Abstract
Background
Although previous research has demonstrated the prognostic value of cardiopulmonary exercise testing (CPX), these studies have exclusively focused on elderly patients with heart failure (HF). Investigations that have comprehensively examined the value of CPX across different age groups are lacking. The purpose of the present investigation was to evaluate the prognostic value of CPX in young, middle-aged and older patients with HF.
Methods
A total of 1605 subjects (age: 59.2 ±13.7 years, 78% male) underwent CPX and were subsequently tracked for major cardiac events. Ventilatory efficiency (VE/VCO2 slope) and peak oxygen consumption (VO2), both absolute and percent-predicted, were determined. The prognostic value of these CPX variables was assessed in ≤45, 46-65 and ≥66 year subgroups.
Results
The three year event rates for major cardiac events in the ≤45, 46-65 and ≥66 year subgroups were 8.8%, 6.0% and 5.7%, respectively. The VE/VCO2 slope (Hazard ratio ≥1.07, p<0.001), peak VO2 (Hazard ratio ≤0.87, p<0.001) and percent-predicted peak VO2 (Hazard ratio ≤0.98, p<0.001) were all significant prognostic markers in each age subgroup. While the VE/VCO2 slope carried the greatest prognostic strength, peak VO2 and percent-predicted peak VO2 were retained in multivariate analyses (Residual Chi-Square≥5.2, p<0.05). With respect to peak VO2, the actual value was the more robust prognostic marker in the ≤45 and ≥66 year subgroups while the percent-predicted expression provided better predictive resolution in subjects who were 46-65 years old.
Conclusions
These results indicate that, irrespective of a patient's age at presentation, CPX provides valuable prognostic information in the HF population.
Keywords: Expired gas analysis, aerobic capacity, ventilatory efficiency
Introduction
Cardiopulmonary exercise testing (CPX) is routinely employed during the clinical assessment of patients with heart failure (HF), primarily to assess prognosis in those being considered for heart transplantation.[1-3] While the HF population is comprised of patients with rather heterogeneous characteristics, the initial body of research strongly establishing the value of CPX was largely been performed without consideration of how specific traits impact the tests' ability to predict adverse events. More recently however, research has examined the potential impact of specific characteristics, and these studies have demonstrated that CPX remains prognostically relevant irrespective of factors such as HF etiology[4-6], sex[7], race[8] and pharmacologic management[9-10]. These investigations collectively help to support the broad application of CPX in the HF population.
The impact of a patient's age on the prognostic value of CPX has also been examined although, to this point, in an incomplete fashion. Specifically, several investigations have consistently demonstrated CPX is safe, reliable and prognostically useful in elderly patients diagnosed with HF.[11-14] While the highest incidence and prevalence of HF is clearly seen in those at an advanced age[15], a substantial number of patients diagnosed with this chronic disease are considerably younger and are also frequently referred for CPX. Thus, determining the prognostic characteristics of CPX in younger patients with HF seems to be a relevant research endeavor.
We are unaware of previous research which has comprehensively examined the impact of age on the prognostic value of CPX in the HF population. Given the premise that an abnormal response in key CPX variables reflects the level of HF disease severity irrespective of age[16], we hypothesized that the prognostic value of this exercise assessment remains prognostically significant across the adult lifespan. The purpose of the present investigation was to therefore examine the prognostic value of CPX in young, middle-aged and elderly patients diagnosed with HF.
Methods
This study was a multi-center analysis including HF patients from the exercise testing laboratories at San Paolo Hospital, Milan, Italy, Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina, USA, LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina, USA, VA Palo Alto Health Care System, Palo Alto, California, USA and Virginia Commonwealth University, Richmond, Virginia, USA. A total of 1605 patients with chronic HF were included. Inclusion criteria consisted of a diagnosis of HF[17] and evidence of left ventricular dysfunction (systolic dysfunction =83%; diastolic dysfunction 17%) by two-dimensional echocardiography obtained within one month of data collection. None of the patients included in this analysis suffered a myocardial infarction within three months of CPX. This investigation was approved by the local ethics committee at each institution and performed in accordance with the declaration of Helsinki. Written informed consent was obtained from subjects prior to study initiation. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology.
CPX Procedure and Data Collection
Symptom-limited CPX was performed on all subjects and pharmacologic therapy was maintained during exercise testing. Conservative ramping protocols were employed at all centers and ventilatory expired gas analysis was performed using a metabolic cart (Medgraphics CPX-D and Ultima, Minneapolis, MN, Sensormedics Vmax29, Yorba Linda, CA or Parvomedics TrueOne 2400, Sandy, UT). Before each test, the equipment was calibrated in standard fashion using reference gases. Minute ventilation (VE), oxygen uptake (VO2), and carbon dioxide output (VCO2) were acquired breath-by-breath, and averaged over 10-second intervals. Peak VO2 and peak respiratory exchange ratio (RER) were expressed as the highest 10-second averaged samples obtained during the exercise test. Percent-predicted peak VO2 was calculated according to normative values proposed by Wasserman and Hansen et al. (one of six equations according to sex and bodyweight) [18-19] Previous work by our group has demonstrated that this set of prediction equations provides optimal prognostic value.[20] VE and VCO2 values, acquired from the initiation of exercise to peak, were input into spreadsheet software (Microsoft Excel, Microsoft Corp., Bellevue, WA) to calculate the VE/VCO2 slope via least squares linear regression (y = mx + b, m=slope). Previous work by our group and others has shown that this method of calculating the VE/VCO2 slope to be prognostically optimal.[16-21]
Endpoints
In the overall cohort, subjects were followed for major cardiac events (mortality, LVAD implantation, heart transplantation) via hospital and outpatient medical chart review. Subjects were followed by the HF programs at their respective institution providing a high likelihood that all events were captured. Any death with a cardiac-related discharge diagnosis was considered an event.
Statistical Analysis
A statistical software package (SPSS 17.0, Chicago, IL) was used to perform all analyses. All continuous data are reported as mean values ± standard deviation (SD). Dichotomous categorical data are reported as percentages or frequency. Pearson product moment correlation assessed the relationship between age and both the VE/VCO2 slope and peak VO2. The correlation between measured and percent-predicted peak VO2 was also assessed. Differences in continuous baseline and CPX variables between no-event and event HF subjects within age subgroups (≤46 years, 46-65 years and ≥66 years) were tested by two-way analysis of variance (ANOVA). Both main (no-event vs. event and age subgroup) and interaction (event status*age subgroup) effects were assessed. When a significant difference was detected amongst age subgroups, post-hoc analysis was performed by Tukey's honestly significant difference test. The chi-square test was used to determine differences in categorical variables between the event status/age subgroups. Receiver operating characteristic (ROC) curve analysis assessed the prognostic classification schemes of the VE/VCO2 slope, peak VO2 and percent-predicted peak VO2 in each age subgroup. Univariate and multivariate Cox regression analyses assessed the prognostic value of the aforementioned CPX variables and key baseline variables. Because of strong co-linearity, peak VO2 and percent-predicted peak VO2 were entered into separate multivariate analyses. A contingency table was used to determine relative risk ratios for major cardiac events according to previously established dichotomous threshold values for the VE/VCO2 slope (</≥36)[22], peak VO2 (≤/>10 ml• kg−1•min−1)[2-10] and percent-predicted peak VO2 (</≥50%)[20] in each age subgroup. Results from the multivariate Cox regression analysis was used to determine which expression of peak VO2 (actual value or percent-predicted) would be used to generate relative risk ratios in each age subgroup. All statistical tests with a p-value <0.05 were considered significant.
Results
Baseline and event characteristics are listed in Table 1. The three year event rates for major cardiac events in the ≤45, 46-65 and ≥66 year subgroups were 8.8%, 6.0% and 5.7%, respectively. When only cardiac mortality was considered, the event rates for the age subgroups were 4.8%, 4.2% and 5.2%. The mean tracking periods for the ≤45, 46-65 and ≥66 year subgroups were 19.3 ±11.4, 22.5 ±12.2 and 24.3 ±11.2 months, respectively. The lowest percentage of cardiac deaths was observed in the ≤45 subgroup while the highest was in the ≥66 year subgroup. As expected, there was a significant difference in age amongst the three subgroups, although no difference was detected between subjects remaining event-free and those suffering a major cardiac event. The percentage of subjects with a non-ischemic HF etiology as well as female subjects was greatest in the ≤45 year subgroup. The percentage of subjects with an ischemic HF etiology was greater in subjects suffering a major cardiac event in the middle-aged and older subgroups. New York Heart Association class was comparable amongst age subgroups, though a significant difference was observed between subjects remaining event-free and those suffering a major cardiac event within each age group. Left ventricular ejection fraction differed amongst the three age subgroups and was lowest in those ≤45 years. Moreover, LVEF was consistently lower in subjects suffering a major cardiac event in each age subgroup. While ACE inhibitor use varied, there was a clear downward trend in beta-blocker use as age progressed, which is consistent with previous findings.[23] With respect to beta-blocker use, 66% of the subjects were prescribed Carvedilol (average daily dose: 30 mg), 23% were prescribed Metoprolol (average daily dose: 80 mg), and 11% were prescribed another pharmacologic agent in this drug class. Lastly, there were no significant age group*event status interactions for any comparison.
Table 1.
Baseline Characteristics and Therapy Distribution According to Age and Event Status
| Overall Group (n=1605) |
≤45 years: No event (n=218) |
≤45 years: Event (n=45) |
46-65 years: No event (n=700) |
46-65 years: Event (n=107) |
≥65 years: No event (n=462) |
≥65 years: Event (n=73) |
|
|---|---|---|---|---|---|---|---|
|
Baseline Characteristics |
|||||||
| Age, yearsa | 59.2 ±13.7 | 36.4 ±6.5 | 35.7 ±8.3 | 57.1 ±5.4 | 57.5 ±5.8 | 73.5 ±5.6 | 74.0 ±5.8 |
| Sex, % Maleb | 78 | 65 | 69 | 80 | 81 | 80 | 100 |
| Etiology, % Isch./Non-Isch.c |
46/54 | 15/85 | 14/86 | 51/49 | 61/39 | 54/46 | 59/41 |
| NYHA Classd | 2.5 ±0.55 | 2.4 ±0.71 | 2.7 ±0.64 | 2.4 ±0.54 | 2.8 ±0.62 | 2.4 ±0.37 | 2.6 ±0.53 |
| LVEF, %e | 32.6=1 ±14.3 | 29.6 ±11.3 | 20.3 ±9.7 | 32.3 ±14.2 | 24.8 ±12.7 | 38.2 ±14.6 | 31.2 ±13.3 |
| Event Type | |||||||
| death/transplant/LVADf | 162/44/19 | --- | 24/14/7 | --- | 74/24/9 | --- | 64/6/3 |
|
Therapy Distribution, % |
|||||||
| ACE Inhibitor | 73 | 81 | 68 | 64 | 62 | 79 | 63 |
| Beta-Blocker | 63 | 78 | 78 | 66 | 61 | 52 | 52 |
All three age groups significantly different (p<0.001); No difference according to event status or interaction effects (p≥0.50)
Significantly greater percent of females in the ≤45 years group compared to both others (p<0.001); Significantly greater percent of males in the ≥65 years group compared to both others (p<0.001)
Percentage of subjects in the ≤45 years group with non-ischemic etiology significantly greater than both others (p<0.001); Percentage of subjects with ischemic etiology suffering a cardiac event significantly greater compared to subjects not suffering a cardiac event in both the 45-65 and ≥65 years groups (p<0.05)
Significant difference within each age group according to event status (p<0.001); No interaction effects (p>0.20)
All three age groups significantly different (p<0.001); Significant difference within each group according to event status (p<0.001); No interaction effects (p>0.90)
Significantly lower percentage of cardiac deaths in ≤45 years group compared to both others (p<0.05); Significantly higher percentage of cardiac deaths in ≥65 years group compared to both others (p<0.001)
For the overall group, the correlations between age and both the VE/VCO2 slope (r= 0.06, p<0.05) and peak VO2 (r = −0.11, p<0.001) were weak although statistically significant. The correlation between measured and percent-predicted peak VO2 was robust (r=0.71, p<0.001), supporting our concerns over co-linearity and inclusion of these aerobic capacity expressions in separate survival analyses. Cardiopulmonary exercise testing results according to age subgroup and event status are listed in Table 2. None of the exercise tests were terminated secondary to ECG criteria for myocardial ischemia. Measured peak VO2 was higher in the ≤45 year subgroup compared to those subjects in the 46-65 and ≥66 year subgroups. Conversely, percent-predicted peak VO2 differed amongst the three age subgroups and was highest in those ≥66 years of age. The VE/VCO2 slope was lower in the ≤45 year group compared to the ≥66 year group. Peak VO2 and percent-predicted peak VO2 were higher and the VE/VCO2 slope was lower in subjects who were event-free compared to those suffering a major cardiac event in each age subgroup. No differences in peak RER were observed. Once again, there were no significant age group*event status interactions for any comparison.
Table 2.
Cardiopulmonary Exercise Test Data According to Age and Event Status
| Overall Group (n=1605) |
≤45 years: No event (n=218) |
≤45 years: Event (n=45) |
46-65 years: No event (n=700) |
46-65 years: Event (n=107) |
≥65 years: No event (n=462) |
≥65 years: Event (n=73) |
|
|---|---|---|---|---|---|---|---|
|
Peak VO2, ml● kg−1●min−1a |
16.7 ±6.6 | 19.2 ±7.7 | 14.0 ±4.7 | 17.4 ±6.6 | 12.3 ±4.3 | 16.5 ±5.8 | 12.4 ±3.8 |
|
Percent-Predicted Peak VO2, %b |
70.8 ±36.9 | 59.5 ±25.5 | 43.3 ±17.6 | 71.3 ±35.2 | 46.0 ±20.5 | 84.6 ±41.6 | 66.4 ±37.5 |
| VE/VCO2 slope c | 34.5 ±9.5 | 32.2 ±9.2 | 39.9 ±10.1 | 33.0 ±8.2 | 42.1 ±11.7 | 34.2 ±8.4 | 42.3 ±11.0 |
| Peak RER | 1.09 ±0.14 | 1.09 ±0.13 | 1.08 ±0.10 | 1.09 ±0.15 | 1.09 ±0.15 | 1.09 ±0.14 | 1.10 ±0.16 |
≤45 years age group significantly different from other two groups (p<0.01); Significant difference within each age group according to event status (p<0.001))
All three age groups significantly different (p<0.001); Significant difference within each age group according to event status (p<0.001)
≤45 years age group significantly different from ≥65 age group (p<0.05); Significant difference within each age group according to event status (p<0.001)
Receiver operating characteristic curve and univariate Cox regression analysis results for CPX variables in the overall group are listed in Table 3. The VE/VCO2 slope, peak VO2 and percent-predicted peak VO2 were all significant predictors of major cardiac events in each age subgroup. When only cardiac-mortality was considered an event, VE/VCO2 slope (Area under ROC curve = 0.71, hazard ratio =1.06, p<0.01), peak VO2 (Area under ROC curve = 0.63, hazard ratio =0.89, p<0.05) and percent-predicted peak VO2 (Area under ROC curve = 0.62, hazard ratio =0.96, p<0.05) once again all remained significant prognostic in each subgroup. When only considering those subjects on a beta-blocking agent, peak VO2, percent predicted peak VO2 and the VE/VCO2 slope all remained significant univariate prognostic markers in each age specific subgroup (Chi square: ≥10.7, p≤0.001).
Table 3.
Receiver Operating Characteristic and Univariate Cox Regression Analysis for Cardiopulmonary Exercise Test Variables According to Age Subgroup: All Events
| Area Under ROC Curve (95% CI) |
p-value | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|---|
| VE/VCO2 Slope | ||||
| ≤45 years | 0.76 (0.69-0.82) | <0.001 | 1.07 (1.04-1.09) | <0.001 |
| 46-65 years | 0.76 (0.71-0.81) | <0.001 | 1.07 (1.05-1.08) | <0.001 |
| ≥65 years | 0.75 (0.70-0.81) | <0.001 | 1.07 (1.05-1.08) | <0.001 |
| Peak VO2 | ||||
| ≤45 years | 0.70 (0.62-0.78) | <0.001 | 0.87 (0.82-0.92) | <0.001 |
| 46-65 years | 0.75 (0.70-0.80) | <0.001 | 0.85 (0.81-0.88) | <0.001 |
| ≥65 years | 0.73 (0.67-0.79) | <0.001 | 0.84 (0.80-0.89) | <0.001 |
| Percent-Predicted Peak VO2 | ||||
| ≤45 years | 0.71 (0.63-0.79) | <0.001 | 0.96 (0.94-0.97) | <0.001 |
| 46-65 years | 0.77 (0.73-0.82) | <0.001 | 0.96 (0.95-0.97) | <0.001 |
| ≥65 years | 0.68 (0.61-0.75) | <0.001 | 0.98 (0.97-0.99) | <0.001 |
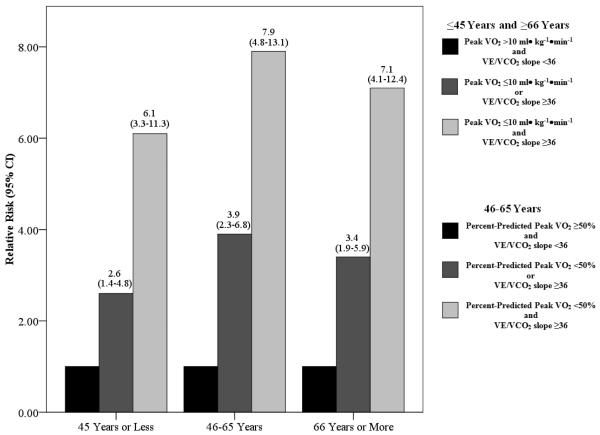
Multivariate Cox regression analyses for CPX variables only are listed in Table 4. The VE/VCO2 slope was the strongest prognostic marker irrespective of age. In all multivariate analyses, peak VO2 and percent-predicted peak VO2 were retained in the regression. Peak VO2 provided stronger prognostic value in the ≤45 and ≥66 year subgroups while percent-predicted peak VO2 was the preferred expression in the 46-65 year subgroup. Figure 1 illustrates the change in relative risk according to CPX values. Compared to subjects with normal responses (a lower VE/VCO2 slope (<36) and higher peak VO2 (>10 ml• kg−1•min−1) or percent-predicted peak VO2 (≥50%)), risk for adverse events substantially increased as subjects had one to two abnormal CPX values. When a peak VO2 threshold of ≤/>10 ml• kg−1•min−1 was substituted for a percent-predicted threshold of </≥50% in the middle-aged group, the relative risks for one and two abnormal values were 3.9 (95% CI: 2.5-6.1) and 6.4 (95% CI: 4.0-10.2), respectively. While the VE/VCO2 slope/peak VO2 combination produced statistically significant (p<0.001) relative risk values in middle-aged subjects, a percent-predicted VO2 threshold of </≥50%, in place of the actual value, provided better predictive resolution in this subgroup.
Table 4.
Multivariate Cox Regression Analysis for Cardiopulmonary Exercise Test Variables According to Age Subgroup
| ≤45 years (n=263; 45 events) | 46-65 years (n=807; 107 events) | ≥65 years (n=535; 73 events) |
|---|---|---|
| Strongest Multivariate Predictor | ||
| VE/VCO2 Slope Chi Square: 38.8, p<0.001* |
VE/VCO2 Slope Chi Square: 119.5, p<0.001* |
VE/VCO2 Slope Chi Square: 72.8, p<0.001* |
| Secondary Multivariate Predictor | ||
| Multivariate Regression #1: Peak VO2 | ||
| Residual Chi-Square: 10.7, p<0.01*,# | Residual Chi-square: 25.9, p<0.001* | Residual Chi-Square: 13.7, p<0.001*,# |
| Multivariate Regression #2: Percent-Predicted Peak VO2 | ||
| Residual Chi-Square: 9.5, p<0.01* | Residual Chi-square: 26.1, p<0.001*,# | Residual Chi-Square: 5.2, p<0.05* |
Retained in multivariate regression
Stronger secondary prognostic marker
Figure 1.
Relative Risk Values in Each Age Subgroup According to Dichotomous Thresholds for Poor CPX Response*
* Compared to referent group, all relative risk ratios were statistically significant (p<0.01)
Multivariate Cox regression results using both CPX and key baseline variables are listed in Table 5. The VE/VCO2 slope was once again the strongest prognostic marker in each age subgroup. Peak VO2 and LVEF were retained in the regression for the ≤45 and ≥66 year subgroups while NYHA class and percent-predicted peak VO2 were retained in the 46-65 year subgroup.
Table 5.
Multivariate Cox Regression Analysis for Baseline and Cardiopulmonary Exercise Test Variables According to Age Subgroup
| ≤45 years (n=263; 45 events) | 46-65 years (n=807; 107 events) | ≥65 years (n=535; 73 events) |
|---|---|---|
| Strongest Multivariate Predictor | ||
| VE/VCO2 Slope Chi Square: 38.8, p<0.001* |
VE/VCO2 Slope Chi Square: 119.5, p<0.001* |
VE/VCO2 Slope Chi Square: 72.8, p<0.001* |
| Secondary Multivariate Predictors | ||
| LVEF Residual Chi-Square: 17.0, p<0.001* Univariate Chi-Square: 20.1, p<0.001# |
NYHA Class Residual Chi-square: 11.8, p<0.01* Univariate Chi-Square: 29.0, p<0.001# |
Peak VO2 Residual Chi-Square: 9.5, p<0.01* Univariate Chi-Square: 23.6, p<0.001# |
| Peak VO2 Residual Chi-Square: 7.4, p<0.01* Univariate Chi-Square: 16.9, p<0.001# |
Percent-Predicted Peak VO2 Residual Chi-Square: 7.3, p<0.01* Univariate Chi-Square: 37.3, p<0.001# |
LVEF Residual Chi-Square: 8.6, p<0.01* Univariate Chi-Square: 16.3, p=0.02# |
| NYHA Class Residual Chi-square: 0.10, p=0.76 Univariate Chi-Square: 9.1, p<0.01# |
LVEF Residual Chi-square: 2.3, p=0.13 Univariate Chi-Square: 25.6, p<0.001# |
NYHA Class Residual Chi-square: 1.8, p=0.12 Univariate Chi-Square: 10.4, p<0.01# |
| HF Etiology Residual Chi-Square: 0.03, p=0.87 Univariate Chi-Square: 0.11, p=0.74 |
HF Etiology Residual Chi-Square: 0.67, p=0.41 Univariate Chi-Square: 3.4, p=0.06 |
HF Etiology Residual Chi-Square: 0.86, p=0.34 Univariate Chi-Square: 2.2, p=0.14 |
Retained in multivariate regression
Significant Univariate Predictor
Discussion
The results of the present study both addresses an area of research that has not been previously investigated as well as reinforcing the findings of previous investigations. The novel finding of the current investigation is the robust prognostic value of CPX in younger patients with HF. We are unaware of any previous investigation demonstrating the prognostic significance of ventilatory efficiency and aerobic capacity in a HF cohort with a mean age in the fourth decade of life. Additionally, our results indicate younger patients with HF referred for CPX are more likely to be female, be diagnosed with a non-ischemic etiology, present with a lower LVEF and be prescribed a beta-blocking agent. Despite these differences in baseline characteristics, the prognostic value of CPX variables in younger subjects was significant and strikingly similar to the two older cohorts, which will be addressed in a subsequent section. The majority of previous investigations in this area have investigated cohorts with a mean age greater than 50 years.[16] The robust prognostic value of CPX in the middle aged cohort was therefore an expected finding, as the majority of previous investigations in this area primarily included subjects who were middle-aged or older. Three previous investigations have demonstrated the prognostic significance of CPX exclusively in elderly HF cohorts.[12-14] These studies all demonstrated the prognostic value of both the VE/VCO2 relationship and peak VO2 in cohorts with a mean age greater than 70 years. The largest of these investigations included 188 subjects and 67 adverse events.[13] The results of the present investigation are consistent with the findings of these previous investigations but in a much larger cohort (n>500) with a greater number of adverse events (n=73), therefore greatly bolstering the concept that CPX is prognostic in elderly patients with HF. Lastly, peak VO2, percent-predicted peak VO2 and the VE/VCO2 slope all maintained prognostic value in each subgroup when only those subjects prescribed a beta-blocking agent were considered, which is consistent with previous investigations that did not consider age.[9-10]
A progressively increasing VE/VCO2 slope and decreasing peak VO2 reflects the degree of disease severity in patients with HF.[24-27] Given the ability of these CPX variables to reflect the degree of pathophysiology, we postulated that an abnormal CPX response would provide prognostic information irrespective of age, a hypothesis supported by our results. Although the correlation between age and both the VE/VCO2 slope and peak VO2 was statistically significant, the relationship was considerably weaker than what is commonly observed in apparently healthy cohorts.[19-28] In fact, the correlations were so weak that there statistical significance can only be attributed to the large number of subjects included in the present study (~1600) and likely has no physiologic/clinical importance This finding in itself indicates the VE/VCO2 and peak VO2 response in patients with HF are more a function disease severity than age-related trends that are observed in apparently healthy individuals. In all three age-based subgroups, subjects who remained event free had a significantly higher measured and percent-predicted peak VO2 and a significantly lower VE/VCO2 slope compared to subjects suffering a major cardiac event. Consistent with these mean differences, the VE/VCO2 slope, peak VO2 and percent-predicted peak VO2 were all found to be robust univariate and multivariate prognostic markers in young, middle-aged and older subjects with HF. Moreover, the stepwise increase in relative risk for adverse events according to established prognostic thresholds for these CPX variables was strikingly similar across age subgroups; subjects with both an abnormal peak VO2 and abnormal VE/VCO2 slope have 6-8 times the risk of adverse events (Figure 1). Therefore, it appears an abnormally elevated VE/VCO2 slope and/or diminished peak VO2 reflects poor prognosis in a consistent manner across the adult lifespan in patients diagnosed with HF.
The majority of previous literature comparing the prognostic power of the VE/VCO2 slope and peak VO2 has demonstrated that the former is superior to the latter. Nevertheless, a multivariate approach, assessing both ventilatory efficiency and aerobic capacity, is thought to provide a higher level of predictive resolution compared to assessment of either variable in isolation.[16] The current findings support the collective assessment of the VE/VCO2 peak VO2 irrespective of patient age. For example, a VE/VCO2 slope of 42 in combination with a peak VO2 of 9.0ml• kg−1 may be equally ominous in two patients whose ages are 35 and 75 years, respectively. In addition, while both the measured and percent-predicted peak VO2 expressions were significant univariate predictors of major cardiac events, there appears to be an age-related influence on which one provides optimal prognostic information. The measured peak VO2 value may be preferable to percent-predicted expressions in young and older patients with HF while the opposite is true for middle-aged individuals. This finding may be attributable to the ability of peak VO2 prediction equations to provide a more accurate representation of what is truly a normal value in middle-aged subjects compared to those who are either younger or elderly. Irrespective of these findings, both measured and percent-predicted peak VO2 should be reported in HF patients undergoing a CPX, as both values demonstrate prognostic value. Future research is needed to confirm the influence of age on the prognostic characteristics of different expressions of aerobic capacity in this population.
Demonstrating the prognostic value of CPX in younger patients with HF was a novel finding. This younger subgroup was however, comprised of the smallest number of subjects and had the fewest adverse events, diminishing the strength of conclusions that can be drawn at the present time. Future research is needed to confirm our findings in younger patients with HF. As with research examining the prognostic characteristics of elderly patients, several investigations from independent research groups are needed to support the results of the present investigation. I
In conclusion, CPX continues to be an important clinical assessment portending powerful prognostic information in patients with HF. The HF population is comprised of patients with rather diverse characteristics, one of them being the age at which they are diagnosed with this chronic disease. The results of the present study indicate CPX provides consistently powerful prognostic information across the adult lifespan in patients with HF.
Acknowledgments
Coats AJ. Ethical authorship and publishing. Int J Cardiol 2009; 131: 149-50.
Supported in part by NIH grants R37AG18915 and P60AG10484
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) J Am Coll Cardiol. 1997;30:260–311. doi: 10.1016/s0735-1097(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons RJ, Balady GJ, Timothy BJ, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002;40:1531–1540. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 3.Jessup M, Abraham WT, et al. 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 4.Arena R, Myers J, Abella J, Peberdy MA. Influence of Heart Failure Etiology on the Prognostic Value of Peak Oxygen Consumption and Minute Ventilation/Carbon Dioxide Production Slope. Chest. 2005;128:2812–2817. doi: 10.1378/chest.128.4.2812. [DOI] [PubMed] [Google Scholar]
- 5.Guazzi M, Myers J, Arena R. Cardiopulmonary Exercise Testing in the Clinical and Prognostic Assessment of Diastolic Heart Failure. Journal of the American College of Cardiology. 2005;46:1883–1890. doi: 10.1016/j.jacc.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 6.Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Arena R. Exercise oscillatory breathing in diastolic heart failure: prevalence and prognostic insights. European Heart Journal. 2008;29:2751–2759. doi: 10.1093/eurheartj/ehn437. [DOI] [PubMed] [Google Scholar]
- 7.Guazzi M, Arena R, Myers J. Comparison of the prognostic value of cardiopulmonary exercise testing between male and female patients with heart failure. International Journal of Cardiology. 2006;113:395–400. doi: 10.1016/j.ijcard.2005.11.105. [DOI] [PubMed] [Google Scholar]
- 8.Arena R, Myers J, Abella J, et al. Prognostic characteristics of cardiopulmonary exercise testing in caucasian and African American patients with heart failure. Congest Heart Fail. 2008;14:310–315. doi: 10.1111/j.1751-7133.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- 9.Arena RA, Guazzi M, Myers J, Abella J. The Prognostic Value of Ventilatory Efficiency with Beta-Blocker Therapy in Heart Failure. Med Sci Sports Exerc. 2007;39:213–219. doi: 10.1249/01.mss.0000241655.45500.c7. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill JO, Young JB, Pothier CE, Lauer MS. Peak Oxygen Consumption as a Predictor of Death in Patients With Heart Failure Receiving {beta}-Blockers. Circulation. 2005;111:2313–2318. doi: 10.1161/01.CIR.0000164270.72123.18. [DOI] [PubMed] [Google Scholar]
- 11.Scardovi AB, Coletta C, De MR, et al. The cardiopulmonary exercise test is safe and reliable in elderly patients with chronic heart failure. J Cardiovasc Med (Hagerstown) 2007;8:608–612. doi: 10.2459/01.JCM.0000281698.53983.4e. [DOI] [PubMed] [Google Scholar]
- 12.Davies LC, Francis DP, Piepoli M, Scott AC, Ponikowski P, Coats AJ. Chronic heart failure in the elderly: value of cardiopulmonary exercise testing in risk stratification. Heart. 2000;83:147–151. doi: 10.1136/heart.83.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicoira M, Davos CH, Florea V, et al. Chronic heart failure in the very elderly: clinical status, survival, and prognostic factors in 188 patients more than 70 years old. Am Heart J. 2001;142:174–180. doi: 10.1067/mhj.2001.115796. [DOI] [PubMed] [Google Scholar]
- 14.Mejhert M, Linder-Klingsell E, Edner M, Kahan T, Persson H. Ventilatory variables are strong prognostic markers in elderly patients with heart failure. Heart. 2002;88:239–243. doi: 10.1136/heart.88.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics--2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 16.Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. 2008;13:245–269. doi: 10.1007/s10741-007-9067-5. [DOI] [PubMed] [Google Scholar]
- 17.Radford MJ, Arnold JM, Bennett SJ, et al. ACC/AHA Key Data Elements and Definitions for Measuring the Clinical Management and Outcomes of Patients With Chronic Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards): Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Failure Society of America. Circulation. 2005;112:1888–1916. doi: 10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- 18.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 19.Wasserman K, Hansen JE, Sue DY, Stringer W, Whipp BJ. Normal Values. In: Weinberg R, editor. Principles of Exercise Testing and Interpretation. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 160–182. [Google Scholar]
- 20.Arena R, Myers J, Abella J, et al. Determining the Preferred Percent-Predicted Equation for Peak Oxygen Consumption in Patients With Heart Failure. Circ Heart Fail. 2009;2:113–120. doi: 10.1161/CIRCHEARTFAILURE.108.834168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bard RL, Gillespie BW, Clarke NS, Egan TG, Nicklas JM. Determining the Best Ventilatory Efficiency Measure to Predict Mortality in Patients with Heart Failure. The Journal of Heart and Lung Transplantation. 2006;25:589–595. doi: 10.1016/j.healun.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 22.Arena R, Myers J, Abella J, et al. Development of a Ventilatory Classification System in Patients With Heart Failure. Circulation. 2007;115:2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 23.Maggioni AP, Sinagra G, Opasich C, et al. Treatment of chronic heart failure with {beta} adrenergic blockade beyond controlled clinical trials: the BRING-UP experience. Heart. 2003;89:299–305. doi: 10.1136/heart.89.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reindl I, Wernecke KD, Opitz C, et al. Impaired ventilatory efficiency in chronic heart failure: possible role of pulmonary vasoconstriction. Am Heart J. 1998;136:778–785. doi: 10.1016/s0002-8703(98)70121-8. [DOI] [PubMed] [Google Scholar]
- 25.De Feo S, Franceschini L, Brighetti G, et al. Ischemic etiology of heart failure identifies patients with more severely impaired exercise capacity. International Journal of Cardiology. 2005;104:292–297. doi: 10.1016/j.ijcard.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Passino C, Poletti R, Bramanti F, Prontera C, Clerico A, Emdin M. Neuro-hormonal activation predicts ventilatory response to exercise and functional capacity in patients with heart failure. Eur J Heart Fail. 2006;8:46–53. doi: 10.1016/j.ejheart.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Tumminello G, Guazzi M, Lancellotti P, Pi+¬rard LA. Exercise ventilation inefficiency in heart failure: pathophysiological and clinical significance. European Heart Journal. 2007;28:673–678. doi: 10.1093/eurheartj/ehl404. [DOI] [PubMed] [Google Scholar]
- 28.Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166:1443–1448. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]