% – 14% in either group indicated that this was the case.
Discussion
Our data suggest a very close match between the drug actually used as the primary drug and the preferred drug, if price and availability were not issues, in opioid dependent impaired health care professionals and other individuals entering treatment. These data, indicate that, in contrast to widely held assumptions, drug accessibility alone is not the sole determinant of regional and other variations in the drugs of choice nor in the discrepancy between predicted abuse liability and actual rates of misuse. Most surprising, in this regard was that drugs with the greatest affinity for the μ opioid receptor, and therefore the greatest potential for abuse-fentanyl, hydromorphone and morphine- were neither the drugs of choice in the real world nor in a perfect world where availability was not a major concern. The most obvious explanation for these unexpected findings is that the intrinsic potential for abuse of these μ agonists, that is so evident in the controlled environment of abuse liability assessments in animals and humans (5–11), may be trumped by other factors that are more important in the decision to use a particular drug. These include: ease of access; the positive and negative subjective effects of the drug; and, in the case of fentanyl and other opioids with significant affinity for the μ opioid receptor, the difficulty in titrating dose to achieve optimal euphoria without overdosing. The latter consideration seems the most important based on the present results and is consistent with the use of terms such as “drop dead” and “suicide packets” to refer to fentanyl products available on the street (16, DEA web site).
To control for the possibility that the endorsement of a preferred drug was constrained by limited prior experience with the drug, we determined the ratio between the number of people who had tried an opioid to get high (denominator) and the number of people who subsequently selected it as their preferred euphorogenic agent (numerator). This measure not only controls the differences in exposure, but we suggest may also serve as an excellent index of the desirability of the drug as a potential drug of choice. Using this index, it is clear that 50% of those who used oxycodone to get high subsequently chose it as their preferred drug of choice. Heroin and hydrocodone were the next closest drugs with 20–25% of those using these drugs to get high endorsing them as their preferred drugs. At the other end of the spectrum, experimentation with the use of fentanyl, morphine and hydromorphone led to very low (<10%) subsequent endorsements as the preferred drug if availability were not an issue. These data suggest that some aspects of the initial use of these potent opioids deterred subsequent use. We postulate that one of the more important factors was that these drugs were perceived to be much too potent to be safe with a poor “therapeutic” index – i.e., the threshold to get high is too close to a toxic dose.
No matter the reason for the discrepancy between the potential and actual abuse liability, it seems clear that it should not be reflexively assumed that a drug – particularly a new drug entity or formulation - with very high predicted abuse potential will actually be abused in practice. This conclusion has particular relevance when the “actual or relative potential for abuse” is evaluated for scheduling under the Controlled Substance Act (factor 1 of the mandated 8 factor analysis). More importantly, our data suggest that fear of abuse of these potent μ agonists, which often influences a physicians' willingness to prescribe them for pain even when their use is strongly indicated, is largely unwarranted.
Other than issues confined to the drug of choice, our data reinforce the growing body of data which indicates that prescription opioid users have a large number of co-morbid physical and psychiatric issues no matter their profession, including chronic pain (>85%) with moderate intensity, mental disorders (55–60%), alcoholism (≈40%), and nicotine dependence(60 – 70%). These data suggest not only that any pain management program should be multidisciplinary in its approach, but also that the existence and severity of psychiatric co-morbidity, nicotine use and alcoholism might be predictive of those pain patients most at risk for abuse of their medications.
Based on a number of published studies, (19–21,31–32,)and widely held assumptions, we expected to find two major differences between HC and NHC individuals: first, diversion of opioids from therapeutic channels would be much higher in HC than NHC because of ease of access; and secondly, that highly reinforcing and readily accessible drugs such as fentanyl and hydromorphone would be endorsed more frequently in HC than. While our results lend support to these conclusions, the overall differences between groups were much more modest than was expected. Indeed, based on the totality of our findings, in many respects the major differences between the two groups was their profession.
There are a number of limitations in our studies. Obviously, our sample was skewed heavily toward nurses, and, therefore, is not representative of other health care professionals, such as, for example, anesthesiologists who are thought to have the greatest risk for substance abuse and for fentanyl misuse in particular (19,31). In addition, all of our surveys were self-administered and thus they have most of the problems associated with these methods, particularly the inability to follow up answers with additional questions to clarify any ambiguities and truthfulness. A third limitation is that our study population was confined to those entering treatment in many cases for the fourth or fifth time. Thus, this population, and their profound problems with drugs and other psychopathology may be very different than less ill, “recreational” drug users. While all of these possibilities need to be kept in mind in interpreting our results, it seems doubtful to us that the abuse of the much feared fentanyl or other strong opioids would be greater in recreational users than experienced drug addicts. Moreover, the anonymity provided by the self-administered questionnaire has been shown to produce much more candid and valid drug related responses than interviewer- elicited information regarding substance abuse issues (43).
Take home messages.
Preclinical and clinical abuse liability assessments do not uniformly predict actual abuse in the real world.
Fentanyl, hydromorphone and morphine have great potential for abuse, but little actual abuse.
Safety is the primary determinant of whether and to what extent a drug is misused.
Availability alone does not dictate which drugs will be misused.
Fear of abuse of fentanyl and other potent μ agonist are overstated and should not deter their use in pain management.
Acknowledgments
Sponsor: This research was supported in part by NIH grant 5 R01 DA20791-04 and an unrestricted grant from the RADARS® System.
Footnotes
Conflict of Interest: TJC has a consulting relationship with Tyco-Mallincrodt, St. Louis, MO, a generic drug manufacturer. He is also a member of the non-profit RADARS® System, a national drug abuse and safety surveillance program which has numerous pharmaceutical sponsors. None of the other authors have any conflicts.
References
- 1.Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. J Pharmacol Exp Ther. 2007 Oct;323(1):346–55. doi: 10.1124/jpet.107.119560. Epub 2007 Jul 23. [DOI] [PubMed] [Google Scholar]
- 2.Sirohi S, Dighe SV, Walker EA, Yoburn BC. Pharmacol Biochem Behav. 2008 Nov;91(Issue 1):115–120. doi: 10.1016/j.pbb.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Portenoy RK, Payne R, Passik SK, Lowinson JH, Ruz P, Millman RB, Langrod JG. Substance Abuse: A Comprehensive Textbook. 4th ed. Lippincott, Williams & Wilkens; Philadelphia: 2004. pp. 863–904. Acute and Chronic Pain. [Google Scholar]
- 4.Melzack R. The tragedy of needless pain. Scientific American. 1990;262:27–33. doi: 10.1038/scientificamerican0290-27. [DOI] [PubMed] [Google Scholar]
- 5.Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pchelintsev MV, Gorbacheva EN, Zvartau EE. Simple methodology of assessment of analgesics' addictive potential in mice. Pharmacol Biochem Behav. 1991;39:873–876. doi: 10.1016/0091-3057(91)90046-5. [DOI] [PubMed] [Google Scholar]
- 7.Broadbear JH, Winger G, Woods JH. Self-administration of fentanyl, cocaine and ketamine: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology (Berl) 2004 Nov;176(3–4):398–406. doi: 10.1007/s00213-004-1891-x. Epub 2004 Apr 28. [DOI] [PubMed] [Google Scholar]
- 8.Baylon GJ, Kaplan HL, Somer G, Busto UE, Sellers EM. Comparative abuse liability of intravenously administered remifentanil and fentanyl. J Clin Psychopharmacol. 2000 Dec;20(6):597–606. doi: 10.1097/00004714-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Sellers EM, Schuller R, Romach MK, Horbay GL. Relative abuse potential ofopioid formulations in Canada: a structured field study. J Opioid Manag. 2006 Jul–Aug;2(4):219–27. doi: 10.5055/jom.2006.0034. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald MK. Behavioral economic analysis of drug preference using multiple choice procedure data. Drug Alcohol Depend. 2008 Jan 11;93(1–2):103–10. doi: 10.1016/j.drugalcdep.2007.09.002. Epub 2007 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths R, Bigelow G, Ator N. Principles of initial experimental drug abuse liability assessment in humans. Drug and Alcohol Depend. 70(2003):241–s54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- 12.Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000 Apr 5;283(13):1710–4. doi: 10.1001/jama.283.13.1710. [DOI] [PubMed] [Google Scholar]
- 13.Novak S, Nemeth WC, Lawson KA. Trends in medical use and abuse of sustained-release opioid analgesics: a revisit. Pain Med. 2004 Mar;5(1):59–65. doi: 10.1111/j.1526-4637.2004.04001.x. [DOI] [PubMed] [Google Scholar]
- 14.Cicero TJ, Inciardi JA, Surratt H. Trends in the use and abuse of branded and generic extended release oxycondone and fentanyl products in the United States. Drug Alcohol Depend. 2007 Dec 1;91(2–3):115–20. doi: 10.1016/j.drugalcdep.2007.05.008. Epub 2007 Jun 21. [DOI] [PubMed] [Google Scholar]
- 15.Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of OxyContin and other opioid analgesics in the United States: 2002 – 2004. J Pain. 2005 Oct;6(10):662–72. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Fodale V, Mafrica F, Santamaria L, Coleman JJ. Killer fentanyl: is the fear justified? Expert Opin Drug Saf. 2008 May;7(No.3):213–217. doi: 10.1517/14740338.7.3.213. [DOI] [PubMed] [Google Scholar]
- 17.Clark HW. Center for Mental Health Services/ Center for Substance Abuse Prevention/ Center for Substance Abuse Treatment; Substance Abuse and Mental Health Services Administration. 2006 June;
- 18.Joseph D. U.S. Department of Justice/Drug Enforcement Administration; Drugs of Abuse. 2005
- 19.Bryson E, Silverstein J. Addiction and substance abuse in anesthesiology. Anesthesiology. 2008 Nov;109(5):905–917. doi: 10.1097/ALN.0b013e3181895bc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domino K, Hornbein T, Polissar N, Renner G, Johnson J, Alberti S, Hankes L. Risk factors for relapse in health care professionals with substance use disorders. JAMA. 2005 March 23–30;293(No. 12) doi: 10.1001/jama.293.12.1453. [DOI] [PubMed] [Google Scholar]
- 21.Baldisseri MR. Impaired healthcare professional. Crit Care Med. 2007 Feb;35:S106–16. doi: 10.1097/01.CCM.0000252918.87746.96. [DOI] [PubMed] [Google Scholar]
- 22.Maher-Brisen P. Addiction: an occupational hazard in nursing. AMJ Nurse. 2007 Aug;107(8):78–79. doi: 10.1097/01.NAJ.0000282302.49183.67. [DOI] [PubMed] [Google Scholar]
- 23.Cicero TJ, Dart RC, Inciardi JA, Woody GE, Schnoll S, Muñoz A. The development of a comprehensive risk-management program for prescription opioid analgesics: researched abuse, diversion and addiction related surveillance (RADARS®) Pain Med. 2007 Mar;8(2):157–70. doi: 10.1111/j.1526-4637.2006.00259.x. [DOI] [PubMed] [Google Scholar]
- 24.Cicero TJ, Adams EH, Geller A, Inciardi JA, Munoz A, Schnoll SH, Senay EC, Woody GE. A postmarketing surveillance program to monitor Ultram® (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend. 1999 Nov 1;57(1):7–22. doi: 10.1016/s0376-8716(99)00041-1. [DOI] [PubMed] [Google Scholar]
- 25.Arfken CL, Cicero TJ. Postmarketing surveillance for drug abuse. Drug Alcohol Depend. 2003 Jun 5;70(3 Suppl):S97–105. doi: 10.1016/s0376-8716(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 26.Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002–2004. J Pain. 6(2005):662–672. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Cicero TJ, Surratt H, Inciardi JA. Relationship between therapeutic use and abuse of opioid analgesics in rural, suburban and urban locations in the United States. Pharmacoepidemiol. Drug. Saf. 2007;16(8):827–40. doi: 10.1002/pds.1452. [DOI] [PubMed] [Google Scholar]
- 28.Cicero TJ. Prescription Drug Abuse and its Relationship to Pain Management. Advances in Pain Management. 2008;2(1):17–29. [Google Scholar]
- 29.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 30.Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 3(1978):235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 31.Rosenblum A, Marsch L, Joseph H, Portenoy R. Opioids and the treatment of chronic pain: controversies, current status and future directions. Exp Clin Psychopharmacol. 2008 Oct;16(5):405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz N, Fernandez K, Chang A, Benoit C, Butler SF. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain. 2008 Jul–Aug;24(6):528–35. doi: 10.1097/AJP.0b013e318167a087. [DOI] [PubMed] [Google Scholar]
- 33.Fendrich M, Yun Soo Kim J. Multiwave analysis of retest artifact in the National Longitudinal Survey of Youth drug use. Drug Alcohol Depend. 2001;62:239–253. doi: 10.1016/s0376-8716(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 34.Schnoll SH, Weaver MF. Addiction and pain. American Journal of Addiction. 2003;12:27–35. [PubMed] [Google Scholar]
- 35.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–7000. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 36.Smith MY, Bailey JE, Woody GE, Kleber HD. Abuse of buprenorphine in the United States: 2003–2005. J Addict Dis. 2007;26:107–111. doi: 10.1300/J069v26n03_12. [DOI] [PubMed] [Google Scholar]
- 37.Riegel B. Pain, the fifth vital sign. [accessed 13, October 2009];Burn survivors throughout the world inc. www.burnsurvivorstw.org/articles/pain.
- 38.Manchikanti L. Health care reform in the United States: Radical surgery needed now more than ever. Pain Physician. 2008;11:13–42. [PubMed] [Google Scholar]
- 39.McQuay H. Opioids in pain Management. Lancet. 1999;353:2229–2232. doi: 10.1016/S0140-6736(99)03528-X. [DOI] [PubMed] [Google Scholar]
- 40.Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 41.Streltzer J, Johansen L. Prescription drug dependence and evolving beliefs about chronic pain management. American Journal of Psychiatry. 2006;163:594–598. doi: 10.1176/ajp.2006.163.4.594. [DOI] [PubMed] [Google Scholar]
- 42.Streltzer J, Kosten TR. Methadone maintenance therapy and chronic pain. Journal of the American Medical Association. 2003;290:2403. doi: 10.1001/jama.290.18.2403-a. [DOI] [PubMed] [Google Scholar]
- 43.Pooler D, Sheheen F, Davidson J. Professional impairment: a history and one state's response. J Addict Dis. 2009;28(2):113–23. doi: 10.1080/10550880902772415. [DOI] [PubMed] [Google Scholar]