Abstract
Study Objective
To identify ovarian autoantigens associated with ovarian autoantibodies.
Design
Hypothesis generating prospective study.
Setting
Urban infertility referral centers and academic research institution.
Patients
74 patients with infertility, 19 patients with premature ovarian failure, 16 healthy control women.
Interventions
None.
Main outcome measures
Identification of autoantigens.
Results
In order to identify major antigens for ovarian autoimmunity, 74 sera from women with unexplained infertility were screened for ovarian autoantibodies (AOA) by immunoassay and one-dimensional Western blot. The majority of sera had immuno-reactions at 50-56kDa. Six representative positive infertility sera were used to identify antigens between 40-60kD by two-dimensional Western blot and mass spectrometry. Antigens included aldehyde (retinal) dehydrogenases (ALDH1A1, ALDH1A2, ALDH7A1), protein disulfide-isomerase A3 (PDIA3), vimentin (VIME), α-enolase (ENO1), phosphoglycerate dehydrogenase and selenium binding protein 1 (SBP1). 60% (n=24/40) of infertility and POF sera were positive for recombinant ALDH1A1, SBP1 or enolase. 80.7% (n=21/26) of AOA positive sera had antibodies to one or more of the three antigens, while only 7% (n=1/14) of AOA negative sera had antibodies to recombinant proteins.
Conclusion
ALDH1A1 and SBP1 are unique to ovarian autoimmunity associated with infertility and POF, and may provide the basis for specific tests to identify patients with ovarian autoimmunity.
Keywords: ovary, autoantigens, autoimmune, infertility, POF
INTRODUCTION
Evidence for an autoimmune disease that targets the ovary has accumulated since the concept was first suggested over 40 years ago (1-3). There are numerous reports of anti-ovarian antibodies associated with premature ovarian failure (POF) (4-8) and infertility (9-13) .
We developed the first immunoassays to test for AOA and for anti-oocyte antibodies in POF and infertility (1, 8, 9). The tests used a microsomal fraction of the ovary, and were validated with respect to tissue specificity (8) and infertility categories (13, 14). Forty to fifty percent of women with POF or unexplained infertility have AOA (8, 9). AOA are associated with lower pregnancy rates in women undergoing infertility treatment (15, 16) and AOA identify women at risk of ovarian failure independent of reproductive hormone levels (17). Also, AOA are associated with poor estradiol responses to hormone stimulation (9) and differentiate poor responders with autoimmunity from those with poor responses due to aging (9, 18). Overall, these studies suggest that standard treatment outcomes for infertility are less effective in the presence of ovarian autoimmunity.
The identification of infertility or POF patients with ovarian autoimmunity could lead to alternative treatment strategies compared to those due to environmental factors, chemotherapy or radiation, or genetic causes. Reversal of infertility or POF associated with AOA by immune suppression has been reported (15, 19-21). Recent advances in the use of anti-B cell therapies for autoimmunity might apply to ovarian autoimmunity associated with infertility and POF (22). However, an antibody test based on specific antigens is needed to more precisely identify patients that might benefit from alternate treatments.
Sundblad et al identified alpha-enolase as an autoantigen women with POF (23). Pires et al reported HSP90 as an autoantigen in infertility (24). While these autoantibodies may be associated with infertility or POF, both antigens are also found in other conditions (25, 26) and healthy individuals (27, 28). This suggests additional antigens are needed in order to better characterize the antibody signature associated with an autoimmune etiology for infertility and POF.
The objective was to identify candidate autoantigens using immuno-proteomics. In order to focus the identification of antigens on the more frequent immunoreactions, the predominant immunoreactions of sera with ovarian proteins were determined using sera from women with unexplained infertility. Selected recombinant autoantigens were then tested for reaction with both infertility and POF sera to confirm their reaction with these autoantigens.
MATERIAL AND METHODS
Patients
Patients at Rush University Medical Center and the University of Ulm were enrolled following protocols approved by the respective Institutional Review Boards. Unexplained infertility patients (n=74) had normal results on standard clinical evaluation, including a normal semen analysis, postcoital test, ovulation (luteal phase progesterone) and tubal patency. Patients with unexplained infertility were 31.0 ± 4.1 years old and had normal day 3 FSH levels (6.5 ± 1.9 mIU/ml). TSH levels were normal (1.4 ± 1.1 IU/mL). The average duration of infertility was 3.2 ± 2.0 years. The average number of prior in vitro fertilization (IVF) cycles was 1.0 ± 1.1. Premature ovarian failure patients (POF) (n=19) had an average age of 30.7±6.6 years and experienced menopause at an average age of 26.6±9.1 years. FSH levels were elevated (64.0±37.8 mIU/mL). TSH levels were normal (1.2±1.1 IU/mL). Only two patients had previous hormone stimulation (for IUI) and none had IVF. Control sera (n=16) were obtained from normally cycling women or postmenopausal women without a history of diagnosed infertility or autoimmune disease and were 35.6 ± 10.6 years old.
Serum and tissue
Blood was collected into a red top tube and the separated serum was stored (−70°C). Normal human ovaries removed at hysterectomy were obtained through the National Disease Research Interchange (Philadelphia, PA). The ovaries used for immunoassay and gel electrophoresis were from women with an average age of 47.7±4.2 years.
Tissue from three ovaries was pooled and homogenized as described previously (8) resulting in a 1,000xg supernatant (29, 30). The supernatant (0.5 ml/500 mg tissue weight) was incubated with protein-G/magnetic bead complexes (30 minutes, 20°C) (Miltenyi Biotech) to remove excess immunoglobulin. The protein content of the supernatant was measured (Bradford assay; BioRad) with bovine serum albumin (BSA) as a standard (Sigma).
The homogenate was used to coat the wells of immunoassay plates (200ug/well/0.1 mL phosphate buffer, pH 7.0). Sera were screened for AOA using the previously described assay (8, 18). Optical density (OD) values two standard deviations (SD) greater than the control mean were considered positive (p<0.05).
Gel electrophoresis and Western Blot
For one-dimensional gel electrophoresis (1-DE), the ovarian extract was mixed with SDS-PAGE lysis buffer (2% SDS, 25% glycerol, 62.5 mM Tris-HCl, pH 6.8) as described previously (29). Protein (250 μg/gel) was resolved in discontinuous 10% Tris-HCl SDS-PAGE preparative well gels (BioRad) with a molecular weight standard (MagicMarker Mix, Invitrogen), and stained with Sypro Ruby (Invitrogen). Digital images were obtained with a Chemidoc XRS Imaging System (BioRad).
For two-dimensional gel electrophoresis (2-DE), proteins were passively rehydrated into IPG strips (16 hours, 20°C) in rehydration buffer and focused as described previously (29). Each IPG strip was loaded on a 10% SDS-Tris HCl gel and resolved as for 1-DE.
For 1-DE or 2-DE Western blot, proteins were transferred (13V, 25 minutes) to nitrocellulose (0.45 μm; BioRad), and blots blocked overnight (16 hours, 4°C) in Tris buffered Starting Block (Pierce) containing 0.05% Tween-20. For 1-DE, the blot was transferred to a multiscreen apparatus (BioRad) according to the manufacturer’s instructions. Sera (1:100) were applied (1 hour, 22°C), the blot removed, washed and incubated with horseradish peroxidase-conjugated goat anti-human immunoglobulin (1:10,000, 1 hour, 22°C; Jackson ImmunoResearch). For 2-DE Western blots the blots were blocked and washed as above, and incubated in serum (1:500). The chemiluminescence reaction was visualized with SuperSignal West Dura Extended Duration substrate (Pierce) and the image analyzed as above.
The molecular sizes of bands in 1-DE Western blots were estimated with QuantityOne and PDQuest software (BioRad) for frequency analysis. Gel images were analyzed to determine Rf values of bands. The molecular weight of each band was calculated from a standard Rf curve generated from the molecular weight standards. Rf values were normalized to a positive sera included in every blot. Previous immunoassays used either rat or human ovarian proteins (correlation coefficient = 0.9, p<0.001) and both human and rat ovarian proteins were used for frequency analysis with identical results using GraphPad Prism (v3) software.
Mass Spectrometry and protein identification
Six representative sera were used to identify antigens. Two to three 2-DE Western blots per serum (15 total) were used to develop spot summaries to locate immunoreactive protein in gels. Proteins were excised, trypsin (Pierce) digested and peptides microsequenced bLC MS/MS using a C18 ProteoPrep nano-HPLC column attached to a NewObjective nano-ESI source interfaced to a ThermoFinnigan LTQ ion trap mass spectrometer. MS/MS spectra for m/z 440 - 2000 were obtained using ESI voltage 2.1kV,MS/MS, isolation width 1.5 m/z, activation Q 0.25, activation time 30 msec and collision energy 35% . Peptide sequence identified with SEQUEST and was searched against human proteins in GenBank v.156. The proteins were ranked according to their protein score. The criteria for selection were proteins with a molecular weight of approximately 50kDa, with greater than 10 Flicka hits (4 peptide sequence hits or more has a 95% confidence level) and a sequence coverage of more than 25%.
Recombinant Protein Immunoassays
Recombinant proteins were produced by expressing the full length mRNA expression ready clone using the PET28 Expression vector in E Coli. Histidine-tagged protein was purified using a Ni-NTA (Qiagen) column, eluted with 200mM imidazole, and further purified by size exclusion chromatography (Superdex 200 16/60; Pharmacia) and ion exchange (Hi-Trap Q column; Pharmacia). The purity of the recombinant protein was verified by 2-DE.
Immunoassays were performed by standard methods. In summary, immunoassay plates (Nunc Maxisorp) were coated (16 hours, 4°C) with recombinant protein (50ng/well) in carbonate buffer (50mM, pH 9.7). Plates were washed with PBS (pH 7.4) containing 0.05% TritonX-100 and nonspecific binding sites were blocked with PBS containing 5% BSA (1 hour, 22°C). Patient sera were diluted (1:100/0.1mL/well) in PBS containing 1% BSA, incubated (90 minutes, 22°C), and autoantibody detected with goat anti-human FAB specific-alkaline phosphatase (AP) (Sigma) reacted with AP substrate (Sigma). OD values (405nm) greater than 2SD above the mean control OD value were considered positive (p<0.05). The Student’s t-test with equal variance was used to identify significant differences.
RESULTS
Frequency distribution of immunoreactive bands
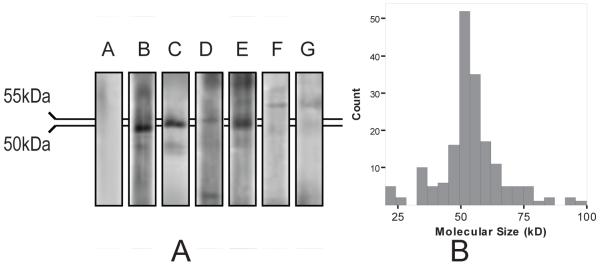
Sera positive for AOA (68%, 50/74) reacted with bands from 20kD-110kD with an average of 4.1±1.5 (SD) bands per serum in 1-DE Western blots (Figure 1A). 74% of the immunoreactions occurred at 50-56 kDa (Figure 1B). Six representative infertility sera that exhibited typical reactions at 40-60kD were selected for 2-DE Western blots and antigen identification.
Figure 1.
(A & B) (A) One-dimensional Western blot showing examples of immunoreactions against human ovarian proteins (250 μg/gel). A negative control serum (Panel A) and examples of positive sera (Panels B-G) are shown.(B) Frequency distribution of the molecular size of immunoreactive bands among positive sera from women with unexplained infertility (n=50/74). The most frequent bands were at 50-56kDa. The data shown were detected using rat ovarian proteins. The frequency distribution was similar for human proteins.
Identification of autoantigens using infertility sera
Similar to 1-DE blots, there was a predominance of reactions around 50kDa in 2-DE Western blots (Figure 2). Proteins with a molecular weight of 40-60kD identified (Table 1) using micro-sequencing included aldehyde dehydrogenase family members (ALDH1A1, ALDH1A2, ALDH7A1), Selenium Binding Protein 1 (SBP1), vimentin, α-enolase (ENO1), protein disulfide-isomerase A3 (PDIA3) precursor and D-3-phosphoglycerate dehydrogenase (3PGDH). Immunoreactive spots near 40kD were also analyzed and contained annexin A2 (AnxA2; molecular weight 38.8kD; 25 hits, 52% coverage), carbonic anhydrase 1 (CA1; molecular weight 28.8kD; 9 hits, 49.5% coverage) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; molecular weight 36kD; 15 hits, 41% coverage).
Figure 2.
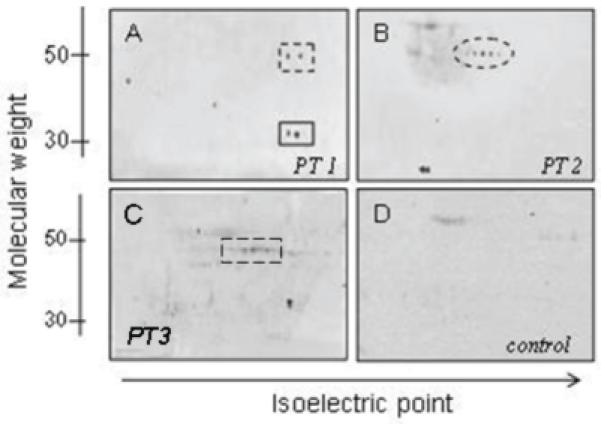
Sera from women with unexplained infertility react with multiple proteins in two-dimensional Western blots. Examples of sera (1:200 dilution) reactions of three different patients (PT 1-3) are shown (Panels A-C). A control incubation with human ovarian protein in which patient sera was omitted (second antibody control) shows no significant reaction (Panel D). Panel A: Upper spot at about 50kDa shows α-enolase (dotted box). Lower spot shows glyceraldehyde-3-phosphate dehydrogenase reaction at 36kDa (solid box). Panel B: Spots at about 50kDa correspond to aldehyde dehydrogenase (dotted oval). Panel C: spots at about 50kDa correspond to Selenium Binding Protein 1 (dotted box).
Table 1.
Autoantigens (40-60kD) identified from human ovary using LC-MS/MS
| Identity | AC | Mw (kD) | pI | Coverage (%) | Flicka hit |
|---|---|---|---|---|---|
| Aldehyde dehydrogenase 1A1 | AAH01505 | 54.8 | 6.3 | 38.8 | 27 |
| Aldehyde dehydrogenase 1A2 | ABC40749 | 56.7 | 5.8 | 40.5 | 14 |
| Aldehyde dehydrogenase 7A1 (Antiquitin) | AAH02515 | 58.7 | 6.3 | 27.9 | 13 |
| Alpha-Enolase | CAA34360 | 47.1 | 7.3 | 65.0 | 31 |
| Phosphoglycerate dehydrogenase | AAH00303 | 56.6 | 6.3 | 25.4 | 13 |
| Protein disulfide-isomerase A3 | BAA11928 | 56.8 | 6 | 67.7 | 40 |
| Selenium-binding protein 1 | AAH09084 | 52.4 | 6.1 | 44.9 | 20 |
| Vimentin | AAH00163 | 53.6 | 5 | 61.0 | 30 |
Abbreviations: LC-MS/MS =high pressure liquid chromatography coupled tandem mass spectrometry; AC= accession number in GenBank; Mw=molecular weight; pI=isoelectric point; Coverage=the percent of peptide sequences matched; Flicka hit=the number of unique peptide matches
Reaction of infertility and POF sera with selected recombinant proteins
The two antigens that appear to be unique to ovarian autoimmunity, ALDH1A1 and SBP1, as well as α-enolase, identified in this study and in a previous study, were tested for reaction with both infertility (n=21) and POF (n=19) sera. The OD values for infertility and POF sera differed significantly from controls (Figure 3). Using the cutoff value based on controls, 55.0% (n=22/40) of sera had anti-SBP1 antibodies, 40% (n=16/40) had anti-enolase antibodies and 52.5% (n=21/40) of sera had anti-ALDH1A1 antibodies. Overall, 60% (24/40) had antibodies to one or more of the three antigens.
Figure 3.
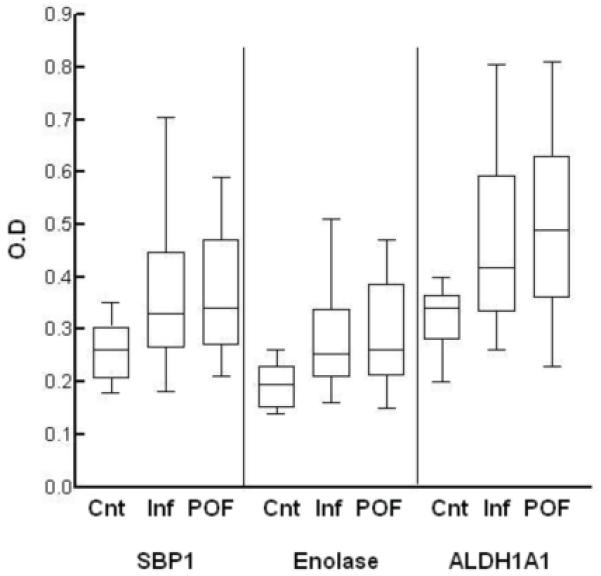
Immunoassay of patient sera against recombinant SBP1, Enolase and ALDH1A1. The box plot shows the median (horizontal line), data in the 50th percentile (box) and data range (T-bars) of the optical density (OD) values for control sera (Cnt), infertility sera (Inf) and premature ovarian failure (POF) sera for each protein. The OD values differed significantly from controls for infertility (SBP1, p=0.020; enolase, p=0.009; ALDH1A1, p=0.026) and POF (SBP1, p=0.019; enolase, p=0.009; ALDH1A1, p=0.019). There was no significant difference between OD density values for infertility and POF for each protein (p>0.6). Applying the OD cutoff value for a positive antibody result based on the control mean OD (0.37 for SBP; 0.28 for enolase; 0.46 for ALDH1A1), infertility and POF sera were positive for SBP1 (55%), enolase (40%) and ALDH1A1 (52.5%). 80.7% (n=21/26) of sera positive for AOA, but only 7% (1/14) of those originally negative for AOA had autoantibodies to one or more of the three antigens.
The data were further examined for significant differences between infertility and POF (Figure 3). There was no significant difference between OD values for infertility and POF sera for any of the antigens. The proportion of sera positive for individual antigens was similar for infertility and POF respectively (SBP1, 47.6% and 63.2%; enolase, 33.3% and 47.4%; ALDH1A1, 47.6% and 57.9%).
Based on AOA immunoassay results (26 AOA positive, 14 AOA negative), 80.7% (n=21/26) of sera positive for AOA had antibodies to one or more of the three antigens. Only 7% (n=1/14) of sera negative for AOA had antibodies to any of the recombinant proteins.
DISCUSSION
Antigens associated with the most frequent immunoreactions for ovarian autoimmunity associated with unexplained infertility were identified. Two proteins, ALDH1A1 and SBP1, are potentially novel antigens, since they have not been identified previously in association with an autoimmune disease. Serum from infertility and POF patients reacted with recombinant SBP1, ALDH1A1 and enolase(a previously identified antigen (23)) confirming that these proteins are potential antigens in ovarian autoimmunity. Although only unexplained infertility sera were used to discover the antigens, POF sera reacted with the same antigens to a similar extent, suggesting they are a continuum of the same disorder.
Aldehyde dehydrogenases (31) are involved in retinoid metabolism. Retinoids are essential for female reproduction and are involved in steroid production (32), oocyte maturation (33) and early embryo development (34). Retinoids reduce FSH-induced FSH and LH receptor expression in rat granulosa cells (35, 36) and granulosa cell maturation (37). ALDH1A1, the cytosolic form of aldehyde (retinal) dehydrogenase, is ubiquitously distributed in epithelial cells, binds thyroid hormone and is induced by estrogen (31). ALDH1A1 is an upstream regulator of CYP17, a P450 enzyme involved in gonadal steroid hormone synthesis (38). Thus, as a target of an autoimmune reaction, alteration of ALDH1A1 expression or function could have multiple effects on reproductive processes.
SBP1 is a cytoplasmic protein found predominantly in epithelial cells, including the surface epithelium of the ovary (39). Selenium supplementation reduces thyroid autoantibodies, selenium is essential for testes function (40) and depletion of selenium in follicular fluid of women with unexplained fertility was reported (41). Interestingly, a conjugated peptide of SBP1, also known as SP56, reduced fertility in mice by 50% in pre-clinical trials of contraceptive vaccines (42). Thus, although little is known of the function of SBP1 (43), one could speculate that autoantibodies might have a detrimental effect on fertility.
Most of the other identified proteins are antigens common to autoimmune diseases or autoimmune responses to tumors. Vimentin, a major intermediate filament protein, is an autoantigen for chronic inflammatory diseases such as rheumatoid arthritis (44). Vimentin is an early phase antigen, and α-enolase is a later phase antigen, in an animal model of lupus (45). Alpha-enolase was recently reported as an autoantigen associated with POF (23). Alpha-enolase is an autoantigen common to a number of systemic autoimmune and inflammatory diseases and is a marker associated with endometriosis and various tumors (46, 47).
Heat shock protein 90 (HSP90) was previously identified as an autoantigen in women with POF (24). Since it is a 90kD protein, it is likely that we did not identify HSP90 because of our experimental design which focused on antigens below 70kD. Furthermore, HSP90 is associated with oocytes (24, 48), and this study focused on ovarian tissue and would not be likely to identify oocytes antigens.
Steroidogenic enzyme CYP21, side chain cleavage (CYP11A1) and 17alpha hydroxylase (CYP17) (49-51), have been identified as autoantigens in women with POF when POF occurs with Addison’s disease, autoimmune polyendocrine syndrome (APS I & II) or diabetes. However, antibodies to CYP21, CYP17 and CYP11 and 3-β-hydroxysteroid dehydrogenase (3-β HSD) were not found in isolated POF (52, 53). In the current study P450 steroidogenic enzymes were not identified. We also tested the reaction of infertility and POF sera with recombinant CYP17alpha and did not find a significant antibody reaction (data not shown). This is consistent with previous reports that antibodies to steroidogenic enzymes were not major antigens in isolated POF (1, 54, 55).
Most autoimmune diseases have several antigens that characterize the disease (56-58). The number of antibodies, rather than individual antibodies, is considered the best predictor of progression from sub-clinical to clinical diabetes (59). Often, antibodies appear years before significant organ damage (56, 59-62). Potentially, a test for ovarian autoimmunity could be used as part of an infertility evaluation to identify women with a risk of autoimmunity so that steps could be taken to prevent follicular destruction or retrieve oocytes before major damage occurs.
In summary, we identified antigens frequently associated with serum autoantibodies in women with unexplained infertility and POF. This is the first study of ovarian autoantigens showing similar antibodies in infertility and POF. Clearly, further evaluation of antigens is needed to determine potential associations with clinical variables. These markers could serve as additional predictors of infertility treatment success in addition to hormone evaluation, could provide a basis for selection of patients for genetic studies and may contribute to an understanding of the pathophysiology of this autoimmune disease.
ACKNOWLEDGEMENTS
We thank Dr. Qishan Lin, Center for Functional Genomics, Proteomics Core Facility for mass spectrometry services. Supported by NIH grant R01 AI 055060 (JL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Luborsky J. Ovarian autoimmune disease and ovarian autoantibodies. J Womens Health Gend Based Med. 2002;11(7):585–99. doi: 10.1089/152460902760360540. [DOI] [PubMed] [Google Scholar]
- 2.Forges T, Monnier-Barbarino P, Faure GC, Bene MC. Autoimmunity and antigenic targets in ovarian pathology. Hum Reprod Update. 2004;10(2):163–75. doi: 10.1093/humupd/dmh014. [DOI] [PubMed] [Google Scholar]
- 3.Tuohy VK, Altuntas CZ. Autoimmunity and premature ovarian failure. Curr Opin Obstet Gynecol. 2007;19(4):366–9. doi: 10.1097/GCO.0b013e328220e90c. [DOI] [PubMed] [Google Scholar]
- 4.Austin G, Coulam C, Ryan R. A search for antibodies to luteinizing hormone receptors in premature ovarian failure. Mayo Clin Proc. 1979;54(6):394–400. [PubMed] [Google Scholar]
- 5.Coulam C, Ryan R. Prevalence of circulating antibodies directed toward ovaries among women with premature ovarian failure. Am J Reprod Immunol Microbiol. 1985;9(1):23–24. doi: 10.1111/j.1600-0897.1985.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 6.Damewood MD, Zacur HA, Hoffman GJ, Rock JA. Circulating antiovarian antibodies in premature ovarian failure. Obstet Gynecol. 1986;68(6):850–854. [PubMed] [Google Scholar]
- 7.Fenichel P, Sosset C, Barbarino-Monnier P, Gobert B, Hieronimus S, Bene M, et al. Prevalence, specificity and significance of ovarian antibodies during spontaneous premature ovarian failure. Hum Reprod. 1997;12(12):2623–2628. doi: 10.1093/humrep/12.12.2623. [DOI] [PubMed] [Google Scholar]
- 8.Luborsky JL, Visintin I, Boyers S, Asare T, Caldwell B, DeCherney A. Ovarian antibodies detected by immobilized antigen immunoassay in patients with premature ovarian failure. J Clin Endocrinol Metab. 1990;70(1):69–75. doi: 10.1210/jcem-70-1-69. [DOI] [PubMed] [Google Scholar]
- 9.Meyer WR, Lavy G, DeCherney AH, Visintin I, Economy K, Luborsky JL. Evidence of gonadal and gonadotropin antibodies in women with a suboptimal ovarian response to exogenous gonadotropin. Obstet Gynecol. 1990;75(5):795–9. [PubMed] [Google Scholar]
- 10.Shivers C, Dunbar B. Autoantibodies to zona pellucida: a possible cause for infertility in women. Science. 1977;197(4308):1082–1084. doi: 10.1126/science.70076. [DOI] [PubMed] [Google Scholar]
- 11.Singh J, Mhaskar A. Enzyme-linked immunosorbent determination of autoantibodies to zona pellucida as a possible cause of infertility in women. J Immunol Methods. 1985;79(1):133–141. doi: 10.1016/0022-1759(85)90399-0. [DOI] [PubMed] [Google Scholar]
- 12.Barbarino-Monnier P, Gobert B, Guillet-Rosso F, Bene MC, Landes P, Faure G. Antiovary antibodies, repeated attempts, and outcome of in vitro fertilization. Fertil Steril. 1991;56(5):928–932. doi: 10.1016/s0015-0282(16)54667-6. [DOI] [PubMed] [Google Scholar]
- 13.Luborsky J, Llanes B, Davies S, Binor Z, Radwanska E, Pong R. Ovarian autoimmunity: greater frequency of autoantibodies in premature menopause and unexplained infertility than in the general population. Clin Immunol. 1999;90(3):368–74. doi: 10.1006/clim.1998.4661. [DOI] [PubMed] [Google Scholar]
- 14.Luborsky JL, Shatavi S, Adamczyk P, Chiong C, Llanes B, Lafniztzegger J, et al. Polycystic ovary syndrome and ovarian autoimmunity--assessment of ovarian antibodies by EIA. J Reprod Immunol. 1999;42(1):79–84. doi: 10.1016/s0165-0378(98)00080-1. [DOI] [PubMed] [Google Scholar]
- 15.Forges T, Monnier-Barbarino P, Guillet-May F, Faure GC, Bene MC. Corticosteroids in patients with antiovarian antibodies undergoing in vitro fertilization: a prospective pilot study. Eur J Clin Pharmacol. 2006;62(9):699–705. doi: 10.1007/s00228-006-0169-0. [DOI] [PubMed] [Google Scholar]
- 16.Luborsky J, Pong R. Pregnancy outcome and ovarian antibodies in infertility patients undergoing controlled ovarian hyperstimulation. Am J Reprod Immunol. 2000;44(5):261–5. doi: 10.1111/j.8755-8920.2000.440502.x. [DOI] [PubMed] [Google Scholar]
- 17.Luborsky J, Roussev R, Coulam C. Ovarian antibodies, FSH and inhibin are independent markers associated with unexplained infertility. Human Reprod. 2000;15(5):1046–1051. doi: 10.1093/humrep/15.5.1046. [DOI] [PubMed] [Google Scholar]
- 18.Luborsky JL, Thiruppathi P, Rivnay B, Roussev R, Coulam C, Radwanska E. Evidence for different aetiologies of low estradiol response to FSH: age-related accelerated luteinization of follicles or presence of ovarian autoantibodies. Hum Reprod. 2002;17(10):2641–9. doi: 10.1093/humrep/17.10.2641. [DOI] [PubMed] [Google Scholar]
- 19.Corenblum B, Rowe T, Taylor PJ. High-dose, short-term glucocorticoids for the treatment of infertility resulting from premature ovarian failure. Fertil Steril. 1993;59(5):988–91. doi: 10.1016/s0015-0282(16)55915-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Huang P, Huang X. Analysis on the treatment of 1,020 patients with immunologic infertility. Zhonghua Fu Chan Ke Za Zhi. 1999;34(4):234–6. [PubMed] [Google Scholar]
- 21.Barbarino-Monnier P, Gobert B, Guillet-May F, Bene MC, Barbarino A, Foliguet B, et al. Ovarian autoimmunity and corticotherapy in an in-vitro fertilization attempt. Hum Reprod. 1995;10(8):2006–7. doi: 10.1093/oxfordjournals.humrep.a136225. [DOI] [PubMed] [Google Scholar]
- 22.Levesque MC. Translational Mini-Review Series on B Cell-Directed Therapies: Recent advances in B cell-directed biological therapies for autoimmune disorders. Clin Exp Immunol. 2009;157(2):198–208. doi: 10.1111/j.1365-2249.2009.03979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundblad V, Bussmann L, Chiauzzi VA, Pancholi V, Charreau EH. Alpha-enolase: a novel autoantigen in patients with premature ovarian failure. Clin Endocrinol (Oxf) 2006;65(6):745–51. doi: 10.1111/j.1365-2265.2006.02661.x. [DOI] [PubMed] [Google Scholar]
- 24.Pires ES, Khole VV. A block in the road to fertility: autoantibodies to heat-shock protein 90-beta in human ovarian autoimmunity. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 25.Vidal CI, Mintz PJ, Lu K, Ellis LM, Manenti L, Giavazzi R, et al. An HSP90-mimic peptide revealed by fingerprinting the pool of antibodies from ovarian cancer patients. Oncogene. 2004;23(55):8859–67. doi: 10.1038/sj.onc.1208082. [DOI] [PubMed] [Google Scholar]
- 26.Gitlits VM, Toh BH, Sentry JW. Disease association, origin, and clinical relevance of autoantibodies to the glycolytic enzyme enolase. J Investig Med. 2001;49(2):138–45. doi: 10.2310/6650.2001.34040. [DOI] [PubMed] [Google Scholar]
- 27.Pashov A, Kenderov A, Kyurkchiev S, Kehayov I, Hristova S, Lacroix-Desmazes S, et al. Autoantibodies to heat shock protein 90 in the human natural antibody repertoire. Int Immunol. 2002;14(5):453–61. doi: 10.1093/intimm/14.5.453. [DOI] [PubMed] [Google Scholar]
- 28.Li WH, Zhao J, Li HY, Liu H, Li AL, Wang HX, et al. Proteomics-based identification of autoantibodies in the sera of healthy Chinese individuals from Beijing. Proteomics. 2006;6(17):4781–9. doi: 10.1002/pmic.200500909. [DOI] [PubMed] [Google Scholar]
- 29.Barua A, Edassery S, Abramowicz J, Dirks A, Bahr J, Hales D, et al. Prevalence of anti-tumor antibodies in the laying hen model of human ovarian cancer. International Journal of Gynecological Cancer. 2009;19(4):500–507. doi: 10.1111/IGC.0b013e3181a39db1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barua A, Bradaric MJ, Kebede T, Espinosa S, Edassery SL, Bitterman P, et al. Antiovarian and anti-tumor antibodies in women with ovarian cancer. Am J Reprod Immunol. 2007;57:243–249. doi: 10.1111/j.1600-0897.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 31.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4(6):697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347–81. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- 33.Mohan M, Thirumalapura NR, Malayer J. Bovine cumulus-granulosa cells contain biologically active retinoid receptors that can respond to retinoic acid. Reprod Biol Endocrinol. 2003;1:104. doi: 10.1186/1477-7827-1-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minegishi T, Hirakawa T, Kishi H, Abe K, Tano M, Abe Y, et al. The mechanisms of retinoic acid-induced regulation on the follicle-stimulating hormone receptor in rat granulosa cells. Biochim Biophys Acta. 2000;1495(3):203–11. doi: 10.1016/s0167-4889(00)00003-3. [DOI] [PubMed] [Google Scholar]
- 36.Minegishi T, Hirakawa T, Kishi H, Abe K, Ibuki Y, Miyamoto K. Retinoic acid (RA) represses follicle stimulating hormone (FSH)-induced luteinizing hormone (LH) receptor in rat granulosa cells. Arch Biochem Biophys. 2000;373(1):203–10. doi: 10.1006/abbi.1999.1528. [DOI] [PubMed] [Google Scholar]
- 37.Hattori M, Takesue K, Nishida N, Kato Y, Fujihara N. Inhibitory effect of retinoic acid on the development of immature porcine granulosa cells to mature cells. J Mol Endocrinol. 2000;25(1):53–61. doi: 10.1677/jme.0.0250053. [DOI] [PubMed] [Google Scholar]
- 38.Livera G, Pairault C, Lambrot R, Lelievre-Pegorier M, Saez JM, Habert R, et al. Retinoid-sensitive steps in steroidogenesis in fetal and neonatal rat testes: in vitro and in vivo studies. Biol Reprod. 2004;70(6):1814–21. doi: 10.1095/biolreprod.103.021451. [DOI] [PubMed] [Google Scholar]
- 39.Huang KC, Park DC, Ng SK, Lee JY, Ni X, Ng WC, et al. Selenium binding protein 1 in ovarian cancer. Int J Cancer. 2006;118:2433–40. doi: 10.1002/ijc.21671. [DOI] [PubMed] [Google Scholar]
- 40.Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol. 2005;184(3):455–65. doi: 10.1677/joe.1.05971. [DOI] [PubMed] [Google Scholar]
- 41.Paszkowski T, Traub AI, Robinson SY, McMaster D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin Chim Acta. 1995;236(2):173–80. doi: 10.1016/0009-8981(95)98130-9. [DOI] [PubMed] [Google Scholar]
- 42.Hardy CM, Clydesdale G, Mobbs KJ. Development of mouse-specific contraceptive vaccines: infertility in mice immunized with peptide and polyepitope antigens. Reproduction. 2004;128(4):395–407. doi: 10.1530/rep.1.00276. [DOI] [PubMed] [Google Scholar]
- 43.Behne D, Kyriakopoulos A. Mammalian selenium-containing proteins. Annu Rev Nutr. 2001;21:453–73. doi: 10.1146/annurev.nutr.21.1.453. [DOI] [PubMed] [Google Scholar]
- 44.El-Gabalawy HS, Wilkins JA. Anti-Sa antibodies: prognostic and pathogenetic significance to rheumatoid arthritis. Arthritis Res Ther. 2004;6(2):86–9. doi: 10.1186/ar1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thebault S, Gilbert D, Hubert M, Drouot L, Machour N, Lange C, et al. Orderly pattern of development of the autoantibody response in (New Zealand White x BXSB)F1 lupus mice: characterization of target antigens and antigen spreading by two-dimensional gel electrophoresis and mass spectrometry. J Immunol. 2002;169(7):4046–53. doi: 10.4049/jimmunol.169.7.4046. [DOI] [PubMed] [Google Scholar]
- 46.Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci. 2001;58(7):902–20. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30(3):142–50. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Calvert ME, Digilio LC, Herr JC, Coonrod SA. Oolemmal proteomics--identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane. Reprod Biol Endocrinol. 2003;1(1):27. doi: 10.1186/1477-7827-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Betterle C, Dalpra C, Greggio N, Volpato M, Zanchetta R. Autoimmunity in isolated Addison’s disease and in polyglandular autoimmune diseases type 1, 2 and 4. Ann Endocrinol (Paris) 2001;62(2):193–201. [PubMed] [Google Scholar]
- 50.Dorman J, Steenkiste A, Foley T, Strotmeyer E, Burke J, Kuller L, et al. The menopause in type 1 diabetic women: is it premature? Diabetes. 2001;50:1857–1862. doi: 10.2337/diabetes.50.8.1857. [DOI] [PubMed] [Google Scholar]
- 51.Winqvist O, Karlsson F, Kampe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison’s disease. Lancet. 1992;339(8809):1559–1562. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
- 52.Soderbergh A, Myhre AG, Ekwall O, Gebre-Medhin G, Hedstrand H, Landgren E, et al. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 2004;89(2):557–62. doi: 10.1210/jc.2003-030279. [DOI] [PubMed] [Google Scholar]
- 53.Falorni A, Laureti S, Candeloro P, Perrino S, Coronella C, Bizzarro A, et al. Steroid-cell autoantibodies are preferentially expressed in women with premature ovarian failure who have adrenal autoimmunity. Fertil Steril. 2002;78(2):270–9. doi: 10.1016/s0015-0282(02)03205-3. [DOI] [PubMed] [Google Scholar]
- 54.Chen S, Sawicka J, Betterle C, Powell M, Prentice L, Volpato M, et al. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison’s disease, and premature ovarian failure. J Clin Endocrinol Metab. 1996;81(5):1871–1876. doi: 10.1210/jcem.81.5.8626850. [DOI] [PubMed] [Google Scholar]
- 55.Reimand K, Peterson P, Hyoty H, Uibo R, Cooke I, Weetman AP, et al. 3beta-hydroxysteroid dehydrogenase autoantibodies are rare in premature ovarian failure. J Clin Endocrinol Metab. 2000;85(6):2324–6. doi: 10.1210/jcem.85.6.6630. [DOI] [PubMed] [Google Scholar]
- 56.Scofield RH. Autoantibodies as predictors of disease. Lancet. 2004;363(9420):1544–6. doi: 10.1016/S0140-6736(04)16154-0. [DOI] [PubMed] [Google Scholar]
- 57.Tsirogianni A, Pipi E, Soufleros K. Specificity of islet cell autoantibodies and coexistence with other organ specific autoantibodies in type 1 diabetes mellitus. Autoimmun Rev. 2009;8(8):687–91. doi: 10.1016/j.autrev.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 58.Sinclair D. Analytical aspects of thyroid antibodies estimation. Autoimmunity. 2008;41(1):46–54. doi: 10.1080/08916930701619466. [DOI] [PubMed] [Google Scholar]
- 59.Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008;41(1):11–8. doi: 10.1080/08916930701619169. [DOI] [PubMed] [Google Scholar]
- 60.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 61.Arbuckle MR, James JA, Kohlhase KF, Rubertone MV, Dennis GJ, Harley JB. Development of anti-dsDNA autoantibodies prior to clinical diagnosis of systemic lupus erythematosus. Scand J Immunol. 2001;54(1-2):211–9. doi: 10.1046/j.1365-3083.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 62.Greenbaum CJ, Sears KL, Kahn SE, Palmer JP. Relationship of beta-cell function and autoantibodies to progression and nonprogression of subclinical type 1 diabetes: follow-up of the Seattle Family Study. Diabetes. 1999;48(1):170–5. doi: 10.2337/diabetes.48.1.170. [DOI] [PubMed] [Google Scholar]