Abstract
The neuronal repellent SLIT2 is repressed in a number of cancer types primarily through promoter hypermethylation. SLIT2, however, has not been studied in prostate cancer. Through genome-wide location analysis we identified SLIT2 as a target of Polycomb group (PcG) protein EZH2. The EZH2-containing Polycomb repressive complexes bound to the SLIT2 promoter inhibiting its expression. SLIT2 was down-regulated in a majority of metastatic prostate tumors exhibiting a negative correlation with EZH2. This repressed expression could be restored by methylation inhibitors or EZH2-suppressing compounds. In addition, a low level of SLIT2 expression was associated with aggressive prostate, breast and lung cancers. Functional assays showed that SLIT2 inhibited prostate cancer cell proliferation and invasion. Thus, this study demonstrated for the first time epigenetic silencing of SLIT2 in prostate tumors, and supported SLIT2 as a potential biomarker for aggressive solid tumors. Importantly, PcG-mediated repression may serve as a precursor for the silencing of SLIT2 by DNA methylation in cancer.
Keywords: Polycomb group proteins, EZH2, SLIT2, prostate cancer, epigenetic silencing, DNA hypermethylation
Introduction
SLIT2, a human homologue of the Drosophila Slit2 gene, belongs to the SLIT family of large secreted proteins including also SLIT1 and SLIT3. The SLIT proteins are evolutionary conserved and contain an N-terminal signal peptide, four leucine-rich tandom repeats, seven or nine EGF repeats, a laminin G domain, and a C-terminal cysteine knot (Rothberg et al., 1988). A critical role of the SLIT proteins is to function as chemorepellents on navigating axons and migrating neurons during development (Wong et al., 2002). It mediates the repulsive cues and guides away the projection of normal axons and developing neurons (Brose et al., 1999). While SLIT1 is predominantly expressed in the nervous system, SLIT2 is also expressed in non-neuronal tissues such as lung, breast, kidney, and heart, suggesting innovative roles in addition to axon guidance (Wu et al., 2001).
Emerging evidence suggests that, outside the nervous system, the neuronal repellent SLIT2 inhibits the migration of a number of other cell types towards a variety of chemotactic factors. For example, SLIT2 inhibits CXCL12-induced chemotaxis of leukocytes (Wu et al., 2001) and transendothelial migration of T cells (Prasad et al., 2007). In breast cancer cells, SLIT2 mediates inhibition of CXCR4-induced chemotaxis, chemoinvasion, and adhesion, thus implicating a role in preventing tumor metastasis (Prasad et al., 2004). Tumor suppressor activities of SLIT2 have been reported in a number of cancer types (Tseng et al., 2010). Both SLIT2 overexpression and SLIT2-containing conditioned medium have been shown to suppress breast cancer cell growth in vitro (Dallol et al., 2002). In medulloblastoma SLIT2 inhibits cell invasion through interfering with the CDC42 and RAC1 pathways (Werbowetski-Ogilvie et al., 2006). More recently, SLIT2 was shown to increase apoptosis, decrease cell proliferation, and inhibit cell migration and invasion in vitro in squamous cell carcinoma and fribrosarcoma (Kim et al., 2008). In addition, overexpression of SLIT2 suppresses xenograft tumor growth and inhibits the metastasis of tumor cells after intravenous inoculation in nude mice, providing compelling evidence for SLIT2 as a tumor suppressor gene (Kim et al., 2008; Prasad et al., 2008).
Consistent with its tumor suppressor activity, SLIT2 was shown down-regulated, primarily through DNA hypermethylation, in a number of cancer types. The CpG islands in the promoter region of the SLIT2 gene were hypermethylated in 59% of gliomas with concordant down-regulated expression (Dallol et al., 2003a). In another study, promoter hypermethylation of SLIT2 was shown in 29% of neuroblastomas, 38% of Wilms’ tumors, and 25% of renal cell carcinomas (Astuti et al., 2004). Frequent epigenetic silencing of SLIT2, with corresponding decrease in SLIT2 expression, has also been reported in a majority of lymphocytic leukemia (Dunwell et al., 2009), 72% of primary colorectal cancers (Dallol et al., 2003b), 83.3% of primary hepatocellular carcinomas (Jin et al., 2009), and almost all lung adenocarcinoma (Dammann et al., 2005b). In addition, SLIT2 promoter hypermethylation was detected in 59% of breast cancers and, importantly, in their paired serum DNA, suggesting its potential as a noninvasive biomarker (Dallol et al., 2002; Sharma et al., 2007). SLIT2 expression and function, however, have not been studied in prostate tumors.
Polycomb group (PcG) proteins are transcriptional repressors that inhibit developmental regulators in embryonic stem cells and silence tumor suppressor genes in cancer (Mathews et al., 2009). They function through multimeric chromatin-associated Polycomb Repressive Complexes (PRCs), including at least PRC1 and PRC2. Core components of PRC1 include Bmi1, Ring1, Ring2 and HPC2, while PRC2 is mainly composed of SUZ12, EED, and EZH2, with EZH2 enzymatically catalyzing the methylation of Lysine 27 of Histone H3 (3mH3K27) (Simon and Kingston, 2009). EZH2 is a bona fide oncogene that mediates cancer cell proliferation and invasion and is frequently found up-regulated in a number of cancer types including prostate and breast cancers (Varambally et al., 2002). EZH2 is thought to promote tumorigenesis via epigenetic silencing of a group of tumor suppressor genes, including ADRB2, CDH1, PSP94 and DAB2IP (Beke et al., 2007; Cao et al., 2008; Chen et al., 2005; Yu et al., 2007b). However, a majority of Polycomb target genes in cancer cells remain unknown.
In this study, genome-wide location analysis of prostate cancer cells revealed SLIT2 as a top target gene of EZH2-mediated H3K27 trimethylation. We show that SLIT2 is down-regulated in prostate cancer by epigenetic mechanisms and represents a potent prognostic biomarker that merits further evaluation in large patient cohorts. In addition, overexpression of SLIT2 inhibits prostate cancer cell proliferation and invasion. Our study is the first to demonstrate epigenetic silencing of SLIT2 in prostate cancer and establishes a novel mechanism for SLIT2 repression in cancer involving PcG proteins, suggesting that PcG-mediated chromatin change may in general serve as a precursor for the silencing of tumor suppressor genes by DNA methylation.
Results
SLIT2 is a target of EZH2-mediated H3K27 trimethylation in prostate cancer
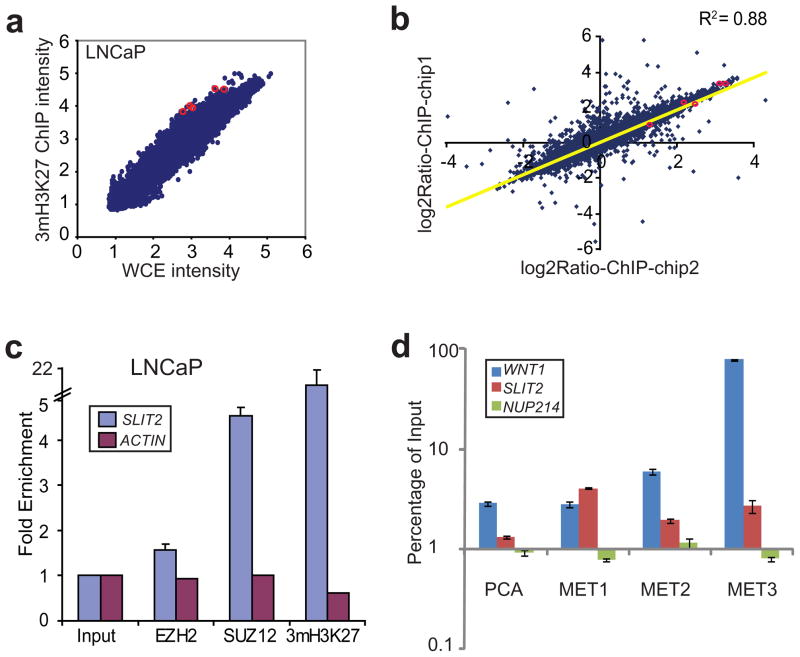
To investigate target genes of PcG proteins in prostate cancer, we performed genome-wide location analysis of SUZ12 and 3mH3K27 in the LNCaP prostate cancer cells (Figure 1a). Out of approximately 80,000 probes present on the promoter array, 7326 showed significant enrichment (P < 0.0001) in the ChIP sample relative to the whole cell extract (WCE). There were only 15 probes with more than 10 fold enrichment, out of which two mapped to the regulatory regions of the same gene, SLIT2. To evaluate the reproducibility of the assay, ChIP-on-chip of 3mH3K27 were replicated using two independent ChIP-enriched DNA fragments. Importantly, the enrichment ratios from both experiments were highly reproducible with r2 = 0.88 (Figure 1b). SLIT2 remained among the most enriched targets of 3mH3K27 in both replicates and all five probes within the SLIT2 promoter region ranked among the top 5% most-enriched targets of SUZ12 and 3mH3K27 (Figure S1).
Figure 1. PRC2 binds to the SLIT2 promoter leading to H3K27 trimethylation.
(a) ChIP-on-chip analysis of 3mH3K27 in LNCaP prostate cancer cells. ChIP-on-chip was done using Agilent promoter arrays and analyzed by Agilent ChIP analytics software. Scatter plot shows ChIP-enriched intensity (processed signals) relative to control whole cell extract (WCE) for all genes in log10 scale. Red circles highlight the probes corresponding to the SLIT2 promoter. (b) Replicate ChIP-on-chip analysis of 3mH3K27 show high correlation. Replicate ChIP-on-chip hybridization was performed using DNA independently enriched by an anti-3mH3K27 antibody in LNCaP prostate cancer cells. Log2Ratio of ChIP-enriched over WCE intensity from each experiment were plotted. Red circles highlight the probes corresponding to the SLIT2 promoter. (c) The SLIT2 promoter is occupied by the PRC2 proteins and trimethylated at H3K27. ChIP assay was performed in LNCaP using antibodies against EZH2, SUZ12 and 3mH3K27. QPCR was performed to evaluate enrichment of SLIT2 over the input whole cell extract DNA. ACTIN was used as a negative control gene for PRC2 binding. (d) One localized and three metastatic (MET) prostate cancer tissues were subjected to ChIP using anti-3mH3K27 antibody. ChIP-enriched DNA and the input DNA were first amplified through ligation-mediated PCR. Equal amounts (50 ng) of amplified ChIP DNA and the input DNA were then subjected to PCR, and enrichment by ChIP was assessed relative to the input DNA. Error bar: n=3, mean ± SEM. The WNT1 promoter and the intragenic region of NUP214 were used as positive and negative controls, respectively.
To confirm this genome-wide location data, we applied ChIP-PCR which couples the conventional ChIP assay with quantitative PCR using gene-specific primers to examine Polycomb occupancy on the SLIT2 promoter. ChIP was performed in the LNCaP cells using antibodies against EZH2, SUZ12 and 3mH3K27. Importantly, our data showed 1.6, 4.7, and 21.5 fold of enrichment by EZH2, SUZ12 and 3mH3K27, respectively, thus confirming SLIT2 as a target of PRC2 (Figure 1c). The difference in fold enrichment largely reflects the quality of the antibodies for ChIP experiments. This repressive H3K27me3 mark can be effectively reduced by histone deacetylase (HDAC) inhibitor SAHA (Figure S2), being consistent with the notion that EZH2-mediated H3K27 methylation requires HDAC activity (van der Vlag and Otte, 1999). As PRC2 binding is known to recruit PRC1 leading to widespread H3K27me3 (Sparmann and van Lohuizen, 2006), we tested whether PRC1 binds to the SLIT2 promoter. Interestingly, ChIP-PCR using antibodies against PRC1 proteins BMI1, RING1, and RING2 revealed significant enrichment at the SLIT2 promoter (Figure S3). To determine whether this protein-DNA interaction holds true in vivo, we performed ChIP analysis of 3mH3K27 in one localized (PCA) and three metastatic prostate tumors (MET). Importantly, the SLIT2 promoter contained strong 3mH3K27 modification especially in metastatic prostate tumors (Figure 1d). Taken together, our data demonstrate that SLIT2 recruits PRC2 and PRC1 complex proteins resulting in nucleosomes harboring repressive histone marks.
SLIT2 expression is negatively regulated by EZH2
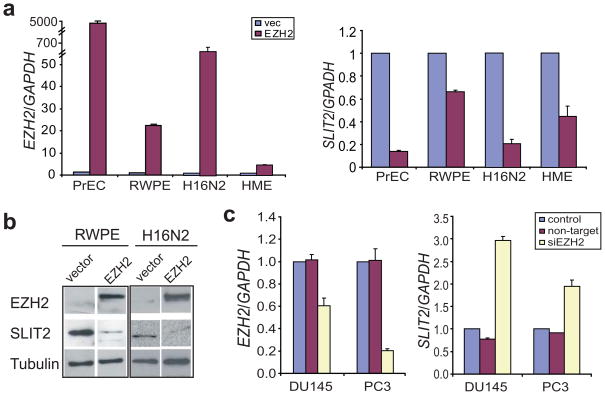
To investigate the consequence of recruiting PRCs to the SLIT2 promoter, we tested the level of SLIT2 expression following EZH2 de-regulation in vitro. As EZH2 is expressed at low levels in benign cells and is primarily up-regulated in aggressive tumors, we over-expressed EZH2 in several benign prostate and breast cell lines including PrEC, RWPE, H16N2 and HME. Importantly, consistent with its binding by the PRC2 complex SLIT2 was significantly down-regulated by EZH2 over-expression in all 4 cell lines (Figure 2a). To confirm that this regulation holds true at the protein level, we performed immunoblot analysis of SLIT2 and EZH2 in the RWPE and H16N2 cells infected with EZH2-overexpressing adenovirus. Our results demonstrated clear repression of the SLIT2 protein following EZH2 overexpression (Figure 2b). Next, we performed RNA interference of EZH2 in metastatic prostate cancer cell lines DU145 and PC3, and achieved a respective 1.7 and 5.2 fold reduction in EZH2 expression. Concordantly, SLIT2 expression was significantly de-repressed leading to 1.8 and 2.9 fold increases in DU145 and PC3 cells, respectively (Figure 2c and S4). It is important to note that the level of EZH2 knockdown and the response of SLIT2 expression to EZH2 knockdown vary between cell lines, probably due to other regulatory mechanisms specific to each cell type.
Figure 2. SLIT2 expression is negatively regulated by EZH2.
(a) EZH2 overexpression represses the transcript level of SLIT2. Benign immortalized prostate cell lines, PrEC and RWPE, and breast cell lines, H16N2 and HME, were infected with adenovirus overexpressing EZH2 or empty vector, and analyzed for EZH2 and SLIT2 mRNA levels by QRT-PCR. (b) Immunoblot analysis of EZH2 and SLIT2 in benign immortalized prostate cell line (RWPE) and breast cell line (H16N2) following infection with EZH2 adenovirus or vector control for 48 hrs. The β-tubulin protein was used as a loading control. (c) QRT-PCR analysis of EZH2 and SLIT2 transcripts in DU145 and PC3 prostate cancer cells following RNA interference of EZH2.
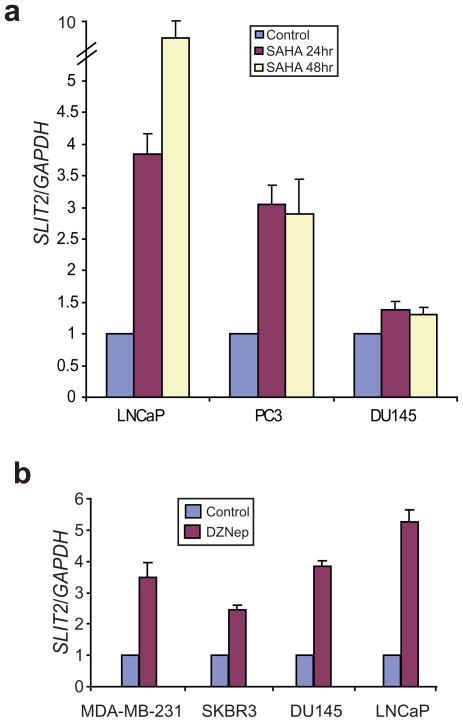
Since HDAC inhibitor SAHA reduces repressive H3K27me3 mark on the SLIT2 promoter, we examined the level of SLIT2 expression following SAHA treatment. Interestingly, SLIT2 was marked de-repressed by SAHA for approximately 9.7, 3.0 and 1.4 fold in LNCaP, PC3 and DU145 cells, respectively (Figure 3a). This de-repression is not due to non-specific effects of SAHA on cell cycle arrest and presumably less DNA/protein synthesis (Figure S5). Previous studies have identified a PRC2-inhibiting compound DZNep that is able to de-repress EZH2 target genes (Tan et al., 2007). We thus tested whether DZNep is able to inhibit EZH2-mediated repression of SLIT2. Interestingly, our results demonstrated marked up-regulation of SLIT2 following DZNep treatment in breast and prostate cancer cell lines including MDA-MB-231, SKBR3, DU145 and LNCaP (Figure 3b). Therefore, SLIT2 is a target of EZH2-mediated transcriptional repression and can be reactivated by PRC2 inhibitors.
Figure 3. Inhibiting EZH2 function de-represses SLIT2.
(a) SLIT2 expression is up-regulated by a histone deacetylase (HDAC) inhibitor SAHA. Prostate cancer cell lines LNCaP, PC3 and DU145 were treated with 5 μM of SAHA for 24 and 48 hours, and analyzed by qRT-PCR. (b) Marked up-regulation of SLIT2 by the PRC2-inhibiting compound DZNep. Breast (MDA-MB-231 and SKBR3) and prostate (DU145 and LNCaP) cancer cells were treated with 5 μM DZNep for 48 hours and analyzed by qRT-PCR. Expression of target gene was normalized to the amount of the GAPDH housekeeping gene.
SLIT2 promoter hypermethylation in prostate cancer
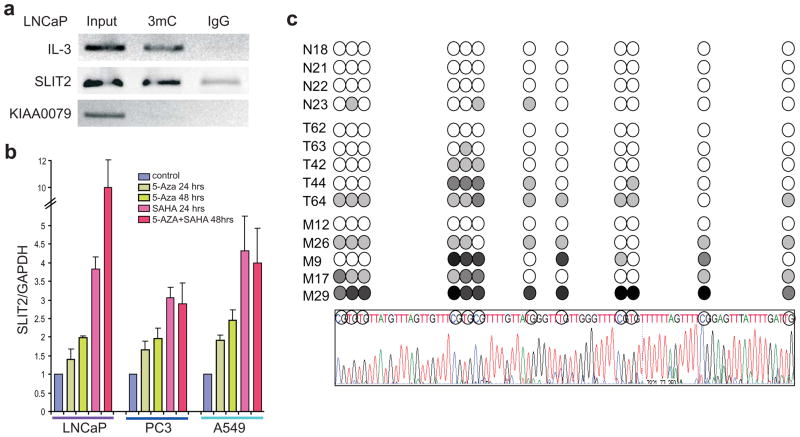
As hypermethylation of the CpG islands in the SLIT2 promoter has been a classical mechanism for SLIT2 repression in cancer, we examined whether the SLIT2 promoter is hypermethylated in prostate cancer cells. We first carried out ChIP experiments using an antibody specific to methylcytidines and performed qPCR analysis using primers specific to the SLIT2 promoter. The promoter region of IL3, a previously reported methylation target bound by DNMT1 (Liu et al., 2005), was used as a positive control. Similar to IL3, the SLIT2 promoter was substantially more enriched by the antibody against 5-methylcytidine than the IgG control (Figure 4a).
Figure 4. DNA methylation of SLIT2 promoter in prostate cancer.
(a) The SLIT2 promoter was enriched by 5-methylcytidine. MeDIP was performed in the LNCaP cells using an anti-5-methylcytidine antibody and IgG control. The IL-3 gene was used as a positive control and KIAA0079 as a negative control. (b) Methylation inhibitors reactivate SLIT2 expression. The LNCaP, PC3 and A549 cells were treated with 5 μM HDAC inhibitor SAHA and/or 5 μM DNA methylation inhibitor 5-Aza for 24 or 48hrs. Total RNA was isolated and analyzed by qRT-PCR. SLIT2 expression was normalized to GAPDH. Error bars represent mean ± SEM. (c) Detection of SLIT2 promoter region methylation in prostate tumor samples. DNA samples were bisulfite-modified and the SLIT2 promoter region was amplified. At least 5 clones per sample were subject to bisulfite sequencing. The circles indicate the CpG dinucleotides shown in the sequence chromatograph. The shades of gray indicate detection of methylated CpG in 20%, 40%, 60%, 80% or 100% of the clones. N is for normal, T for localized tumor, and M for Metastatic prostate tumors. An example sequence trace of one clone is shown at the bottom of the panel. Circled bases represent the CpG dinucleotides analyzed above.
As promoter hypermethylation can be reduced by the DNA methylation inhibitor 5-Aza-2′-deoxycytidine (5-Aza) leading to gene reactivation, we treated LNCaP and PC3 prostate cancer cells with 5-Aza and monitored SLIT2 level. We used the A549 lung cancer cell line as a positive control as previous studies have reported SLIT2 promoter hypermethylation in almost all lung adenocarcinoma (Dammann et al., 2005a). QRT-PCR analysis revealed that 5-Aza significantly de-repressed SLIT2 in all 3 cell lines tested, further supporting SLIT2 as a target of DNA hypermethylation (Figure 4b).
To determine the status of SLIT2 promoter hypermethylation in vivo, we performed bisulfite sequencing of the SLIT2 promoter region in a set of 4 benign prostate tissues, 5 localized and 5 metastatic prostate cancer tissues. Our results showed that the CpG islands in the SLIT2 promoter were rarely methylated in benign samples but the level of hypermethylation dramatically increased in localized and metastatic prostate tumors (Figure 4c).
SLIT2 is down-regulated in metastatic prostate cancer
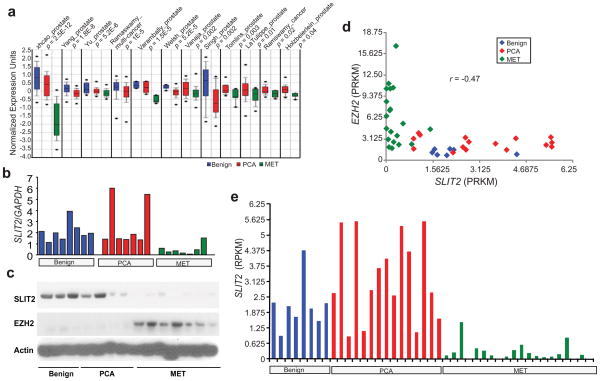
We have now shown that SLIT2 is a target for repression by EZH2-mediated histone methylation as well as a promoter hypermethylation in prostate cancer using in vitro cell line models. We next examined whether SLIT2 expression is down-regulated in prostate tumors in vivo. We first analyzed microarray profiling datasets of prostate cancer tissues using Oncomine (www.oncomine.com). SLIT2 was found significantly down-regulated, in particular, in metastatic prostate cancer in multiple microarray cancer profiling datasets (Figure 5a). In addition, the expression levels of SLIT2 and EZH2 showed a highly significant (r < −0.4; P < 0.0001) anti-correlation supporting EZH2 repression of SLIT2 in vivo (Figure S6). To confirm this, we performed qRT-PCR analysis of SLIT2 and EZH2 in a set of 8 benign prostatic tissues, 7 localized and 7 metastatic prostate tumors. Concordantly, SLIT2 was remarkably down-regulated (P < 0.001 by t-test) in metastatic prostate tumors (Figure 5b). Furthermore, immunoblot analysis of SLIT2 and EZH2 in a set of 3 benign, 4 localized and 7 metastatic prostate tumors confirmed that the SLIT2 protein is highly expressed in benign tissues, decreased in localized and almost absent in metastatic prostate cancer tissues (Figure 5c). By contrast, EZH2 protein is overexpressed in metastatic prostate cancers. In addition, our recent RNA-Seq analysis of 8 benign, 15 localized prostate cancer, and 20 metastatic prostate tumors likewise revealed negatively correlated (r = −0.47) expression of EZH2 and SLIT2 (Figure 5d). None of the 20 metastatic prostate tumors expressed high levels of SLIT2 (Figure 5e).
Figure 5. SLIT2 down-regulation in prostate cancer.
(a) SLIT2 expression in multiple cancer microarray datasets available in Oncomine (www.oncomine.com). (b) QRT-PCR analysis of SLIT2 and EZH2 transcripts in a cohort of 8 benign prostate hyperplasia (Benign), 7 localized prostate cancer (PCA), and 7 metastatic prostate cancer (MET) tissues. Expression of target genes was normalized to the amount of the GAPDH housekeeping gene. SLIT2 markedly (p = 0.0004 by t-test) lower in metastatic prostate cancers. (c) Immunoblot of SLIT2 in a cohort of 3 benign prostate hyperplasia (Benign), 4 localized prostate cancer (PCA), and 7 metastatic prostate cancer (MET) tissues. (d) Negative correlation (r = −0.47) between the transcript levels of EZH2 and SLIT2 in a cohort of prostate cancer analyzed by RNA-Seq. Scatter plot of EZH2 and SLIT2 expression was shown from all profiled samples in our in-house RNA-Seq analysis of 8 benign, 15 localized and 20 metastatic prostate tumors. Expression values are in RPKM (the number of mappable sequencing reads per million per killobase of cDNA sequence) (e) SLIT2 expression (in RPKM) analyzed by RNA-Seq as in (d). SLIT2 is downregulated in metastatic prostate cancers (p<0.001 by t-test).
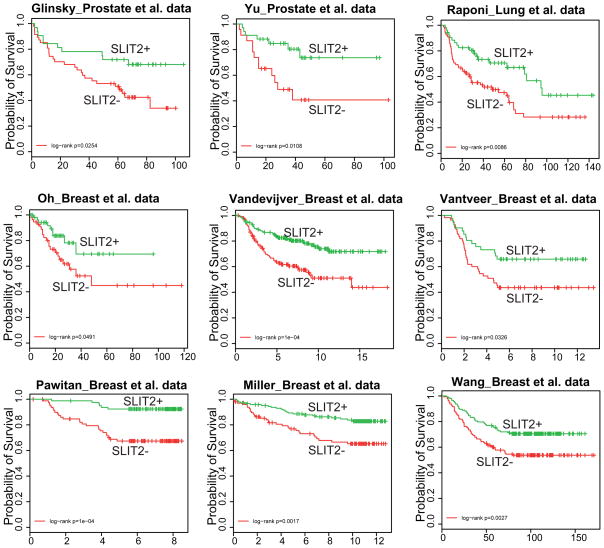
Low level of SLIT2 expression is associated with aggressive tumors
We next examined whether the expression level of SLIT2 is associated with prostate cancer patient survival by first exploring publically available microarray profiling datasets of localized prostate tumors with varying disease outcome (Glinsky et al., 2004; Yu et al., 2004). For each dataset, primary prostate tumors were first classified into two groups based on the expression level of the SLIT2 gene. Kaplan-Meier analysis was used to evaluate survival differences between the two groups and revealed that, for both datasets, the two groups differed significantly in clinical outcome (p = 0.025 for Glinsky et al. dataset and p = 0.011 for Yu et al. dataset) (Figure 6). As epigenetic silencing of SLIT2 has also been reported in lung and breast cancers, we evaluated its power in predicting survival of lung and breast cancer patients. Similarly, Kaplan-Meier analyses showed that a low level of SLIT2 expression was significantly associated with more aggressive disease in multiple cancer datasets including the Raponi et al. lung cancer dataset, and the Oh et al., the van de Vijver et al., the Vant Veer et al., the Pawitan et al., the Miller et al., and the Wang et al. breast cancer datasets (Miller et al., 2005; Oh et al., 2006; Pawitan et al., 2005; van’t Veer et al., 2002; van de Vijver et al., 2002; Wang et al., 2005).
Figure 6. The expression level of SLIT2 predicts clinical outcome in multiple cancer microarray profiling datasets.
Samples of each dataset were classified into 2 groups based on the transcript levels of SLIT2. The two groups were assessed for survival differences by Kaplan-Meier (KM) analysis. KM plots are shown for the Glinsky et al. and the Yu et al. prostate cancer datasets (Glinsky et al., 2004; Yu et al., 2004), the Raponi et al. lung cancer dataset (Raponi et al., 2006), and the Oh et al., the Vandevijver et al., the Van ’t Veer et al., the Pawitan et al., the Miller et al., and the Wang et al. breast cancer datasets (Miller et al., 2005; Oh et al., 2006; Pawitan et al., 2005; van ’t Veer et al., 2002; van de Vijver et al., 2002; Wang et al., 2005).
To confirm this at the protein level, we performed tissue microarray analysis (TMA) of SLIT2 in a cohort of 169 tumor cores from 79 patients. Univariate outcome analysis showed that the SLIT2 level is significantly (P = 0.04) associated with the hazard of PSA recurrence (Table S1). Kaplan-Meier analysis of recurrence-free survival demonstrated that the lower level of SLIT2 protein is associated with poorer clinical outcomes in prostate cancer patients (Figure S7). Multivariate outcome analysis also indicated a trend of SLIT2 in predicting the risk of PSA recurrence (Table S2), thus suggesting that SLIT2 has some association with clinical outcome but not at an independent level.
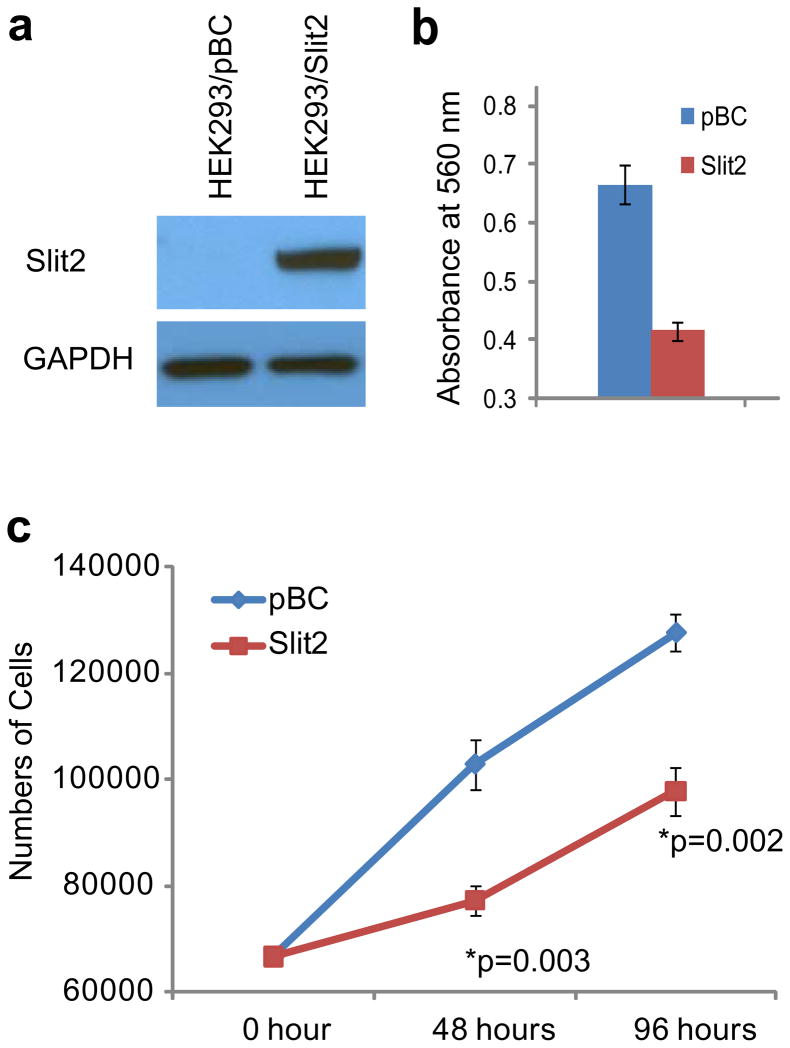
To shed some light on the mechanisms underlying SLIT2 association with clinical outcome, we examined the role of SLIT2 in prostate cancer. As SLIT2 is a secreted protein, we obtained SLIT2-conditioned medium from HEK293 cells stably overexpressing SLIT2 (Figure 7a). Compared to the conditioned medium from control cells (HEK293/pBC), SLIT2-containing conditioned medium significantly inhibited LNCaP cell invasion (Figure 7b) and proliferation (Figure 7c).
Figure 7. SLIT2 inhibits prostate cancer cell invasion and proliferation.
(a) Immunoblot analysis of SLIT2 in HEK293/SLIT2 stable cells and the control HEK293/pBC cells. (b) SLIT2 overexperssion inhibits LNCaP cell invasion. LNCaP cells were incubated with conditioned medium from HEK293/SLIT2 or HEK293/pBC control cell for 48 hr and subjected to Boyden Chamber Invasion assay. (c) SLIT2 overexpression reduces LNCaP cell proliferation. Cell proliferation assay was performed in LNCaP cells incubated in conditioned medium from HEK293/SLIT2 or HEK293/pBC for 48 and 96 hours.
Discussion
The PcG proteins were initially discovered for their roles in body patterning in Drosophila and have been shown to be critical to the cellular memory system, through which epigenetic modifications encode inheritable cellular identity (Kanno et al., 2008). More recently, PRC2 proteins including SUZ12 and EZH2 have been implicated in maintaining the pluoripotency of embryonic stem cells by silencing developmental regulators (Lee et al., 2006). De-regulated expression of the PcG protein EZH2 has been documented in a variety of tumor types and is associated with poor clinical outcomes (Simon and Lange, 2008). Oncogenic properties of EZH2 were believed to be mediated by its epigenetic silencing of a group of tumor suppressor genes in cancer (Yu et al., 2007b). Identification of EZH2 target genes may facilitate the understanding of its function as well as discovering novel biomarkers and therapeutic targets. In this study we report neuronal repellent SLIT2 as a novel target of EZH2-medated epigenetic silencing in prostate cancer.
SLIT2 functions as a neural repellent in the central nervous system guiding axon elongation and branching through repulsive cues (Brose et al., 1999; Wang et al., 1999). During malignancy, it acts as a chemo-repellent in multiple cancer types by inhibiting chemotaxis, cell migration and invasion, thus demonstrating properties of tumor suppressor genes (Prasad et al., 2004). It is frequently down-regulated in a variety of cancer types including colorectal, lung and breast cancers. Hypermethylation of the CpG islands in the SLIT2 promoter is a well-established mechanism for SLIT2 repression in tumors. In this study, we report, for the first time, a novel mechanism involving Polycomb-mediated histone modification to epigenetic silencing of SLIT2 in prostate cancer. We found that PcG proteins bind the SLIT2 promoter in prostate cancer cell lines as well as prostate tumors inhibiting its expression. This repressed expression can be reactivated by HDAC inhibitors or EZH2-inhibiting compounds. In addition, we showed that DNA hypermethylation of the SLIT2 promoter is present in prostate cancer, like in many other cancers. Importantly, our results support the recently formulated hypothesis that PcG-mediated repression maybe a prerequisite step toward the silencing of tumor suppressor genes by DNA hypermethylation (Ohm et al., 2007; Sparmann and van Lohuizen, 2006).
Although SLIT2 has been shown to be down-regulated in a number of tumor types, it has, thus far, not been demonstrated in the context of prostate cancer, despite that prostate cancer is a leading cause of cancer-related death in American men. Our study provides a timely report that SLIT2 is repressed in prostate carcinoma and, in particular, in a majority of metastatic prostate tumors. This down-regulated expression was observed in multiple microarray profiling datasets of prostate tumors and confirmed by qRT-PCR, RNA-Seq, and immunblot analysis. In addition, we show that the levels of SLIT2 expression are anti-correlated with that of EZH2. Importantly, while high levels of EZH2 are associated with aggressive prostate and breast cancers, low levels of SLIT2, by contrast, predict poor clinical outcomes. Interestingly, an earlier study of breast cancer has demonstrated epigenetic silencing of SLIT2 in both breast cancer tissues and paired serum samples (Sharma et al., 2007). Therefore, in future studies it would be of great interest to examine the expression of SLIT2 in the sera and urine of prostate cancer patients and determine its value as a non-invasive prognostic biomarker in prostate cancer.
In summary, through genome-wide location analysis of PcG proteins we identified SLIT2 as a direct target of EZH2-mediated H3K27 trimethylation in prostate cancer. We also showed that the CpG islands in the SLIT2 promoter are hypermethylated in prostate cancer, like in many other cancer types. Functional assays implicated the role of SLIT2 in inhibiting prostate cancer cell growth and invasion. In addition, our study suggests that SLIT2 may be a potent noninvasive prognostic biomarker of prostate cancer.
Materials and Methods
Cell culture
The prostate and breast cell lines were obtained from the American Type Culture Collection (ATCC). LNCaP, DU145, PC3, MDA-MB-231, SKBR3 cells were cultured in RPMI supplemented with 10% fetal bovine serum (Invitrogen). The PrEC (Lonza) and RWPE (ATCC) cells were grown in their respective medium specified by manufacturer. HME and H16N2 cells were grown in Ham’s F12 supplemented with 0.1% BSA, 0.5 μg/ml fungizone, 5 μg/ml gentamycin, 5 mM ethanolamine, 10 mM HEPES, 5 μg/ml transferrin, 10 μM T3, 50 μM selenium, 5 μg/ml insulin, 1 μg/ml hydrocortisone, and 10 ng/ml EGF. HEK293 cells stably expression SLIT2 (HEK293/SLIT2) were provided by Dr. Jane Y. Wu (Wu et al., 2001) and grown in DMEM supplemented with 10% FBS and 200 μg/ml G418 (Sigma). For inhibitor treatment, prostate cancer cells were treated with 5 μM DZNep, 5 μM SAHA, and/or 5 μM 5-Aza for indicated length of time. EZH2 adenovirus infection and RNA interference were performed as previously described (Yu et al., 2007a). At 48 hours after adenovirus infection or RNA interference, cells were harvest for RNA isolation using TRIzol (Invitrogen).
ChIP and ChIP-on-chip
Chromatin immunoprecipitation (ChIP) and ChIP-on-chip were performed as previously described (Yu et al., 2007a). Antibodies (5 μg) used for ChIP included monoclonal anti-EZH2 (BD), polyclonal anti-SUZ12 (Upstate), and polyclonal anti-H3K27me3 (Upstate) antibodies. ChIP-on-chip was done using the 44k, 2-set Agilent proximal promoter arrays according to the manufacturer’s protocols. The ChIP-on-chip data has been deposited to NCBI GEO database with accession number GSE9069.
Bisulfite conversion and methylation analysis
DNA Samples were bisulfite-modified before PCR amplification and sequencing according to previously described procedures with slight modifications (Dallol et al., 2003a). The region analyzed was upstream of the SLIT2 translation start site in accession number NT_006316. More precisely, the region PCR amplified was chr4:19,863,915-19,864,324 and the region sequenced was chr4:19,863,964-19,864,050 according to the March 2006 NCBI Build 36.1. The primers used to PCR amplify the SLIT2 promoter regions were 5′-AGTTTAGAGTYGTGYGTTTTTAGAAT-3′ (forward) and 5′-CAAAAACTCCTTAAACAACTTTAAATCCTAAAA-3′ (reverse). The PCR conditions were 95°C for 10 min, followed by 40 cycles of 1 min denaturation at 95°C, 1 min annealing at 54°C, and 2 min extension at 74°C using Qiagen HotStarTaq DNA Polymerase. The PCR products were cloned into the pGEM-T Easy system (Promega) before sequencing. For each sample, at least 5 clones were sequenced using the T7 vector primers. The methylation index (MI) is calculated as a percentage using the equation: number of CpG dinucleotides methylated/total number of CpG dinucleotides sequenced × 100.
MeDIP
Methylated DNA immunoprecipitation (MeDIP) was performed in genomic DNA using an anti-5-methylcytidine antibody (BI-MECY_0100, Eurogentec) or IgG control (Santa Cruz) according to previously published protocols (Weber et al., 2005). Quantitative PCR was used to determine the enrichment of target genomic regions using gene-specific primers. Enrichment in the MeDIP sample was evaluated relative to the IgG sample, using input DNA as a positive control for PCR amplification.
Quantitative RT-PCR (qRT-PCR)
Q-PCR was performed using Power SYBR Green Mastermix (Applied Biosystems) on an Applied Biosystems 7300 Real Time PCR machine as previously described (Yu et al., 2007a). All primers were designed using Primer 3 and synthesized by Integrated DNA Technologies and are listed in Table S3. All PCR were performed in triplicates.
Tissue Microarray Analysis (TMA)
TMA analysis was done in the University of Michigan Prostate Cancer Specialized Program of Research Excellence Tissue Core (SPORE), with informed consent of the patients and prior institutional review board approval. The tissue microarray contained 360 cores from 105 patients. Out of these, 169 cores, from 79 patients, were evaluable, the remainder being either lost or purely stromal (191). Standard biotin-avidin complex immunohistochemistry was performed using a SLIT2 antibody (HPA023088, Sigma) and the staining was scored as previously described (Yu et al., 2007a). Briefly, product score was used as an overall measure of staining percentage and intensity. Specifically, intensity was coded by an integer ranging from 1–4 (none=1, weak=2, moderate=3, strong=4) and this number was multiplied by staining percentage to produce the product score for each core. These core-level product scores were then aggregated into a patient-level product score by taking the median score over the set of tumor cores for each patient as the measure of SLIT2 expression for each patient/tissue type combination.
RNA-Seq analysis
Total RNA was isolated from prostate tissues and prepared into libraries for next-generation sequencing according to the manufacturer’s protocol (Illumina). Massively parallel sequencing was performed using the Genome Analyzer (Illumina) and sequence was aligned to the reference genome using the Illumina pipeline.
Microarray data Analysis
All microarray expression studies and corresponding patient information were extracted from ONCOMINE database. Log2 transformed intensities of SLIT2 and EZH2 genes were then mean-centered across samples per gene per study and a linear model was fitted to estimate the association significance. For Kaplan-Meier survival analysis, patient samples in each study with SLIT2 expression values greater its median intensity were grouped as “SLIT2+”, otherwise as “SLIT2−”. Survival analysis was then performed in R 2.9 (http://www.r-project.org) and p-value was reported by log-rank test.
Cell Invasion and Proliferation Assays
Cell invasion assay was performed with Boyden Chamber (Corning) coated with Matrigel (BD biosciences) as previously described (Yu et al., 2007a). Briefly, LNCaP cells were harvested and resuspended in RPMI 1640 medium without serum but with HEK293/pBC- or HEK293/SLIT2-conditioned medium at a density of 106 cells/ml. Lower chamber of the transwell was filled with 600ul RPMI 1640 medium with 10% FBS and with HEK293/pBC- or HEK293/SLIT2-conditioned medium. LNCaP cell suspension was applied onto the matrigel membrane, and incubated at 37°C for 24 to 48 hrs. Cells migrated through membrane were stained with crystal violet, and further dissolved in acetic acid and read at OD 560nm. Cell proliferation assay was done as previously described (Yu et al., 2007a).
Supplementary Material
Acknowledgments
This work was supported in part by the NIH Prostate Specialized Program of Research Excellence grant P50CA69568 and P50CA090386, Early Detection Research Network grant UO1 111275 (to A.M.C.), and the NIH 1R01CA132874-01A1 (to A.M.C.) and K99CA129565-01A1 (to J.Y.), and the U.S. Department of Defense PC051081 (to A.M.C.), and PC080665 (to J.Y.). A.M.C. is supported by a Burroughs Welcome Foundation Award in Clinical Translational Research, a Doris Duke Charitable Foundation Distinguished Clinical Investigator Award, the Prostate Cancer Foundation and the Howard Hughes Medical Institute. A.M.C. is an American Cancer Society Research Professor.
Footnotes
Conflict of Interest
A.M.C. is a cofounder of Compendia Biosciences and serves as head of the scientific advisory board. Other authors declare no potential conflict of interest.
References
- Astuti D, Da Silva NF, Dallol A, Gentle D, Martinsson T, Kogner P, et al. SLIT2 promoter methylation analysis in neuroblastoma, Wilms’ tumour and renal cell carcinoma. Br J Cancer. 2004;90:515–21. doi: 10.1038/sj.bjc.6601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beke L, Nuytten M, Van Eynde A, Beullens M, Bollen M. The gene encoding the prostatic tumor suppressor PSP94 is a target for repression by the Polycomb group protein EZH2. Oncogene. 2007;26:4590–5. doi: 10.1038/sj.onc.1210248. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–84. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Tu SW, Hsieh JT. Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J Biol Chem. 2005;280:22437–44. doi: 10.1074/jbc.M501379200. [DOI] [PubMed] [Google Scholar]
- Dallol A, Da Silva NF, Viacava P, Minna JD, Bieche I, Maher ER, et al. SLIT2, a human homologue of the Drosophila Slit2 gene, has tumor suppressor activity and is frequently inactivated in lung and breast cancers. Cancer Res. 2002;62:5874–80. [PubMed] [Google Scholar]
- Dallol A, Krex D, Hesson L, Eng C, Maher ER, Latif F. Frequent epigenetic inactivation of the SLIT2 gene in gliomas. Oncogene. 2003a;22:4611–6. doi: 10.1038/sj.onc.1206687. [DOI] [PubMed] [Google Scholar]
- Dallol A, Morton D, Maher ER, Latif F. SLIT2 axon guidance molecule is frequently inactivated in colorectal cancer and suppresses growth of colorectal carcinoma cells. Cancer Res. 2003b;63:1054–8. [PubMed] [Google Scholar]
- Dammann R, Schagdarsurengin U, Seidel C, Strunnikova M, Rastetter M, Baier K, et al. The tumor suppressor RASSF1A in human carcinogenesis: an update. Histol Histopathol. 2005a;20:645–63. doi: 10.14670/HH-20.645. [DOI] [PubMed] [Google Scholar]
- Dammann R, Strunnikova M, Schagdarsurengin U, Rastetter M, Papritz M, Hattenhorst UE, et al. CpG island methylation and expression of tumour-associated genes in lung carcinoma. Eur J Cancer. 2005b;41:1223–36. doi: 10.1016/j.ejca.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Dunwell TL, Dickinson RE, Stankovic T, Dallol A, Weston V, Austen B, et al. Frequent epigenetic inactivation of the SLIT2 gene in chronic and acute lymphocytic leukemia. Epigenetics. 2009;4:265–9. doi: 10.4161/epi.9137. [DOI] [PubMed] [Google Scholar]
- Glinsky GV, Glinskii AB, Stephenson AJ, Hoffman RM, Gerald WL. Gene expression profiling predicts clinical outcome of prostate cancer. J Clin Invest. 2004;113:913–23. doi: 10.1172/JCI20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, You H, Yu B, Deng Y, Tang N, Yao G, et al. Epigenetic inactivation of SLIT2 in human hepatocellular carcinomas. Biochem Biophys Res Commun. 2009;379:86–91. doi: 10.1016/j.bbrc.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Kanno R, Janakiraman H, Kanno M. Epigenetic regulator polycomb group protein complexes control cell fate and cancer. Cancer Sci. 2008;99:1077–84. doi: 10.1111/j.1349-7006.2008.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim HK, Zhang H, Li H, Wu TT, Swisher S, He D, et al. Slit2 inhibits growth and metastasis of fibrosarcoma and squamous cell carcinoma. Neoplasia. 2008;10:1411–20. doi: 10.1593/neo.08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–13. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shen T, Huynh L, Klisovic MI, Rush LJ, Ford JL, et al. Interplay of RUNX1/MTG8 and DNA methyltransferase 1 in acute myeloid leukemia. Cancer Res. 2005;65:1277–84. doi: 10.1158/0008-5472.CAN-04-4532. [DOI] [PubMed] [Google Scholar]
- Mathews LA, Crea F, Farrar WL. Epigenetic gene regulation in stem cells and correlation to cancer. Differentiation. 2009;78:1–17. doi: 10.1016/j.diff.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102:13550–5. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DS, Troester MA, Usary J, Hu Z, He X, Fan C, et al. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol. 2006;24:1656–64. doi: 10.1200/JCO.2005.03.2755. [DOI] [PubMed] [Google Scholar]
- Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–42. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawitan Y, Bjohle J, Amler L, Borg AL, Egyhazi S, Hall P, et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res. 2005;7:R953–64. doi: 10.1186/bcr1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Fernandis AZ, Rao Y, Ganju RK. Slit protein-mediated inhibition of CXCR4-induced chemotactic and chemoinvasive signaling pathways in breast cancer cells. J Biol Chem. 2004;279:9115–24. doi: 10.1074/jbc.M308083200. [DOI] [PubMed] [Google Scholar]
- Prasad A, Paruchuri V, Preet A, Latif F, Ganju RK. Slit-2 induces a tumor-suppressive effect by regulating beta-catenin in breast cancer cells. J Biol Chem. 2008;283:26624–33. doi: 10.1074/jbc.M800679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Qamri Z, Wu J, Ganju RK. Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. J Leukoc Biol. 2007;82:465–76. doi: 10.1189/jlb.1106678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raponi M, Zhang Y, Yu J, Chen G, Lee G, Taylor JM, et al. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res. 2006;66:7466–72. doi: 10.1158/0008-5472.CAN-06-1191. [DOI] [PubMed] [Google Scholar]
- Rothberg JM, Hartley DA, Walther Z, Artavanis-Tsakonas S. slit: an EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell. 1988;55:1047–59. doi: 10.1016/0092-8674(88)90249-8. [DOI] [PubMed] [Google Scholar]
- Sharma G, Mirza S, Prasad CP, Srivastava A, Gupta SD, Ralhan R. Promoter hypermethylation of p16INK4A, p14ARF, CyclinD2 and Slit2 in serum and tumor DNA from breast cancer patients. Life Sci. 2007;80:1873–81. doi: 10.1016/j.lfs.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–9. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6:846–56. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–63. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng RC, Lee SH, Hsu HS, Chen BH, Tsai WC, Tzao C, et al. SLIT2 attenuation during lung cancer progression deregulates beta-catenin and E-cadherin and associates with poor prognosis. Cancer Res. 2010;70:543–51. doi: 10.1158/0008-5472.CAN-09-2084. [DOI] [PubMed] [Google Scholar]
- van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet. 1999;23:474–8. doi: 10.1038/70602. [DOI] [PubMed] [Google Scholar]
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, et al. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–84. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–62. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- Werbowetski-Ogilvie TE, Seyed Sadr M, Jabado N, Angers-Loustau A, Agar NY, Wu J, et al. Inhibition of medulloblastoma cell invasion by Slit. Oncogene. 2006;25:5103–12. doi: 10.1038/sj.onc.1209524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Park HT, Wu JY, Rao Y. Slit proteins: molecular guidance cues for cells ranging from neurons to leukocytes. Curr Opin Genet Dev. 2002;12:583–91. doi: 10.1016/s0959-437x(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–52. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Cao Q, Mehra R, Laxman B, Yu J, Tomlins SA, et al. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007a;12:419–31. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R, et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res. 2007b;67:10657–63. doi: 10.1158/0008-5472.CAN-07-2498. [DOI] [PubMed] [Google Scholar]
- Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–9. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.