SUMMARY
Background
Osteopontin (OPN) is a multifunctional protein which has recently been linked to allergic diseases. Clara cell 10-kDa protein (CC10) is another protein linked to allergy, and has been suggested to have an inhibitory role in inflammatory airway diseases. At this time, it is not known whether OPN is involved in allergic rhinitis (AR) or if there is any association between CC10 and OPN in AR.
Objective
To study the expression of OPN and its potential association with CC10 in AR.
Methods
The expression of CC10 and OPN in nasal mucosa of AR patients was investigated. AR animal models were established by employing wild-type and CC10-knockout mice. In some experiments, human recombinant CC10 protein was given to AR mice during either sensitization or challenge. The phenotypic changes were examined by histology and real-time RT-PCR. The direct effect of CC10 on OPN expression in spleen mononuclear cells and on OPN-induced inflammatory cytokine expression in BEAS-2B cells was measured through in vitro cell culture.
Results
OPN expression was up-regulated, with a concomitant down-regulation of CC10, in AR patients, showing a significant negative correlation between their expression. Compared with control mice sensitized with PBS, OPN expression was significantly increased in AR mice; such increase was more prominent in CC10-knockout mice, compared to wild-type. Administration of CC10 during both sensitization and challenge could markedly ameliorate Th2-skewed inflammation and OPN expression in nasal mucosa. CC10 administration at the sensitization phase could also reduce spleen OPN expression. The in vitro study showed that CC10 directly down-regulated OPN expression in spleen mononuclear cells stimulated with OVA and suppressed OPN-induced expression of Th2 cytokines and proinflammatory cytokines in BEAS-2B cells.
Conclusion
In the context of allergic airway responses, CC10 can inhibit OPN expression and suppress the Th2 promoting function of OPN, resulting in CC10’s inhibitory biological effects.
Keywords: allergic rhinitis, Clara cell 10-kDa protein, osteopontin, regulation
Introduction
Osteopontin (OPN) is a multifunctional 34-kDa phosphorylated acidic glycoprotein with a suspected role in the development of several inflammatory diseases, but, to date, the mechanistic aspects of OPN function remain ill-defined. Previously, OPN has been described as a crucial player in Th1-driven processes, and elevated levels of OPN have been found in several Th1-associated diseases including rheumatoid arthritis, multiple sclerosis, and Crohn’s disease (1–3). In addition, recent studies have also addressed the potential contribution of OPN in Th2-mediated diseases (4–9). Increased OPN has been detected in the tear fluids of patients with allergic ocular diseases and in lung biopsies from asthmatic patients (4–8). Our newly published study has demonstrated that OPN expression is upregulated in chronic rhinosinusitis and correlates with eosinophilic infiltration in nasal polyp tissues. Moreover, we have shown that OPN can stimulate the production of inflammatory cytokines in sinonasal mucosa (9). Studies involving OPN ablation via the use of OPN-deficient mice or neutralizing antibodies have demonstrated that during allergic airway responses, OPN can inhibit the migration of plasmacytoid dendritic cells at the sensitization phase and conventional dendritic cells at the challenge phase, respectively, and promote eosinophil migration and airway remodeling (4–8). However, at present, the role of OPN seems to be complex, and contradictory reports have been published regarding its function in allergic airway responses (4–8). More importantly, the regulatory mechanism(s) of OPN in airway diseases remains unknown.
Clara cell 10-kd protein (CC10), a member of the secretoglobin family, is a secretory protein with anti-inflammatory and immunomodulatory effects (10–13). It can antagonize the activity of secretory phospholipase A2, diminish inflammatory cell chemotaxis, downregulate Th2 cell differentiation, and block prostaglandin D2-receptor-mediated nuclear factor-κB activation (10–13). Our previous studies have shown that CC10-de cient mice present an exaggerated Th2-skewed pulmonary inflammatory response to allergen, and that CC10 can directly suppress Th2 cytokine production (10, 12). Reduced CC10 levels have been associated not only with pulmonary diseases but also with sinonasal diseases, such as allergic rhinitis (AR) and chronic rhinosinusitis (14–19). However, the mechanisms underlying the CC10 mediated regulation in these conditions, especially in upper airway diseases, remain to be elucidated.
Given the potential importance of OPN and CC10 in inflammatory airway diseases, it would be of great significance and interest to explore whether OPN is involved in the development of AR and whether this process can be modified via interaction between CC10 and OPN. In the present study, we first investigated the relationship between CC10 and OPN expression in AR patients. We then explored the effect of CC10 on OPN expression during AR processes through the use of CC10-knockout mice and via extrinsic CC10 protein administration. Furthermore, we examined the effect of CC10 on OPN-induced cytokine production in airway epithelial cells in vitro.
Materials and Methods
Animals
Wild-type C57BL/6 mice were purchased from Shanghai Experimental Animal Center (Shanghai, China). Homozygous CC10-knockout mice on C57BL/6 background were obtained from an intercross of heterozygous CC10-knockout mice (kindly provided by Dr. A.B. Mukherjee, National Institute of Health, Bethesda, MD), and germline transmission of the mutant CC10 allele was identified by PCR as described (12). All mice were used following protocols approved by the Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology.
Subjects
This study comprised 12 patients (7 males and 5 females; mean age, 35±16 years, and age range, 26–54 years) with house dust mite induced persistent AR and nasal septal deviation and 12 control patients (8 males and 4 females; mean age, 36±11 years, and age range, 28–46 years) with nasal septal deviation alone. All AR patients had typical symptoms of perennial nasal allergyand a positive skin test result to house dust mite. In this study, subjects were excluded if they had received any oral steroid within 3 months before the surgery. Topical steroids and antihistamines were withheld for a minimum of 1 month before the study. None had received anti-leukotrienes or immunotherapy. These patients were recruited to receive septoplasty, and inferior turbinate mucosal samples were taken during surgery. Surgical samples were processed for immunohistochemistry and real-time RT-PCR study. This study was approved by the Ethical Committee of Tongji Medical College of Huazhong University of Science and Technology. Informed consent was obtained from every subject.
AR mouse model, nasal lavage, tissue preparation, and RNA extraction
Mice (8-week old) were sensitized by s.c. injection of 0.4 ml solution containing 10 μg of OVA (grade V; Sigma, St. Louis, Mo, USA) emulsified in 1.6 mg Alum (Pierce, Rockford, Ill, USA) on days 0 and 7. The sensitized mice were challenged nasally by the administration of 10 μg of OVA in 40 μl PBS (20 μl per each nostril) on day 14 to day 18. Control mice were sensitized with PBS but challenged with OVA. In order to define the role of CC10 in the sensitization or challenge phase, 2.5 μg of human recombinant CC10 protein (R&D Systems, Minneapolis, Minn, USA) was given to mice intraperitoneally 2 hours before each OVA sensitization or 1.4 μg of CC10 protein in 40 μl PBS was administered intranasally (20 μl per each nostril) 2 hours before each OVA challenge, respectively. The control mice were given equivalent amount of PBS only. The doses of human recombinant CC10 were chosen based on published reports (20). Twenty-four hours after the last challenge, nasal lavage fluid (NLF) was collected and total NLF cells and differential cell counts were determined as previously described (21, 12). Sinonasal cavity structure was dissected, decalcified, embedded in paraffin, and sectioned as mentioned elsewhere (21). In some experiments, respiratory sinonasal mucosa was dissected under a microscope and spleen tissues were harvested, and total RNA was extracted by using an RNeasy kit (Qiagen, Valencia, Calif, USA).
Spleen cell preparation and culture
Splenocytes (4×106 cells per condition) isolated from OVA sensitized wild-type mice (8-week old) were cultured in medium consisting of RPMI 1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin, and stimulated with 60 μg/ml OVA or PBS for 48 hours at 37°C in a humidified incubator with 5% CO2. In some experiments, before stimulation, spleen cells were pretreated with human recombinant CC10 at 30 ng/ml for 2 hours. After culture, the cells were harvested and RNAs were extracted.
BEAS-2B cell culture
The BEAS-2B cells (American Type Culture Collection, Manassas, VA, USA) were grown in DMEM/F-12 supplemented with 5% heat-inactivated fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C with 5% CO2 in humidified air. When the cells reached 80–90% confluence, medium was changed to serum-free DMEM/F-12, and the cells were stimulated with OPN at 330 ng/ml (Peprotech, Placentia, Calif, USA). Before the stimulation, the cells were pretreated with or without 30 ng/ml human recombinant CC10 for 2 hours. Five hours later, cells were harvested and total RNA was extracted with Trizol (Invitrogen, San Diego, Calif, USA).
Immunohistochemistry
Immunohistochemical staining was conducted using the streptavidin-peroxidase complex method as previously described (22). Rabbit anti human and mouse OPN (1:200, Beijing Biosynthesis Biotechnology, Beijing, China), rabbit anti mouse CC10 (1:200, Santa Cruz Biotechnology, Santa Cruz, Calif, USA), and rabbit anti-human CC10 (1:200, Santa Cruz Biotechnology) antibodies were used as primary antibodies. Color development was achieved with 3′, 3′-diaminobenzidine, which rendered positive cells brown. Control isotype rabbit IgG was used as a negative control. The numbers of OPN positive cells per square millimeter of epithelium and lamina propria and CC10 positive cells per millimeter of epithelium were counted as previously described (23).
Quantitative real-time PCR
OPN, CC10, IL-1β, TNF-α, IL-4, IL-5, IL-13, and INF-γ mRNA expression was detected by means of quantitative RT-PCR. cDNA was reverse transcribed as stated elsewhere (24). By using the specific primer pairs (Table S1 in the Online supplement) and SYBR Premix Ex Taq kit (TaKaRa Biotechnology, Dalian, China), cDNA equivalent to 40 ng total RNA was used to perform quantitative PCR as mentioned elsewhere (24). Relative gene expression was calculated by using the comparative CT method (24). GAPDH was used as a housekeeping gene for normalization and a “no template” sample was used as a negative control.
Statistical analysis
All results were expressed as the means ± SEM. The Mann-Whitney U test was used for paired sets of data. Spearman’s test was applied to determine correlations. The paired t test was used in cell culture data analysis. The P value for significance was set to .05. Data analysis was performed through the application of SPSS software for Windows (SPSS Inc., Chicago, Ill, USA).
Results
CC10 and OPN expression in AR patients
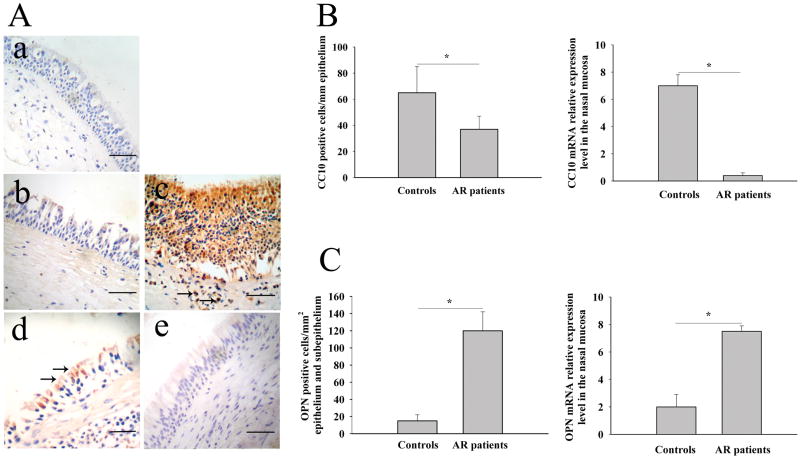
We found that OPN mRNA and protein expression was significantly upregulated, whereas CC10 mRNA and protein expression was significantly downregulated, in nasal tissues from AR patients compared with those from controls (Figure 1). Consistent with our previous reports (9, 23), OPN positive staining was found in epithelial cells and infiltrating cells in lamina propria (Figure 1A, b and c), and CC10 positive staining was mainly demonstrated in epithelial cells (Figure 1A, d and e). When analyzing the relationship between CC10 and OPN expression, we found that OPN mRNA and protein expression was negatively correlated with CC10 mRNA and protein expression in AR patients, respectively (r = −0.43 for mRNA expression and r = −0.59 for protein expression, respectively; and both P < 0.05). However, no significant correlation between OPN and CC10 mRNA and protein expression was found in controls.
Figure 1.
Increased OPN expression and decreased CC10 expression in human AR. (A) Representative photomicrographs of CC10 and OPN immunohistochemical staining of inferior turbinate tissue sections from control and AR patients. a) a section stained with isotype control for anti-OPN and anti-CC10 antibodies; b) OPN staining in control; c) OPN staining in AR, arrows, OPN positive inflammatory cells; d) CC10 staining in control, arrows, CC10 positive epithelial cells; e) CC10 staining in AR. Scale bar, 50 μm. (B) The number of OPN positive cells and the relative expression levels of OPN mRNA in controls and AR patients. (C) The number of CC10 positive cells and the relative expression levels of CC10 mRNA in controls and AR patients.* P < 0.01.
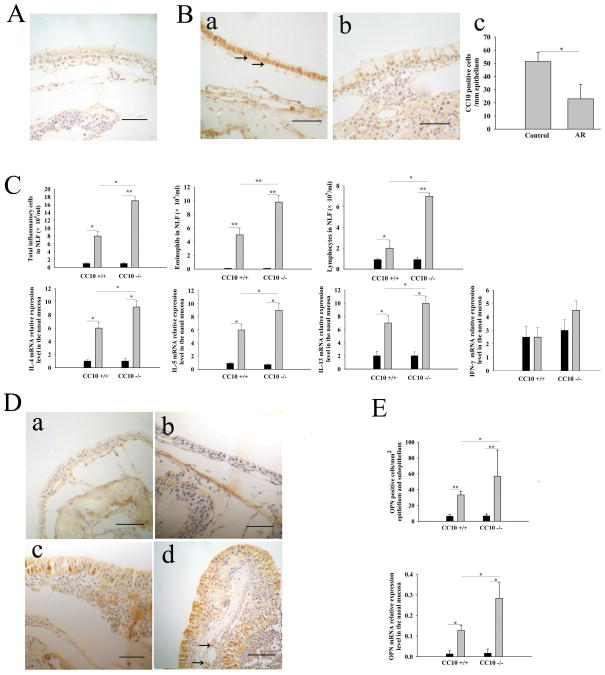
OPN gene expression is augmented in CC10-knockout AR mice
In line with our findings in human subjects, in wild-type mice, we found that CC10 protein expression was significantly decreased after establishment of AR (Figure 2B). Moreover, consistent with our previous report (25), we found that CC10-knockout AR mice displayed exaggerated Th2-skewed eosinophilic inflammation in nasal mucosa compared with wild-type mice (Figure 2C). In sensitized CC10-knockout mice, markedly increased numbers of eosinophils, lymphocytes, and total inflammatory cells in NLF were seen following secondary challenge in comparison with those seen in challenged wild-type mice (Figure 2C). Meanwhile, there was a significantly higher level of IL-4, IL-5, and IL-13 mRNA expression in nasal mucosa in CC10-knockout AR mice than in wild-type AR mice; however, the level of INF-γ mRNA expression did not show significant difference between CC10-knockout and wild-type AR mice (Figure 2C). With respect to OPN expression, a marked increase of OPN protein and mRNA expression level was found in nasal mucosa in AR mice, with an even greater increase in CC10-knockout AR mice (Figure 2, D and E). The immunohistochemical staining patterns of OPN and CC10 in mice were similar to those in humans (Figure 2, B and D). A negative correlation between OPN and CC10 protein expression was discovered in wild-type AR mice (r = −0.6, P < 0.01).
Figure 2.
Decreased CC10 expression and increased OPN expression in AR mice. (A) A representative photomicrograph of nasal tissue sections stained with isotype control for anti-OPN and anti-CC10 antibodies. Scale bar, 50 μm. (B) a) and b) Representative photomicrographs of CC10 immunohistochemical staining of nasal tissue sections from a) wild-type control mice sensitized with PBS and b) wild-type AR mice. Scale bar, 50 μm; arrows, CC10 positive epithelial cells; c) The number of CC10 positive cells in nasal mucosa in wild-type control mice and AR mice. (C) Increased numbers of total inflammatory cells, eosinophils and lymphocytes in NLF and elevated mRNA relative expression levels of IL-4, IL-5, and IL-13 in the nasal mucosa in CC10-knockout AR mice compared with wild-type AR mice. Solid columns = control mice sensitized with PBS; gray columns = AR mice. (D) Representative photomicrographs of OPN immunohistochemical staining of nasal tissue sections from a) wild-type control mice, b) CC10-knockout control mice, c) wild-type AR mice, and d) CC10-knockout AR mice. Scale bar, 50 μm; arrows, OPN positive inflammatory cells. (E) The number of OPN positive cells and the relative expression levels of OPN mRNA in nasal mucosa are increased in CC10-knockout AR mice as compared to wild-type AR mice. Solid columns = control mice; gray columns = AR mice. * P < 0.05 and ** P < 0.01. n = 3 mice per group.
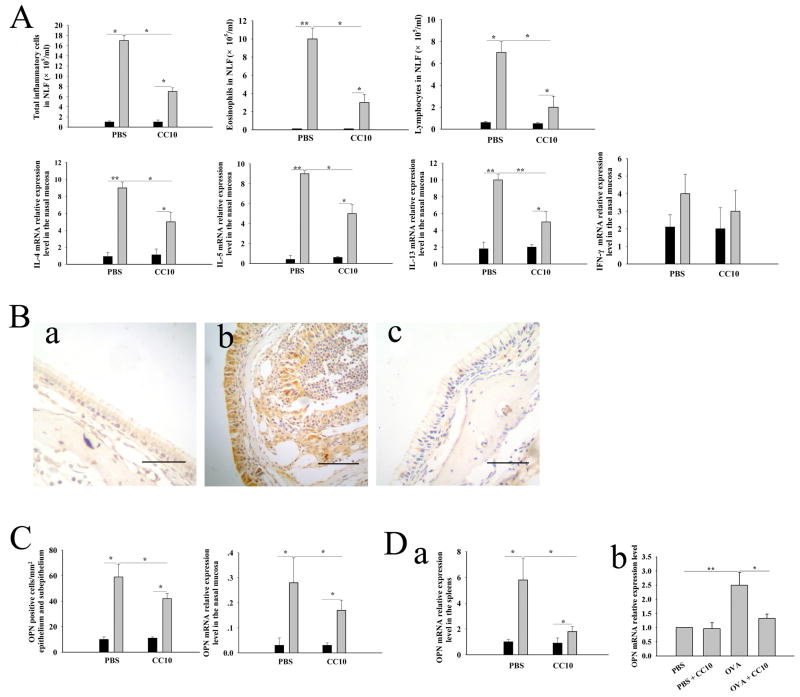
CC10 administration during sensitization decreases OPN expression in spleen and sinonasal mucosa
Since CC10 has been indicated to have anti-inflammatory and immunomodulatory functions, we investigated whether CC10 has a preventive role in AR. For this purpose, human recombinant CC10 protein was administrated to CC10-knockout mice during sensitization. We found that CC10 given during sensitization could dramatically ameliorate Th2-skewed inflammation in nasal mucosa after antigen challenge (Figure 3A). After antigen challenge, CC10-knockout mice treated with CC10 protein during sensitization displayed fewer eosinophils, lymphocytes, and total inflammatory cells in NLF and a lower level of IL-4, IL-5, and IL-13 mRNA expression in nasal mucosa compared with CC10-knockout mice treated with PBS (Figure 3A). Nevertheless, no significant measurable effect of CC10 on the mRNA expression of INF-γ was found (Figure 3A). Regarding the OPN expression, we found that CC10 treatment during sensitization could markedly inhibit OPN expression in nasal mucosa in CC10-knockout AR mice (Figure 3, B and C). More importantly, OPN mRNA expression in spleen was also dramatically suppressed by CC10 treatment in vivo (Figure 3D, a). In order to investigate whether CC10 has a direct effect on OPN expression, we further studied OPN expression in spleen mononuclear cells in vitro. We found that OPN mRNA expression in spleen mononuclear cells from OVA sensitized wild-type mice was increased by 2.5-fold after stimulation with OVA compared with PBS control (Figure 3D, b). CC10 pretreatment before OVA stimulation could reduce the OPN mRNA expression in spleen mononuclear cells to levels comparable to those without OVA stimulation (Figure 3D, b)
Figure 3.
Intraperitoneal administration of CC10 during sensitization decreases OPN expression in sinonasal mucosa and spleens. (A) Decreased numbers of total inflammatory cells, eosinophils, and lymphocytes in NLF and reduced mRNA relative expression levels of IL-4, IL-5, and IL-13 in the nasal mucosa in CC10-knockout AR mice with CC10 treatment compared with those with PBS treatment. Solid columns = control mice sensitized with PBS; gray columns = AR mice. n = 4 to 5 mice per group. (B) Representative photomicrographs of OPN immunohistochemical staining of nasal tissue sections from a) CC10-knockout control mice without AR, b) CC10-knockout AR mice with PBS treatment, and c) CC10-knockout AR mice with CC10 treatment during sensitization. Scale bar, 50 μm. (C) Down-regulated protein and mRNA expression of OPN in nasal mucosal in CC10-knockout AR mice with CC10 treatment compared with those with PBS treatment. Solid columns = control mice; gray columns = AR mice. n = 4 to 5 mice per group. (D) a) Reduced mRNA relative expression levels of OPN in the spleen in CC10-knockout AR mice with CC10 treatment compared with those with PBS treatment. Solid columns = control mice; gray columns = AR mice. n = 4 to 5 mice for each group; b) CC10 suppresses OVA-induced OPN expression by spleen mononuclear cells derived from OVA sensitized wild-type mice in vitro. n = 3. * P < 0.05 and ** P < 0.01.
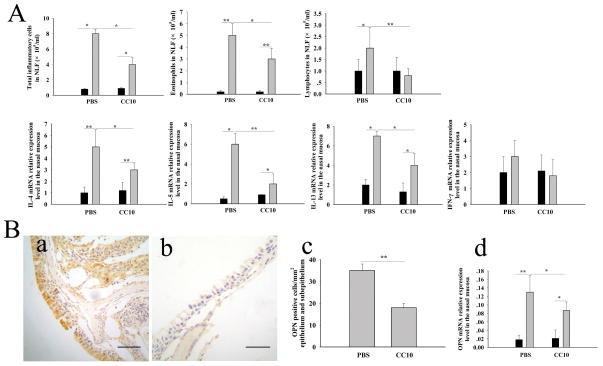
CC10 administration during challenge decreases nasal mucosal OPN expression
Next, we investigated whether CC10 has a therapeutic role in AR. For this purpose, wild-type mice were employed. We found that intranasal CC10 administration before OVA challenge could markedly inhibit Th2-dominated inflammation in nasal mucosa (Figure 4A). As compared to mice treated with PBS, mice receiving CC10 protein during challenge demonstrated a significantly lower level of infiltration of nasal mucosa by inflammatory cells and a markedly down-regulated expression of Th2 cytokines (Figure 4A). In regard to OPN expression, we found a significant decrease of OPN expression in nasal mucosa in AR mice treated with CC10 in comparison with AR mice treated with PBS during challenge (Figure 4B).
Figure 4.
Intranasal CC10 administration during challenge inhibits the OPN expression in nasal mucosa in wild-type mice. (A) Decreased numbers of total inflammatory cells, eosinophils and lymphocytes in NLF and reduced mRNA relative expression levels of IL-4, IL-5, and IL-13 in the nasal mucosa in wild-type AR mice with CC10 treatment compared with those with PBS treatment. Solid columns = control mice sensitized with PBS; gray columns = AR mice. (B) a) and b) Representative photomicrographs of OPN immunohistochemical staining of nasal tissue sections from a) AR mice with PBS treatment and b) AR mice with CC10 treatment. Scale bar, 50 μm; c) Reduced number of OPN positive cells in wild-type AR mice with CC10 treatment compared with those with PBS treatment; d) Down-regulated expression of OPN mRNA in wild-type AR mice with CC10 treatment as compared to those with PBS treatment. Solid columns = control mice; gray columns = AR mice. * P < 0.05 and ** P < 0.01. n = 3 to 5 mice per group.
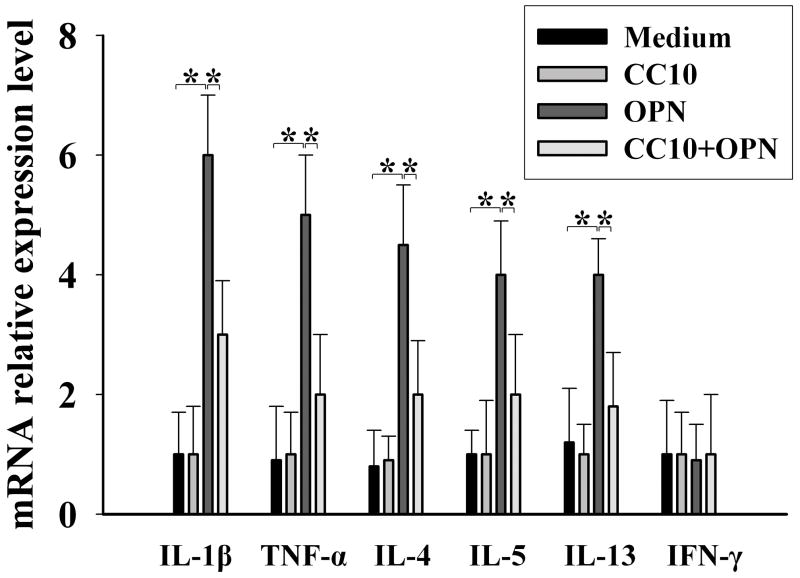
CC10 inhibits OPN-induced cytokine production in airway epithelial cells
Because our previous study demonstrated that OPN can stimulate the production of inflammatory cytokines in human nasal mucosa and that epithelial cells are a major source of OPN in the airway (9), we used the BEAS-2B cell line, derived from human bronchial epithelial cells, to study CC10’s effect on OPN function through measurement of the expression of OPN-induced inflammatory cytokines. We found that OPN significantly upregulated the mRNA expression of IL-4, IL-5, IL-13, IL-1β and TNF-α in BEAS-2B cells (Figure 5). Intriguingly, CC10 pretreatment could markedly inhibit OPN-induced production of these proinflammatory and Th2 cytokines in BEAS-2B cells (Figure 5).
Figure 5.
CC10 inhibits OPN-induced Th2 and proinflammatory cytokine production in BEAS-2B cells. * P < 0.05. n = 5 to 6.
Discussion
OPN’s potential involvement in Th2-associated allergic responses has been the subject of recent investigation, particularly in regard to its role in eosinophilic airway inflammation (4–9). Our recent study showed that OPN protein expression is upregulated in human nasal polyps and correlates with local eosinophilic infiltration (9). Moreover, we found that the OPN protein level is increased in induced sputum from asthmatic patients and that OPN can induce significant migration of human eosinophils (8). Xanthou et al. found that lung biopsies from asthmatics show increased OPN expression in both bronchial epithelial cells and inflammatory cells in the lamina propria, as compared to healthy subjects (4). In line with these previous reports (4, 9), in the present study we found that OPN expression was upregulated in epithelial cells and inflammatory cells in nasal mucosa of AR, confirming the involvement of OPN in AR.
Although the involvement of OPN in a number of pathophysiological events has been revealed by numerous studies, the regulation of OPN expression has not been extensively investigated. Our previous studies have shown that Th1 cytokines can induce, whereas Th2 cytokines may inhibit, the production of OPN in human sinonasal mucosa, monocytes, and monocyte-derived dendritic cells (9, 26). However, the regulation of OPN expression in the context of allergic airway responses is unclear. CC10 is an endogenous molecule that plays an important role in constraining airway inflammatory reactions (10–19). Previous studies have shown decreased CC10 expression in intermittent AR (27). Our present study has further demonstrated that CC10 expression is also significantly down-regulated in nasal mucosa from patients with persistent AR. Our published study has demonstrated that CC10 may attenuate the expression of some proinflammatory molecules, such as chitinase 3-like 1 protein, in allergic airway diseases (25). However, whether CC10 can regulate OPN expression in allergic airway diseases is unknown. In this study, we found that OPN expression was negatively correlated with CC10 expression in human AR. Moreover, by establishing an AR animal model, we found that OPN expression was significantly increased, whereas CC10 expression was significantly decreased, in nasal mucosa in AR mice. This is in agreement with our findings in human subjects and indicates the association between CC10 and OPN in AR. In order to further explore whether there is a cause-effect relationship between CC10 and OPN expression, we took advantage of the availability of CC10-knockout mice and CC10 protein to study the effect of CC10 on OPN expression. After establishment of AR, CC10 knock-out mice exhibited higher levels of OPN expression in comparison with wild-type mice, with concomitant augmented Th2-dominated inflammation in nasal mucosa, indicating that CC10 may have an inhibitory role on Th2-dominated inflammation and OPN expression in AR. Since the development of allergic diseases depends on two steps, sensitization and challenge, we next investigated whether CC10 can exert its effect on Th2-dominated inflammation and OPN expression through affecting these two phases. We found that CC10 administration during both sensitization and challenge could significantly attenuate OPN expression in nasal mucosa and down-regulate inflammatory phenotypes of local mucosa, which added further evidence that CC10 may inhibit OPN expression in AR. Moreover, in the present study, we found that CC10 could decrease OVA-induced expression of OPN in the spleen mononuclear cells in vitro, suggesting that CC10 may have a direct effect on OPN expression. However, one limitation of our current study is that we did not investigate the direct effect of CC10 on OPN expression in airway epithelial cells, which remains a subject for further study. Recent studies suggest that OPN has both a secreted isoform and an intracellular one, each with distinct functions (28). Secreted OPN can function as both an extracellular matrix component and a cytokine. It can recruit inflammatory cells, stimulate cytokine expression, and promote angiogenesis and tissue repair (1–8, 28). On the other hand, the intracellular form of OPN is essential for Toll-like receptor 9 dependent interferon-α response in plasmacytoid dendritic cells (28). In our current study, total mRNA and protein expression of OPN was assessed, which did not allow us to differentiate the secreted and the intracellular isoforms.
Our present study demonstrated that OPN could stimulate proinflammatory and Th2 cytokine production in airway epithelial cells, and that the inhibition of allergic airway inflammation by CC10 administration during both sensitization and challenge was associated with decreased OPN levels, suggesting that OPN may have a pro-allergic role in both sensitization and challenge phases. However, Xanthou et al. found that OPN is pro-allergic during sensitization and anti-allergic during challenge (4). In fact, regarding OPN’s function, contradictory reports have been published. Xanthou et al. found that OPN deficient mice have enhanced Th2-mediated allergic airway responses (4), whereas Kohan et al. revealed that OPN deficient mice display reduced allergic airway inflammation as compared to wild-type mice (7). It is difficult, at present, to reconcile these discrepancies, which may be due, in part, to the difference in experimental protocols, the multi-functional nature of OPN, the complicated structure of OPN, and the existence of several functional motifs and distinct isoforms of OPN (1–8, 28). It is noted that in the study by Xanthou et al., OPN blockade with a neutralizing antibody only allowed the authors to investigate the function of the secreted isoform of OPN. However, in our current study, both secreted and intracellular isoforms of OPN may be involved.
OPN can be expressed by several types of immune cells, including dendritic cells, macrophages, T cells, and eosinophils (1–8, 26, 28, 29). In the present study, we found that CC10 treatment during sensitization markedly decreased OPN expression in spleen in vivo and CC10 dramatically inhibited OVA-induced OPN expression in spleen mononuclear cells in vitro. Currently, the exact cell targets of CC10 in spleen are unclear; however, they are likely to be dendritic cells, macrophages, and T cells, the major producers of OPN (1–8, 26, 28, 29). Studies from us and others suggested that OPN can significantly affect dendritic cell and T cell function, which are crucial in determining the outcome of adaptive immunity (4, 28–30). Therefore, our results suggest that CC10 may affect the antigen sensitization through influencing OPN expression in dendritic cells and T cells, which, in turn, suppress allergic inflammation. In this study, we found that CC10 could decrease the expression of proinflammatory and Th2 cytokines induced by OPN in airway epithelial cells, whereas had no effect on INF-γ expression, which may contribute to the CC10-mediated suppression of allergic airway responses. Besides OPN’s effect on epithelial cells, OPN is also involved in the recruitment of inflammatory cells and the cytokine release by inflammatory cells. The question of whether CC10 can influence those functions of OPN needs further study. Furthermore, since the putative receptor(s) of CC10 has not been identified, the detailed mechanisms underlying CC10’s effect also need further study.
In conclusion, our present study suggests that CC10 may inhibit OPN expression and suppress the Th2 promoting function of OPN in allergic airway diseases, which may contribute, at least in part, to the inhibitory effects of CC10. However, the precise mechanisms remain to be elucidated.
Supplementary Material
Acknowledgments
This study was supported by the National Nature Science Foundation of China (NSFC) grant 30500557 and 30872847, and the program for New Century Excellent Talents in University from the State Education Ministry (NCET-07-0326) to ZL, NSFC grant 30901660 to XL, NSFC grant 30700933 to HXY, and also, in part, by NIH grants (AI052468 and AI073610) to SKH.
The authors thank Ms. Beverly Plunkett in Johns Hopkins Asthma and Allergy Center for careful reading and editing of our manuscript.
Footnotes
Disclosure of conflict of interest: None
References
- 1.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 2.Xu G, Nie H, Li N, et al. Role of osteopontin in amplification and perpetuation of rheumatoid synovitis. J Clin Invest. 2005;115:1060–7. doi: 10.1172/JCI23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O_Regan AW, Nau GJ, Chupp GL, Berman JS. OPN (Eta-1) in cell-mediated immunity: teaching an old dog new tricks. Immunol Today. 2000;21:475–8. doi: 10.1016/s0167-5699(00)01715-1. [DOI] [PubMed] [Google Scholar]
- 4.Xanthou G, Alissafi T, Semitekolou M, et al. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med. 2007;13:570–8. doi: 10.1038/nm1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohan M, Bader R, Puxeddu I, Levi-Schaffer F, Breuer R, Berkman N. Enhanced osteopontin expression in a murine model of allergen-induced airway remodeling. Clin Exp Allergy. 2007;37:1444–54. doi: 10.1111/j.1365-2222.2007.02801.x. [DOI] [PubMed] [Google Scholar]
- 6.Simoes DC, Xanthou G, Petrochilou K, Panoutsakopoulou V, Roussos C, Gratziou C. Osteopontin deficiency protects against airway remodeling and hyperresponsiveness in chronic asthma. Am J Respir Crit Care Med. 2009;179:894–902. doi: 10.1164/rccm.200807-1081OC. [DOI] [PubMed] [Google Scholar]
- 7.Kohan M, Breuer R, Berkman N. Osteopontin induces airway remodeling and lung fibroblast activation in a murine model of asthma. Am J Respir Cell Mol Biol. 2009;41:290–6. doi: 10.1165/rcmb.2008-0307OC. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi A, Kurokawa M, Konno S, et al. Osteopontin is involved in migration of eosinophils in asthma. Clin Exp Allergy. 2009;39:1152–9. doi: 10.1111/j.1365-2222.2009.03249.x. [DOI] [PubMed] [Google Scholar]
- 9.Lu X, Zhang XH, Wang H, et al. Expression of osteopontin in chronic rhinosinusitis with and without nasal polyps. Allergy. 2009;64:104–11. doi: 10.1111/j.1398-9995.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- 10.Hung CH, Chen LC, Zhang Z, et al. Regulation of TH2 responses by the pulmonary Clara cell secretory 10-kd protein. J Allergy Clin Immunol. 2004;114:664–70. doi: 10.1016/j.jaci.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Johansson S, Wennergren G, Aberg N, Rudin A. Clara cell 16-kd protein downregulates T(H)2 differentiation of human naive neonatal T cells. J Allergy Clin Immunol. 2007;120:308–14. doi: 10.1016/j.jaci.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Chen LC, Zhang Z, Myers AC, Huang SK. Cutting edge: altered pulmonary eosinophilic inflammation in mice deficient for Clara cell secretory 10-kDa protein. J Immunol. 2001;167:3025–8. doi: 10.4049/jimmunol.167.6.3025. [DOI] [PubMed] [Google Scholar]
- 13.Mandal AK, Zhang Z, Ray R, et al. Uteroglobin represses allergen-induced inflammatory response by blocking PGD2 receptor-mediated functions. J Exp Med. 2004;199:1317–30. doi: 10.1084/jem.20031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30:469–75. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang KD, Ou CY, Chang JC, et al. Infant frequent wheezing correlated to Clara cell protein 10 (CC10) polymorphism and concentration, but not allergy sensitization, in a perinatal cohort study. J Allergy Clin Immunol. 2007;120:842–8. doi: 10.1016/j.jaci.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Wang SZ, Rosenberger CL, Espindola TM, et al. CCSP modulates airway dysfunction and host responses in an Ova-challenged mouse model. Am J Physiol Lung Cell Mol Physiol. 2001;281:1303–11. doi: 10.1152/ajplung.2001.281.5.L1303. [DOI] [PubMed] [Google Scholar]
- 17.Johansson S, Keen C, Stahl A, Wennergren G, Benson M. Low levels of CC16 in nasal fluid of children with birch pollen-induced rhinitis. Allergy. 2005;60:638–42. doi: 10.1111/j.1398-9995.2005.00775.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang SZ, Rosenberger CL, Bao YX, Stark JM, Harrod KS. Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2003;171:1051–60. doi: 10.4049/jimmunol.171.2.1051. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Lu X, Zhang XH, et al. Clara cell 10-kDa protein expression in chronic rhinosinusitis and its cytokine-driven regulation in sinonasal mucosa. Allergy. 2009;64:149–57. doi: 10.1111/j.1398-9995.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller TL, Shashikant BN, Melby JM, Pilon AL, Shaffer TH, Wolfson MR. Recombinant human Clara cell secretory protein in acute lung injury of the rabbit: effect of route of administration. Pediatr Crit Care Med. 2005;6:698–706. doi: 10.1097/01.pcc.0000165565.96773.08. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Lu X, Cao PP, et al. Histological and immunological observations of bacterial and allergic chronic rhinosinusitis in the mouse. Am J Rhinol. 2008;22:343–8. doi: 10.2500/ajr.2008.22.3184. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Lu X, Wang H, Gao QX, Cui YH. The up-regulated expression of tenascin C in human nasal polyp tissues is related to eosinophil-derived transforming growth factor beta1. Am J Rhinol. 2006;20:629–33. doi: 10.2500/ajr.2006.20.2918. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Kim J, Sypek JP, et al. Gene expression profiles in human nasal polyp tissues studied by means of DNA microarray. J Allergy Clin Immunol. 2004;114:783–90. doi: 10.1016/j.jaci.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Lu X, Wang H, You XJ, Gao QX, Cui YH. Group II subfamily secretory phospholipase A2 enzymes: expression in chronic rhinosinusitis with and without nasal polyps. Allergy. 2007;62:999–1006. doi: 10.1111/j.1398-9995.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Long XB, Cao PP, et al. Clara cell 10-kd protein suppresses chitinase 3-like 1 expression associated with eosinophilic chronic rhinosinusitis. Am J Respir Crit Care Med. 2010;181:908–916. doi: 10.1164/rccm.200904-0597OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konno S, Eckman JA, Plunkett B, et al. Interleukin-10 and Th2 cytokines differetially regulate osteopontin expression in human monocytes and dendritic cells. J Interferon Cytokine Res. 2006;26:562–7. doi: 10.1089/jir.2006.26.562. [DOI] [PubMed] [Google Scholar]
- 27.Benson M, Fransson M, Martinsson T, Naluai AT, Uddman R, Cardell LO. Inverse relation between nasal fluid and Clara cell protein 16 levels and symptoms and signs of rhinitis in allergen-challenged patients with intermittent allergic rhinitis. Allergy. 2007;62:178–83. doi: 10.1111/j.1398-9995.2006.01264.x. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara ML, Lu L, Bu J, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–4. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 30.Kurokawa M, Konno S, Takahashi A, et al. Regulatory role of DC-derived osteopontin in systemic allergen sensitization. Eur J Immunol. 2009;39:3323–30. doi: 10.1002/eji.200838970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.