Abstract
Background
Accessing the interior of live cells with minimal intrusiveness for visualizing, probing and interrogating biological processes has been the ultimate goal of much of the biological experimental development.
Scope of Review
The recent development and use of the bio-functionalized nanoneedles for local and spatially-controlled intracellular delivery brings in exciting new opportunities in accessing the interior of living cells. Here we review the technical aspect of this relatively new intracellular delivery method and the related demonstrations and studies, and provide our perspectives on the potential wide applications of this new nanotechnology-based tool in the biological field, especially on its use for high resolution studies of biological processes in living cells.
Major Conclusions
Different from the traditional micropipette-based needles for intracellular injection, a nanoneedle deploys a sub-100 nm diameter solid nanowire as a needle to penetrate a cell membrane and to transfer and deliver the biological cargo conjugated onto its surface to the target regions inside a cell. Although the traditional micropipette-based needles can be more efficient in delivery biological cargoes, a nanoneedle-based delivery system offers an efficient introduction of biomolecules into living cells with high spatiotemporal resolution but minimal intrusion and damage. It offers a potential solution to quantitatively address biological processes at the nanoscale.
General significance
The nanoneedle-based cell delivery system provides new possibilities for efficient, specific, and precise introduction of biomolecules into living cells for high resolution studies of biological processes, and it has potential application in addressing broad biological questions.
1. Introduction
Nanotechnology has recently found increasing applications in biology by providing new nanotechnology-based tools and materials to probe and manipulate biological processes at the nanoscale (~1 to 100 nm) [1], which is the length scale where many fundamental biological processes occur. For instance, fluorescent semiconductor nanoparticles, or quantum dots [2, 3], have been used as probes to visualize dynamic processes in living cells, including the dynamic movement of singe membrane receptors [4-8], motor proteins [9], nerve growth factors [10], and synaptic vesicles [11, 12]; and magnetic nanoparticles have been used to manipulate individual membrane receptors to control signal transduction in living cells [13].
One-dimensional nanomaterials, such as nanotubes and nanowires, have also been used as intracellular biosensors, delivery carriers, and imaging agents [14-21]. In particular, with their unique physical and chemical properties distinct from both individual molecules and bulk materials, chemically synthesized nanomaterials have presented new opportunities and applications in biology and medicine, from basic biophysical studies at the single-molecule level to the diagnosis and treatment of diseases [22-24]. Additionally, with their needle-like nanoscale geometry and excellent mechanical and electrical properties, these high-aspect ratio nanostructures have been explored as membrane-penetrating nanoneedles that can manipulate and sense biological processes inside cell with minimal intrusiveness and toxicity [24-31]. For example, surface-functionalized nanotubes have been used to deliver biomolecular species into living cells with high spatial and temporal precision [27, 30, 31]. Conductive nanotubes have also been envisioned as an electrochemical nanoprobe to measures electrochemical events, redox environments, and signaling processes occurring inside cells or between neighboring cells [31, 32].
The transfer of biomolecules into living cells is a general practice used to monitor or modify molecule-specific intracellular processes. It provides an efficient way to study the temporal and spatial regulation of protein systems that underlie basic cellular functions [33]. Many methods have been developed for this purpose [33-41]. Each of them has its characteristic advantages and disadvantages with respect to cell viability, transfer efficiency, general applicability, and technical requirements [33]. In this review, we discuss a new type of nanotechnology-based methodology for the introduction of biomolecules into living cells and its potential implications in addressing biological questions.
2. General Description of the Nanoneedle-Based Intracellular Delivery
Similar to a micropipette-based injection system, a nanoneedle-based intracellular delivery system comprises a nanoneedle (a nanotube or a nanowire) on a macroscopic handle (an etched metallic wire or simply a pulled glass micropipette) and a manipulator (a standard piezoelectric micromanipulator) integrated with an inverted optical microscope [26, 27, 30, 31]. Similar also in practice to the use of a standard micropipette-based injection system, the nanoneedle is manipulated with the micromanipulator to penetrate into a target cell under the observation of an optical microscope. The major difference between the nanoneedle-based system and the micropipette-based injection system is that in the nanoneedle-based system a sub-100 nm diameter nanowire is used (compared to the micrometer-sized micropipette used in the injection system) to penetrate the cell membrane, which introduces minimal damage to the cell membrane and minimal disruption to the interior environment of a cell; and the materials to be delivered into the cell are carried by the nanoneedle surface and released through a predesigned surface chemistry [27, 30, 42-44] and not through a pressure-drive injection flow.
Such a configuration also allows the direct visual monitoring of the whole nanoneedle-based delivery process (Fig 1A) and requires no additional setup beyond what a biological science laboratory typical has. The drawback is that its operation is limited by the resolution of the optical microscope; thus, only nanoneedles with relatively large diameter and length (diameter larger than ~30 nm and length larger than ~3 μm) can be visually monitored and thus used. Other configurations have used a nanoneedle mounted on an atomic force microscope (AFM) probe and manipulated by an AFM. The advantage of such an AFM-based nanoneedle system is that the force-displacement dependence behavior when the nanoneedle approaches towards and breaks through the cell membrane, and advances into the cell interior can be quantitatively monitored with very high resolution (Fig. 1B, C) [26-28]. However, the limitation is that the direct visualization of the nanoneedle is difficult as the AFM cantilever blocks the direct view of the nanoneedle, and the nanoneedle motion is restricted along the vertical direction. Some AFM-based systems have also been integrated with a confocal microscope; this allows the three-dimensional imaging of the nanoneedle and the target cell (Fig. 1B, C) [26, 43]. However, because of the long acquisition time needed in confocal imaging, real-time monitoring of the nanoneedle operation and the dynamic cellular processes is difficult [45].
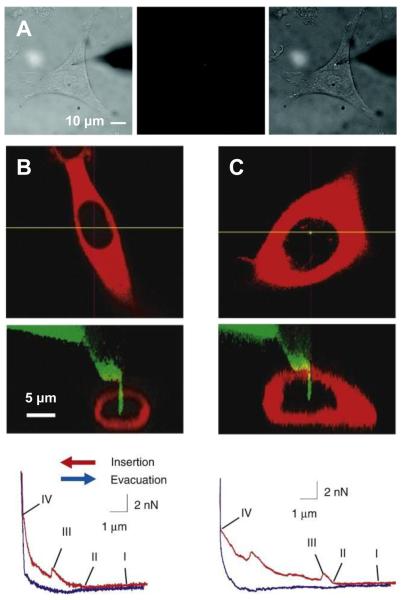
Fig. 1.
Nanoneedle manipulation with a micromanipulator and an AFM system. (A) Optical microscope images of a nanoneedle functionalized with quantum dots when the nanoneedle was manipulated by a micromanipulator to penetrate into the cytoplasm of a living HeLa cell. The whole process was monitored in situ in the bright field (left), the fluorescence (middle), or the combined bright-field and fluorescence (right) image mode. The quantum dots attached on the nanoneedle are shown in red (middle) and bright white (right). The unfocused dark shade on the right side is the tip of the macroscopic needle on which the nanoneedle is attached. (B) Cross-sectional laser-scanning confocal microscope images of a living human mesenchymal stem cell (MSC) expressing red-fluorescent protein (DsRed2-NES) (top) and a Si nanoneedle conjugated with fluorescein isothiocyanate (FITC) with green emission when the nanoneedle was manipulated by an AFM system to penetrate into the nucleus of the MSC cell (middle), and a force-distance curve obtained from the AFM during the nanoneedle operation (bottom). (C) The same observation for a human embryonic kidney cell (HEK293). The nanoneedle contacted the cell membrane (point I), began to feel the repulsive force as it indented the cell (point II), penetrated though the cell membrane (point III), and contacted the substrate (point IV). (A) from Ref. [30] (Copyright 2009 American Chemical Society), (B) and (C) reproduced with permission from Ref. [43] (Copyright 2008 Elsevier).
Overall, an ideal nanoneedle-based delivery system that can control the nanoneedle at the nanoscale resolution, directly visualize the nanoneedle and the target cell, and monitor the force exerted on the nanoneedle would be desirable for the wider use of nanoneedles for biological studies in living cells [45, 46].
In the following, we discuss the fabrication and functionalization of a typical nanoneedle for the intracellular delivery application.
3. Fabrication of Nanoneedles for Intracellular delivery
The most critical component in the nanoneedle-based system is the nanoneedle, which is often attached to a macroscopic structure for the ease of manual handling. For the intracellular delivery purpose, the nanoneedle, in general, needs to have a needle-like structure with nanoscale diameter (less than ~100 nm) and microscale length (~1 to 20 μm, long enough to reach the target area inside a cell), be mechanically rigid to survive the operation in an aqueous media and to penetrate through the cell membrane [25, 28], and have a surface that can be chemically functionalized to attach the cargo on its surface or to convey other functionalities to the nanoneedle [28].
Such a nanoneedle can be typically made by the following two methods. First, chemically-synthesized one-dimensional nanostructures, such as nanotubes and nanowires, can be directly used as nanoneedles [27, 28, 30]. For example, chemically-synthesized nanotubes (carbon nanotubes or boron nitride nanotubes) have ideal properties as nanoneedles for intracellular delivery: they have the needle-like structure with nanoscale diameter (~1 to 100 nm) and microscale length (~1 to 100 μm) [47], large Young's modulus (~1 TPa) and high tensile strength [48-51], while in the meantime are resilient [48, 49, 52, 53]. There are well-developed methods to synthesize such one-dimensional nanostructures with controlled sizes and shapes and they are mostly commercially available. However, the precise alignment and stable assembly of such nanostructures into useful individual nanoneedles are still challenging [25]. Reported methods for the assembly of the nanostructures into needle-like structures include direct attachment of nanotubes by using a manipulator [27, 28, 30, 52-55], catalyst-patterning and direct growth of nanotubes by chemical vapor deposition [56-58], alignment and assembly using dielectrophoresis or magnetic fields [25, 59-66], and transplanting of single nanotubes encapsulated in polymer blocks [67, 68]. However, these methods do not reproducibly produce nanoneedles in large quantity or produce high-aspect ratio “water-stable” nanoneedles (that can survive the meniscus forces involved in the intracellular delivery operation) [25, 28, 54]. Second, a nanoneedle can be fabricated by nanofabrication, such as focused ion beam (FIB) machining [26] and direct-write nanofabrication techniques [69-71]. For example, Nakamura and colleagues have fabricated Si nanoneedles by sharpening Si AFM tips with FIB [26, 42-44, 72-76]. They fabricated Si nanoneedles with diameters of ~200 to 800 nm and lengths of ~5 to 10 μm and demonstrated the capability of these Si nanoneedles to penetrate through both the cellular and nuclear membranes of living cells. However, in general, nanofabrication methods produce nanoneedles with relatively large diameters (in most cases, larger than 100 nm) and short lengths.
4. Functionalization of Nanoneedles for Intracellular Delivery
Several research groups have developed the nanoneedle-based delivery system that uses the outer surface of the nanoneedle for carrying the cargo for intracellular delivery (Fig. 2) [27, 30, 42-44]. This requires that the cargo is conjugated on the surface of the nanoneedle and is able to be released from the surface of the nanoneedle once transferred inside cells. There are various surface chemistry and bioconjugation methods to functionalize the surface of the nanoneedle and conjugate the cargo on it. For example, the surface of the nanoneedle can be directly functionalized (Fig. 2A) [27, 43, 66] or can be first coated with other materials (e.g., gold) and then functionalized (Fig. 2B) [28, 30].
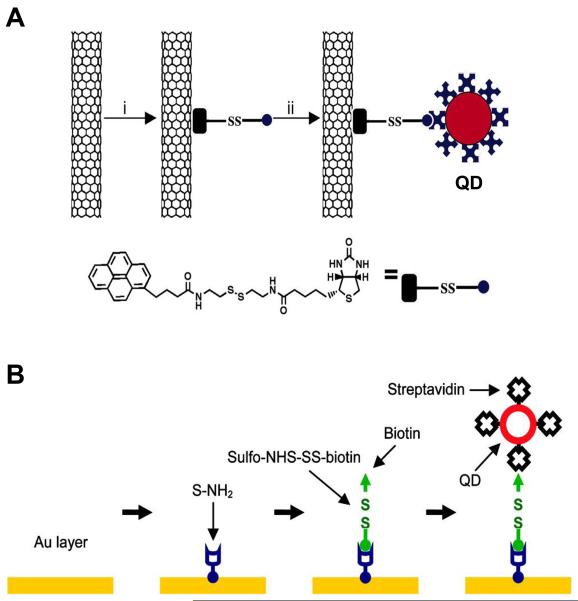
Fig. 2.
Surface functionalization of nanoneedles and loading of cargo. (A) Surface functionalization of a CNT nanoneedle with streptavidin-conjugated quantum dots (QDs) through a linker molecule containing a disulfide bond. (B) Surface functionalization of a gold-coated nanoneedle with streptavidin-conjugated quantum dots (QDs) through a stepwise procedure. (A) reproduced with permission from Ref. [27] (Copyright 2007 National Academy of Sciences, U.S.A.), (B) from Ref. [30] (Copyright 2009 American Chemical Society).
In the case of a carbon nanotube-based nanoneedle, it can be functionalized by either covalent methods or non-covalent methods. The covalent methods use chemical reactions to chemically bond functional groups directly on the surface of the CNT (e.g., carboxyl groups by oxidation) [20, 21, 66, 77]. The non-covalent methods use hydrophobic and π-π interactions [20, 21, 27, 77] to attach functional groups on the CNT surface. For example, Chen et al. [27] used a linker molecule that contains a pyrene moiety and a biotin moiety to functionalize the CNT nanoneedle with nanoparticles: the pyrene moiety binds to the CNT surface through π-π stacking and the streptavidin-coated nanoparticles are attached to the biotin moiety (Fig. 2A).
A Si nanowire-based nanoneedle can be functionalized by forming self-assembly monolayers (SAMs) of alkylsilanes on the Si surface through the silane coupling reaction. For instance, Nakamura and colleagues used (3-aminopropyl)triethoxysilane and (3-mercaptopropyl)trimethoxysilane to functionalize the surface of Si nanoneedles with SAMs with amine and thiol terminal groups and used these functional groups to conjugate proteins, DNA, and polymers [42-44, 73, 75].
A more general approach is to coat the nanoneedle with a common material, such as gold, and subsequently functionalize the coated nanoneedle [28, 30]. This approach is nonspecific to the type of nanoneedles; thus, a “standard” surface chemistry method can be used for various nanoneedles rather than synthesizing linker molecules for a specific type of nanoneedles. The surface coating also increases the mechanical integrity of the nanoneedle structure [28, 30, 78]. A well-studied method is to coat the nanoneedle with a thin layer of gold (~1 to 20 nm in thickness) and form a SAM through the chemisorption of thiols on gold surfaces [28, 30]. For example, Yu and colleagues [30] developed a stepwise procedure to functionalize gold-coated nanotube nanoneedles: they first formed an amine-terminated SAM on the gold-coated nanoneedle, then conjugated a linker molecule containing a disulfide bond within its spacer and a biotin moiety, and finally attached streptavidin-coated nanoparticles on the biotinylated nanoneedle by the binding of streptavidin and biotin (Fig. 2B). Moudgil and colleagues [28] also conjugated gold nanoparticles on gold coated CNT nanoneedles by forming an amine-terminated SAM and subsequently attaching gold nanoparticles by electrostatic interactions.
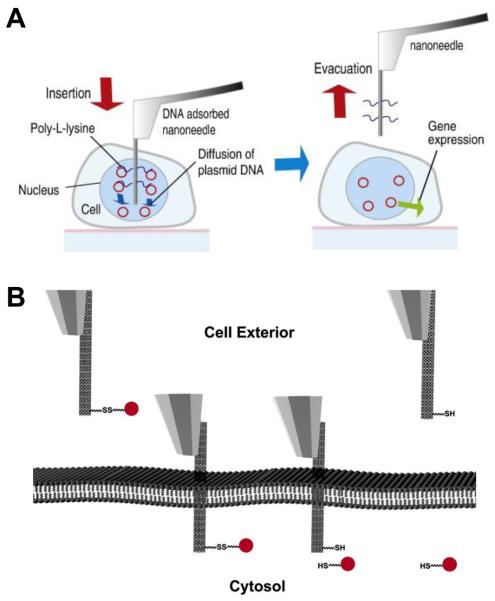
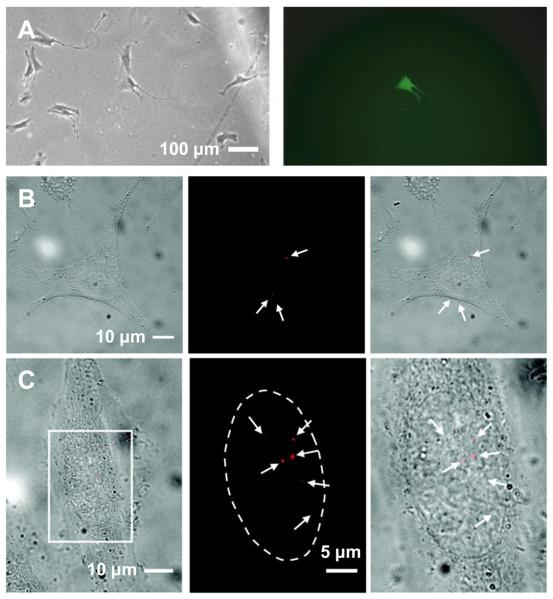
Comparing to the ready availability of numerous surface functionalization methods for conjugating various materials on the nanoneedle, a more challenging task is to design a conjugation strategy that can allow the controlled release of the conjugated materials from the nanoneedle once transferred inside cells (Fig. 3). A simple approach is to rely on the passive desorption of the non-chemically attached cargo from the nanoneedle. For example, Nakamura and colleagues [42-44] attached DNA on the functionalized Si nanoneedle (~200 nm in diameter) through electrostatic interactions and demonstrated the release of the DNA from the nanoneedle through the passive desorption of DNA inside cells (Fig 3A). They demonstrated the delivery of plasmid DNA into the nucleus of single human mesenchymal stem cells (MSCs), human embryonic kidney cells (HEK293), and breast cancer cells (MCF-7), using an AFM-based nanoneedle system. In particular, the direct delivery of green fluorescent protein (GFP) plasmid DNA into the nucleus of single target cells not only selectively expressed GFP in the single target cells but also expressed GFP at a high successful rate (~70% for primary cultured human MSCs), compared favorably with other nonviral gene delivery methods (lipofection ~50% and microinjection ~10% for human MSCs) (Fig 4A) [43]. However, because it is nonspecifically adsorbed on the nanoneedle, the cargo (i.e., DNA in the study) is unselectively desorbed from the nanoneedle both in the media and inside the target cell in this approach. Thus, the delivery process must be completed within several minutes after inserting the nanoneedle into the cell media (before the complete desorption of cargo from the nanoneedle into the media).
Fig. 3.
Schematics of nanoneedle-based intracellular delivery. (A) Gene delivery using a DNA-adsorbed Si nanoneedle. The DAN is passively desorbed from the nanoneedle in the nucleus of the cell. (B) Intracellular delivery though the reductive cleavage of the disulfide bond. The nanoneedle functionalized with cargo via a disulfide bond penetrates the cell membrane. The cargo is released from the nanoneedle through the reduction of the disulfide bond in the cytosol of the cell. The nanoneedle is then retracted. (A) reproduced with permission from Ref. [43] (Copyright 2008 Elsevier), (B) from Ref. [27] (Copyright 2007 National Academy of Sciences, U.S.A.)
Fig. 4.
Nanoneedle-based delivery of molecules into the cytoplasm and nucleus of living cells. (A) GFP expression in a mesenchymal stem cell (MSC) using a DNA-adsorbed nanoneedle (shown in Fig. 3A): bright-field (left) and fluorescence (right) images of the MSC (24 hours after delivery). (B) Delivery of quantum dots into the cytoplasm of a HeLa cell using the nanoneedle-based mechanochemical delivery method: bright field (left), fluorescence (middle) images of the cell after delivery and overlay of the bright field and fluorescence images (right). (C) Delivery into the nucleus of a HeLa cell using the same method in (B): overlay of bright-field and fluorescence images of a cell after delivery (left) and enlarged fluorescence image (middle) and overlay of bright-field and fluorescence images (right) of the marked region in the left image. The arrows indicate quantum dots (red). The dotted line locates the boundary of the nucleus. (A) reproduced with permission from Ref. [43] (Copyright 2008 Elsevier), (B) and (C) from Ref. [30] (Copyright 2009 American Chemical Society).
A more sophisticated method to release cargo specifically inside cells is to use the reductive cleavage of the disulfide bond in the reducing environment of the interior of cells; cells have the regulatory mechanism that maintains the redox equilibrium of the intracellular environment, where the disulfide bond is reductively cleaved into two thiol groups (Fig. 3B) [27, 30]. Using this strategy, Bertozzi and colleagues [27] and Yu and colleagues [30] demonstrated the delivery of a discrete, small number of protein-coated fluorescent quantum dots into living HeLa cells: they attached the quantum dots on the nanoneedle through a linker molecule containing a disulfide bond (Fig. 2B) and demonstrated the release of the quantum dots inside cells by incubating the nanoneedle inside cells for ~15 to 30 minutes (a time required for the reduction of the disulfide bond) (Fig. 3B). Furthermore, Yu and colleagues [30] demonstrated the selective delivery of well-dispersed single quantum dots into the cytoplasm and nucleus and the tracking of the delivered quantum dots inside the cells (Fig. 4 and Fig. 5).
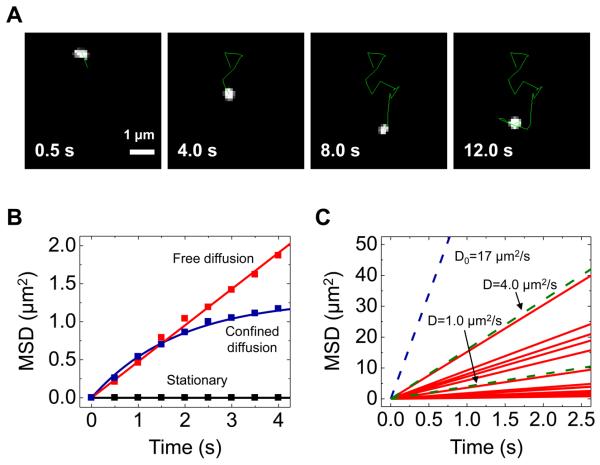
Fig. 5.
Tracking of quantum dots delivered into living HeLa cells. (A) A series of fluorescence images showing the trajectory (the green line) of a tracked QD. (B) Mean-square displacement (MSD) versus time data showing three types of characteristic motion of QDs: free diffusive (red square), confined (blue square), and stationary (black square). The solid red line and blue line are the line fit on the data based on a free diffusion model and a confined diffusion model, respectively. (C) Diffusion coefficient of QDs in the cytoplasm: the diffusion coefficient D (0.08−3.8 μm2/s, mean 0.8 μm2/s, n = 20) was determined by fitting a free diffusion model MSD(t) = 4Dt to the initial few data points of each MSD versus time curve acquired from tracking of different QDs. The solid red lines are the fitted line. The dashed blue line indicates the MSD expected for freely diffusing QDs in aqueous solution (D0 = 17 μm2/s). The dashed green lines are reference lines for D of 1.0 and 4.0 μm2/s. Reproduced from Ref. [30] (Copyright 2009 American Chemical Society).
5. Features of the Nanoneedle-Based Intracellular Delivery
The intracellular delivery using a cell membrane-penetrating nanoneedle has several unique features that may allow new strategies for biological experiments inside living cells. The nanoneedle can deliver a discrete, small number of molecules into a target area of a single target cell at desirable times without any apparent damage to the cell. The general advantages and disadvantages of the nanoneedle-based delivery method in comparison to other common delivery methods are summarized in the Table 1 [33].
Table 1.
Common Cellular Delivery methods
| Method | Target cell | Cargo | Efficiency | Cell survival | Quantitative | Automation | Others |
|---|---|---|---|---|---|---|---|
| Nanoneedle-based delivery | Unlimited | Diversified | High (up to 100%) | High | Great | No | Expensive and technically demanding, limited number of cells, spatial precision in delivery |
| Microinjection [33, 34] | Unlimited | Diversified | High (100%) | Fair | Fair | No | Expensive and technically demanding, limited number of cells |
| Viral infection [31] | Unlimited | DNA only | High | High | Poor | Yes | Virus packing and viral gene expression |
| Electroporation [35] | Limited | Limited | Fair | Fair | Poor | No | Requires specialized equipment works best when cells are in suspension |
| Protein transduction [32] | Unlimited | Diversified | Fair | High | Poor | Yes | Receptor independent Cargo Size restriction, in vivo applications possible Protein unfolding |
| Liposome-mediated transfer [36, 37] | Limited | Diversified | Fair | Fair | Poor | Yes | Interferes with lipid metabolism in vivo applications possible |
The nanoneedle can deliver cargo into living cells with spatial and temporal precision. The ability of the nanoneedle to reach target areas inside cells allows the direct delivery of cargo into target areas or compartments inside cells, not readily achievable by conventional delivery methods. For instance, it is demonstrated that the nanoneedle-based method can selectively deliver quantum dots into either the cytoplasm or the nucleus of living HeLa cells (Fig. 4B, C) [30]. This capability may potentially allow spatially-resolved experiments inside living cells (e.g., inside the nucleus). Because the cargo is released from the nanoneedle inside cells, the spatial resolution of the delivery is mostly determined by the size of the nanoneedle inserted into a cell (< ~100 nm in diameter and ~1 to 4 μm in length) and the spatial precision of the manipulator (e.g., a nanoscale resolution of ~1 nm is achievable with a piezoelectric manipulator, such as an AFM system). The delivery can also be done with high temporal precision (e.g., at desirable times throughout the cell cycle); the temporal resolution of ~15 to 30 minutes is achievable, for example, when the reductive cleavage of the disulfide bond is used as a release mechanism (Fig. 3B). Currently, we are also working on an electrochemical delivery approach, in which the release of the attached cargo can be achieved by applying a pulse of a small electrical potential to the nanoneedle. Such a delivery strategy can significantly enhance the temporal resolution down to the several seconds range. The delivery with such a high temporal precision will allow the manipulation of cellular processes with short time scales, such as signaling transduction and protein transportation inside of cells.
The nanoneedle can deliver a discrete, small number of molecules into cells. For example, the nanoneedle-based method can uniquely deliver well-dispersed single quantum dots into cells [30]; this capability may potentially allow the use of the delivered quantum dots for molecular imaging inside living cells (Fig. 4B, C). Despite their bright and stable fluorescence, ideal for single-molecule imaging, the lack of methods that can deliver singly-dispersed quantum dots into cells has hindered their use for molecular imaging inside living cells, one of the most promising applications of quantum dots [2, 6]. The unique capability of the nanoneedle to precisely deliver only a small number of nanoprobes can minimize the interference of the delivered nanoprobes (e.g., quantum dots and magnetic nanoparticles) with intended experiments in living cells and the effect of such nanoprobes on cellular physiology. For instance, the delivery of a small number of quantum dots significantly reduced the background signal from the out-of-focus quantum dots, enabling the detection and tracking of single quantum dots inside living cells even with a simple epifluorescence microscope (Fig. 5A) [30]. The direct tracking showed that the quantum dots diffused in the cytoplasm of HeLa cells with varying diffusion coefficient D of ~0.1 to 4 μm2/s, indicating the high heterogeneity of physical properties and the molecular crowding of the intracellular environment (Fig. 5B, C) [30]. In contrast, a recent study also showed that endosomal accumulation of quantum dots, introduced into cells via the endocytic pathways, can have several effects on cell physiology [79]. Additionally, spatially-resolved delivery of one or a traceable number of force probes (e.g., magnetic nanoparticles) would be desirable for some cellular and molecular mechanics experiments inside living cells, in which one needs to know where the force is applied [80].
How the nanoneedle affects cellular function and viability is important in any living cell experiments. Most studies showed that the penetration of a nanoneedle into a living cell does not impair the cell viability or membrane integrity. For example, the cell viability tests, using the trypan blue exclusion assay, the Calcein AM assay, and the Annexin V-FITC/propidium iodide assay, or the monitoring of the cell proliferation with nanoneedles with diameter less than 100 nm showed that mammalian cells, including HeLa cells, mouse embryonic stem cells and human embryonic kidney cells (HEK 293), were viable for the duration of the experiments (from several minutes to several days) [27, 29, 30]. Cell viability tested with nanoneedles with different diameters, using the 4,6-diamido-2-phenylindole (DAPI) exclusion assay for human epidermal melanocytes, HEK 293, and breast cancer cells (MCF-7), also suggested that maintaining nanoneedles with diameter less than 400 nm inside cells does not affect the cell viability for at least one hour [42]. However, how the nanoneedle affects the cellular processes and functions needs more thorough examination.
6. Biological Applications of Nanoneedle-Based Intracellular Delivery
While it is exciting to have a functionalized nanoneedle as a new gene/ protein delivery system for cell studies, more remains to be explored to utilize such an elegant yet powerful system for studying different cellular processes. In this section, we highlight a few preliminary attempts and potential applications with the functionalized nanoneedle for intracellular delivery.
Delivery of DNA/RNA molecules for gene expression: high efficiency in gene delivery remains highly desirable for cellular studies; in particular, high efficiency delivery of exogenous genes into many primary cultures as well as stem cells remains a challenge. As mentioned previously, Nakarmura and colleagues [42-44] have demonstrated the direct delivery of DNA into the nucleus of a variety of mammalian cells, including human mensenchymal stem cells (Fig 4A). With improvement on the releasing methods, it is expected that the direct delivery of plasmid DNA into the nucleus of individual cells can lead to a superior successful rate of expression at the single-cell level in comparison to other nonviral gene delivery methods. Due to the high spatial resolution, the functionalized nanoneedle can also be used to deliver exogenous genes into small cellular compartments, such as axonal terminals or dendritic spines, which have their own gene expression systems independent from those in the neuron cell body (soma).
Small signaling molecules for signaling amplification and transduction: one of the advantages of the functionalized nanoneedle-based delivery is that it can deliver a small number of molecules into cells. The signaling molecules have the capability to amplify the signaling cascades in mammalian cells; however, the quantification of signaling amplification is far from being completely understood. While various theoretical methods has been applied to understand quantification of signaling amplification, most experimental approaches are rudimentary, and yield little information on quantification of any specific cellular events in native environment. Using the functionalized nanoneedle-based delivery, one can deliver small diffusible signaling molecules such as second messenger cyclic AMP, cyclic GMP, inositol phosphates, and diacylglycerol, and monitor how individual second messengers can trigger signaling amplification in a specific cellular setting.
Delivery of polypeptides or proteins for examining protein transportation, stability and degradation: the functionalized nanoneedle-based delivery can also be used to directly deliver polypeptides and proteins into specific cellular compartments. Although the direct delivery of polypeptides and proteins has not been demonstrated with this nanoneedle-based delivery system, it should be attainable by modifying the surface functionalization of the nanoneedle for the purpose of attaching protein molecules [27, 30]. Alternatively, one can exploit the physical and chemical properties in a specific targeting cellular environment to allow the proteins to be absorbed on the needle, and desorbed in the cellular environment. The challenge is to develop different functionalized needle surface for the conjugation of diversified polypeptides or proteins onto the nanoneedle. An additional challenge would be to conjugate large proteins on the nanoneedle for delivery due to the difficulties of handling large proteins in vitro.
Upon successful delivery of a few protein molecules with fluorescent markers into a cell, the movement of the delivered proteins can be potentially tracked to understand the protein distribution and functions at different cellular processes such as cell cycle, cell growth, and protein processes and delivery. In addition, the function of the delivered protein can be monitored with live cell imaging by detection of the substrate production or changes in biophysical and chemical properties, such as ion concentrations. Moreover, the stability of the delivered proteins can be tracked inside of cells. This is particular useful to check protein stability of signaling molecules which can undergo rapid degradation under stimulation of hormones and growth factors.
7. Perspectives
Bio-functionalized nanoneedle-based intracellular delivery presents a novel method to deliver biomolecules into cells with high spatio-temporal resolution. The delivery system can deliver biomolecules to virtually any type of cells with minimal intrusiveness, which makes it feasible to deliver biomolecules into low copy cells such as certain sensory neurons isolated from animals, and thus expands the application of cellular studies on these cells. The method also possesses potential advantages in delivering a few molecules with high spatial and temporal precision, which can enable the single-molecule study of a wide range of cellular processes, including cell cycle, cell growth and differentiation, membrane potential and redox environment, signaling transduction and amplification, and gene expression. It will be critical for the further development of this method for the delivery of a broad range of biomolecules from nucleic acids, proteins, to small diffusible molecules. Meanwhile, it is equally important to integrate the nanoneedle-based delivery system with other experimental techniques such as live cell imaging and patch clamping to broaden the biological application of such high-end techniques to tackle biologically important but challenging problems. In the light of its high transfer efficiency and flexibility in the kind of molecules that can be transferred, it would be interesting to explore the possibilities of automation for this nanoneedle-based delivery, which would allow high-throughput screening required for systematic large scale genome projects.
Beyond intracellular delivery, the nanoneedle can also be used in many other ways for biological experiments in living cells [31]. For example, one may use a conductive nanoneedle, electrically insulated throughout its sidewall but exposed at the very end of its tip, as a nanoscale electrochemical probe to measure electrochemical reactions and signaling processes occurring inside cells or between neighboring cells [32, 81, 82]. As the cellular probes based on glass micropipettes (e.g., used for patch-clamp and microelectrode techniques) have revolutionized cellular electrophysiology, the nanoneedle nanoprobe capable of spatially-resolved electrochemical measurements would further enhance our understanding of related cellular processes.
Acknowledgements
The work was supported by NSF (Grant DMI 0328162, CBET 0933223, and CBET 0731096), the Grainger Foundation, and NIH (Grant GM072744 and HL082646)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitesides GM. The ‘right’ size in nanobiotechnology. Nature Biotechnology. 2003;21:1161. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 2.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alivisatos AP, Gu W, Larabell C. QUANTUM DOTS AS CELLULAR PROBES. Annual Review of Biomedical Engineering. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 4.Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion Dynamics of Glycine Receptors Revealed by Single-Quantum Dot Tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 5.Howarth M, Takao K, Hayashi Y, Ting AY. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7583–7588. doi: 10.1073/pnas.0503125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannai H, Levi S, Schweizer C, Dahan M, Triller A. Imaging the lateral diffusion of membrane molecules with quantum dots. Nat. Protocols. 2007;1:2628–2634. doi: 10.1038/nprot.2006.429. [DOI] [PubMed] [Google Scholar]
- 7.Howarth M, Liu W, Puthenveetil S, Zheng Y, Marshall LF, Schmidt MM, Wittrup KD, Bawendi MG, Ting AY. Monovalent, reduced-size quantum dots for imaging receptors on living cells. Nat Meth. 2008;5:397–399. doi: 10.1038/nmeth.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roullier V, Clarke S, You C, Pinaud F, Gouzer Gr, Schaible D, Marchi-Artzner Vr, Piehler J, Dahan M. High-Affinity Labeling and Tracking of Individual Histidine-Tagged Proteins in Live Cells Using Ni2+ Tris-nitrilotriacetic Acid Quantum Dot Conjugates. Nano Letters. 2009;9:1228–1234. doi: 10.1021/nl9001298. [DOI] [PubMed] [Google Scholar]
- 9.Courty S, Luccardini C, Bellaiche Y, Cappello G, Dahan M. Tracking Individual Kinesin Motors in Living Cells Using Single Quantum-Dot Imaging. Nano Letters. 2006;6:1491–1495. doi: 10.1021/nl060921t. [DOI] [PubMed] [Google Scholar]
- 10.Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li W-P, Mobley WC, Chu S. One at a time, live tracking of NGF axonal transport using quantum dots. Proceedings of the National Academy of Sciences. 2007;104:13666–13671. doi: 10.1073/pnas.0706192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Cao Y-Q, Tsien RW. Quantum dots provide an optical signal specific to full collapse fusion of synaptic vesicles. Proceedings of the National Academy of Sciences. 2007;104:17843–17848. doi: 10.1073/pnas.0706906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Li Y, Tsien RW. The Dynamic Control of Kiss-And-Run and Vesicular Reuse Probed with Single Nanoparticles. Science. 2009;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannix RJ, Kumar S, Cassiola F, Montoya-Zavala M, Feinstein E, Prentiss M, Ingber DE. Nanomagnetic actuation of receptor-mediated signal transduction. Nat Nano. 2008;3:36–40. doi: 10.1038/nnano.2007.418. [DOI] [PubMed] [Google Scholar]
- 14.Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand J-P, Prato M, Kostarelos K, Bianco A. Functionalized Carbon Nanotubes for Plasmid DNA Gene Delivery. Angewandte Chemie International Edition. 2004;43:5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- 15.Cai D, Mataraza JM, Qin Z-H, Huang Z, Huang J, Chiles TC, Carnahan D, Kempa K, Ren Z. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nature Methods. 2005;2:449–454. doi: 10.1038/nmeth761. [DOI] [PubMed] [Google Scholar]
- 16.Kam NWS, O'Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Tam UC, Czlapinski JL, Lee GS, Rabuka D, Zettl A, Bertozzi CR. Interfacing Carbon Nanotubes with Living Cells. Journal of the American Chemical Society. 2006;128:6292–6293. doi: 10.1021/ja060276s. [DOI] [PubMed] [Google Scholar]
- 18.Kostarelos K, Lacerda L, Pastorin G, Wu W, Wieckowski Sebastien, Luangsivilay J, Godefroy S, Pantarotto D, Briand J-P, Muller S, Prato M, Bianco A. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nano. 2007;2:108. doi: 10.1038/nnano.2006.209. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Wu P, Rousseas M, Okawa D, Gartner Z, Zettl A, Bertozzi CR. Boron Nitride Nanotubes Are Noncytotoxic and Can Be Functionalized for Interaction with Proteins and Cells. Journal of the American Chemical Society. 2009;131:890–891. doi: 10.1021/ja807334b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu F, Gu L, Meziani MJ, Wang X, Luo PG, Veca LM, Cao L, Sun Y-P. Advances in Bioapplications of Carbon Nanotubes. Advanced Materials. 2009;21:139–152. [Google Scholar]
- 21.Liu Z, Tabakman S, Welsher K, Dai H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Research. 2009;2:85. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang M, Huang J-L, Lieber CM. Fundamental Electronic Properties and Applications of Single-Walled Carbon Nanotubes. Accounts of Chemical Research. 2002;35:1018. doi: 10.1021/ar0101685. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson K, Tran L, Jimenez L, Duffin R, Newby D, Mills N, MacNee W, Stone V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Particle and Fibre Toxicology. 2005;2:10. doi: 10.1186/1743-8977-2-10. PMCIDdoi:10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albert P, Ana Gallegos S, Ramon C-D, Jordi P, Miquel S, Luigi C, Andrew PW. Modeling the structure-property relationships of nanoneedles: A journey toward nanomedicine. Journal of Computational Chemistry. 2009;30:275–284. doi: 10.1002/jcc.21041. [DOI] [PubMed] [Google Scholar]
- 25.Kouklin NA, Kim WE, Lazareck AD, Xu JM. Carbon nanotube probes for single-cell experimentation and assays. Applied Physics Letters. 2005;87:173901. [Google Scholar]
- 26.Obataya I, Nakamura C, Han, Nakamura N, Miyake J. Nanoscale Operation of a Living Cell Using an Atomic Force Microscope with a Nanoneedle. Nano Letters. 2005;5:27–30. doi: 10.1021/nl0485399. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Kis A, Zettl A, Bertozzi CR. A cell nanoinjector based on carbon nanotubes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8218–8222. doi: 10.1073/pnas.0700567104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vakarelski IU, Brown SC, Higashitani K, Moudgil BM. Penetration of Living Cell Membranes with Fortified Carbon Nanotube Tips. Langmuir. 2007;23:10893–10896. doi: 10.1021/la701878n. [DOI] [PubMed] [Google Scholar]
- 29.Kim W, Ng JK, Kunitake ME, Conklin BR, Yang P. Interfacing Silicon Nanowires with Mammalian Cells. Journal of the American Chemical Society. 2007;129:7228. doi: 10.1021/ja071456k. [DOI] [PubMed] [Google Scholar]
- 30.Yum K, Na S, Xiang Y, Wang N, Yu M-F. Mechanochemical Delivery and Dynamic Tracking of Fluorescent Quantum Dots in the Cytoplasm and Nucleus of Living Cells. Nano Letters. 2009;9:2193–2198. doi: 10.1021/nl901047u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yum K, Wang N, Yu M-F. Nanoscale. 2010 doi: 10.1039/b9nr00231f. DOI: 10.1039/b1039nr00231f. [DOI] [PubMed] [Google Scholar]
- 32.Yum K, Cho HN, Hu J, Yu M-F. Individual Nanotube-Based Needle Nanoprobes for Electrochemical Studies in Picoliter Microenvironments. ACS Nano. 2007;1:440. doi: 10.1021/nn700171x. [DOI] [PubMed] [Google Scholar]
- 33.Stephens DJ, Pepperkok R. The many ways to cross the plasma membrane. Proceedings of the National Academy of Sciences. 2001;98:4295–4298. doi: 10.1073/pnas.081065198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barry MA, Hofherr SE, Chen CY, Senac JS, Hillestad ML, Shashkova EV. Systemic delivery of therapeutic viruses. Current Opinion in Molecular Therapeutics. 2009;11:411–420. [PubMed] [Google Scholar]
- 35.Rapoport M, Lorberboum-Galski H. TAT-based drug delivery system - new directions in protein delivery for new hopes? Expert Opinion on Drug Delivery. 2009;6:453–463. doi: 10.1517/17425240902887029. PMCID19413454. [DOI] [PubMed] [Google Scholar]
- 36.Celis JE. Microinjection of somatic cells with micropipettes: Comparison with other transfer techniques. Biochemical Journal. 1984;223:281. doi: 10.1042/bj2230281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis TE. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- 38.Knight DE, Scrutton MC. Gaining access to the cytosol: The technique and some applications of electropermeabilization. Biochemical Journal. 1986;234:497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregoriadis G. Engineering liposomes for drug delivery: Progress and problems. Trends in Biotechnology. 1995;13:527. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 40.Sells MA, Li J, Chernoff J. Delivery of protein into cells using polycationic liposomes. BioTechniques. 1995;19:72. [PubMed] [Google Scholar]
- 41.Derfus AM, Chan WCW, Bhatia SN. Intracellular Delivery of Quantum Dots for Live Cell Labeling and Organelle Tracking. Advanced Materials. 2004;16:961–966. [Google Scholar]
- 42.Han S, Nakamura C, Obataya I, Nakamura N, Miyake J. Gene expression using an ultrathin needle enabling accurate displacement and low invasiveness. Biochemical and Biophysical Research Communications. 2005;332:633. doi: 10.1016/j.bbrc.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 43.Han S-W, Nakamura C, Kotobuki N, Obataya I, Ohgushi H, Nagamune T, Miyake J. High-efficiency DNA injection into a single human mesenchymal stem cell using a nanoneedle and atomic force microscopy. Nanomedicine. 2008;4:215. doi: 10.1016/j.nano.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Han S-W, Nakamura C, Imai Y, Nakamura N, Miyake J. Monitoring of hormonal drug effect in a single breast cancer cell using an estrogen responsive GFP reporter vector delivered by a nanoneedle. Biosensors and Bioelectronics. 2009;24:1219. doi: 10.1016/j.bios.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Chaudhuri O, Parekh SH, Lam WA, Fletcher DA. Combined atomic force microscopy and side-view optical imaging for mechanical studies of cells. Nat Meth. 2009;6:383. doi: 10.1038/nmeth.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mueller-Falcke C, Gouda SD, Kim S, Kim S-G. A nanoscanning platform for bio-engineering: an in-plane probe with switchable stiffness. Nanotechnology. 2006;17:S69. doi: 10.1088/0957-4484/17/4/011. PMCID0957-4484-17-4-011. [DOI] [PubMed] [Google Scholar]
- 47.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56. [Google Scholar]
- 48.Falvo MR, Clary GJ, Taylor RM, Chi V, Brooks FP, Washburn S, Superfine R. Bending and buckling of carbon nanotubes under large strain. Nature. 1997;389:582. doi: 10.1038/39282. [DOI] [PubMed] [Google Scholar]
- 49.Wong EW, Sheehan PE, Lieber CM. Nanobeam Mechanics: Elasticity, Strength, and Toughness of Nanorods and Nanotubes. Science. 1997;277:1971–1975. [Google Scholar]
- 50.Yu M-F, Lourie O, Dyer MJ, Moloni K, Kelly TF, Ruoff RS. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science. 2000;287:637–640. doi: 10.1126/science.287.5453.637. [DOI] [PubMed] [Google Scholar]
- 51.Yu M-F, Files BS, Arepalli S, Ruoff RS. Tensile Loading of Ropes of Single Wall Carbon Nanotubes and their Mechanical Properties. Physical Review Letters. 2000;84:5552. doi: 10.1103/PhysRevLett.84.5552. PMCID10.1103/PhysRevLett.84.5552. [DOI] [PubMed] [Google Scholar]
- 52.Dai H, Hafner JH, Rinzler AG, Colbert DT, Smalley RE. Nanotubes as nanoprobes in scanning probe microscopy. Nature. 1996;384:147–150. [Google Scholar]
- 53.Hafner JH, Cheung C-L, Oosterkamp TH, Lieber CM. High-Yield Assembly of Individual Single-Walled Carbon Nanotube Tips for Scanning Probe Microscopies. Journal of Physical Chemistry B. 2001;105:743. [Google Scholar]
- 54.Akita S, Nishijima H, Nakayama Y, Tokumasu F, Takeyasu K. Carbon nanotube tips for a scanning probe microscope: their fabrication and properties. Journal of Physics D: Applied Physics. 1999;32:1044. PMCID0022-3727-32-9-316. [Google Scholar]
- 55.Martinez J, Yuzvinsky TD, Fennimore AM, Zettl A, Garcia R, Bustamante C. Length control and sharpening of atomic force microscope carbon nanotube tips assisted by an electron beam. Nanotechnology. 2005;16:2493. PMCID0957-4484-16-11-004. [Google Scholar]
- 56.Hafner JH, Cheung CL, Lieber CM. Growth of nanotubes for probe microscopy tips. Nature. 1999;398:761. [Google Scholar]
- 57.Cheung CL, Hafner JH, Lieber CM. Carbon nanotube atomic force microscopy tips: Direct growth by chemical vapor deposition and application to high-resolution imaging. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3809–3813. doi: 10.1073/pnas.050498597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yenilmez E, Wang Q, Chen RJ, Wang D, Dai H. Wafer scale production of carbon nanotube scanning probe tips for atomic force microscopy. Applied Physics Letters. 2002;80:2225. [Google Scholar]
- 59.Tang J, Gao B, Geng H, Velev OD, Qin LC, Zhou O. Assembly of 1D Nanostructures into Sub-micrometer Diameter Fibrils with Controlled and Variable Length by Dielectrophoresis. Advanced Materials. 2003;15:1352–1355. [Google Scholar]
- 60.Hall A, Matthews WG, Superfine R, Falvo MR, Washburn S. Simple and efficient method for carbon nanotube attachment to scanning probes and other substrates. Applied Physics Letters. 2003;82:2506. [Google Scholar]
- 61.Tang J, Yang G, Zhang Q, Parhat A, Maynor B, Liu J, Qin L-C, Zhou O. Rapid and Reproducible Fabrication of Carbon Nanotube AFM Probes by Dielectrophoresis. Nano Letters. 2004;5:11. doi: 10.1021/nl048803y. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Tang J, Yang G, Qiu Q, Qin LC, Zhou O. Efficient Fabrication of Carbon Nanotube Point Electron Sources by Dielectrophoresis. Advanced Materials. 2004;16:1219–1222. [Google Scholar]
- 63.Freedman JR, Mattia D, Korneva G, Gogotsi Y, Friedman G, Fontecchio AK. Magnetically assembled carbon nanotube tipped pipettes. Applied Physics Letters. 2007;90:103108. [Google Scholar]
- 64.Wei H, Craig A, Huey BD, Papadimitrakopoulos F, Marcus HL. Electric field and tip geometry effects on dielectrophoretic growth of carbon nanotube nanofibrils on scanning probes. Nanotechnology. 2008;19:455303. doi: 10.1088/0957-4484/19/45/455303. PMCID0957-4484-19-45-455303. [DOI] [PubMed] [Google Scholar]
- 65.Wei H, Kim SN, Zhao M, Ju S-Y, Huey BD, Marcus HL, Papadimitrakopoulos F. Control of Length and Spatial Functionality of Single-Wall Carbon Nanotube AFM Nanoprobes. Chemistry of Materials. 2008;20:2793–2801. [Google Scholar]
- 66.Jouzi M, Kerby MB, Tripathi A, Xu J. Nanoneedle Method for High-Sensitivity Low-Background Monitoring of Protein Activity. Langmuir. 2008;24:10786–10790. doi: 10.1021/la703630a. [DOI] [PubMed] [Google Scholar]
- 67.El-Aguizy TA, Jeong J-h, Jeon Y-B, Li WZ, Ren ZF, Kim S-G. Transplanting carbon nanotubes. Applied Physics Letters. 2004;85:5995. [Google Scholar]
- 68.Kim S, Lee HW, Kim S-G. Transplanting assembly of carbon-nanotube-tipped atomic force microscope probes. Applied Physics Letters. 2009;94:193102. [Google Scholar]
- 69.Suryavanshi AP, Yu M-F. Probe-based electrochemical fabrication of freestanding Cu nanowire array. Applied Physics Letters. 2006;88:083103. [Google Scholar]
- 70.Suryavanshi AP, Yu M-F. Electrochemical fountain pen nanofabrication of vertically grown platinum nanowires. Nanotechnology. 2007;18:105305. PMCID0957-4484-18-10-105305. [Google Scholar]
- 71.Suryavanshi AP, Hu J, Yu MF. Meniscus-Controlled Continuous Fabrication of Arrays and Rolls of Extremely Long Micro- and Nano-Fibers. Advanced Materials. 2008;20:793–796. [Google Scholar]
- 72.Han SW, Nakamura C, Obataya I, Nakamura N, Miyake J. A molecular delivery system by using AFM and nanoneedle. Biosensors and Bioelectronics. 2005;20:2120. doi: 10.1016/j.bios.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 73.Obataya I, Nakamura C, Han S, Nakamura N, Miyake J. Direct insertion of proteins into a living cell using an atomic force microscope with a nanoneedle. NanoBiotechnology. 2005;1:347. doi: 10.1021/nl0485399. [DOI] [PubMed] [Google Scholar]
- 74.Obataya I, Nakamura C, Han S, Nakamura N, Miyake J. Mechanical sensing of the penetration of various nanoneedles into a living cell using atomic force microscopy. Biosensors and Bioelectronics. 2005;20:1652. doi: 10.1016/j.bios.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 75.Kihara T, Yoshida N, Mieda S, Fukazawa K, Nakamura C, Ishihara K, Miyake J. Nanoneedle Surface Modification with 2-Methacryloyloxyethyl Phosphorylcholine Polymer to Reduce Nonspecific Protein Adsorption in a Living Cell. NanoBiotechnology. 2007;3:127. [Google Scholar]
- 76.Kihara T, Nakamura C, Suzuki M, Han S-W, Fukazawa K, Ishihara K, Miyake J. Development of a method to evaluate caspase-3 activity in a single cell using a nanoneedle and a fluorescent probe. Biosensors and Bioelectronics. 2009;25:22. doi: 10.1016/j.bios.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 77.Tasis D, Tagmatarchis N, Bianco A, Prato M. Chemistry of Carbon Nanotubes. Chemical Reviews. 2006;106:1105. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- 78.Patil A, Sippel J, Martin GW, Rinzler AG. Enhanced Functionality of Nanotube Atomic Force Microscopy Tips by Polymer Coating. Nano Letters. 2004;4:303. [Google Scholar]
- 79.Tekle C, van Deurs B, Sandvig K, Iversen T-G. Cellular Trafficking of Quantum Dot-Ligand Bioconjugates and Their Induction of Changes in Normal Routing of Unconjugated Ligands. Nano Lett. 2008;8:1858–1865. doi: 10.1021/nl0803848. [DOI] [PubMed] [Google Scholar]
- 80.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun P, Laforge FO, Abeyweera TP, Rotenberg SA, Carpino J, Mirkin MV. Nanoelectrochemistry of mammalian cells. Proceedings of the National Academy of Sciences. 2008;105:443–448. doi: 10.1073/pnas.0711075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schrlau MG, Dun NJ, Bau HH. Cell Electrophysiology with Carbon Nanopipettes. ACS Nano. 2009;3:563–568. doi: 10.1021/nn800851d. [DOI] [PubMed] [Google Scholar]