Abstract
Background
Ex vivo manufacture of red blood cells from stem cells is a potential means to ensure an adequate and safe supply of blood cell products. Advances in somatic cell reprogramming of human induced pluripotent stem cells have opened the door to generating specific cells for cell therapy. Human induced pluripotent stem cells represent a potentially unlimited source of stem cells for erythroid generation for transfusion medicine.
Design and Methods
We characterized the erythroid differentiation and maturation of human induced pluripotent stem cell lines obtained from human fetal (IMR90) and adult fibroblasts (FD-136) compared to those of a human embryonic stem cell line (H1). Our protocol comprises two steps: (i) differentiation of human induced pluripotent stem cells by formation of embryoid bodies with indispensable conditioning in the presence of cytokines and human plasma to obtain early erythroid commitment, and (ii) differentiation/maturation to the stage of cultured red blood cells in the presence of cytokines. The protocol dispenses with major constraints such as an obligatory passage through a hematopoietic progenitor, co-culture on a cellular stroma and use of proteins of animal origin.
Results
We report for the first time the complete differentiation of human induced pluripotent stem cells into definitive erythrocytes capable of maturation up to enucleated red blood cells containing fetal hemoglobin in a functional tetrameric form.
Conclusions
Red blood cells generated from human induced pluripotent stem cells pave the way for future development of allogeneic transfusion products. This could be done by banking a very limited number of red cell phenotype combinations enabling the safe transfusion of a great number of immunized patients.
Keywords: progenitor cells, embryonic stem cells, hiPSC, hESC, erythroid differentiation, hemoglobin, red blood cells
Introduction
More than 80 million units of whole blood are collected from donors worldwide every year. Supply constraints and uncertain screening for infectious diseases are of particular concern in developing and transitional nations [World Heath Organization (2004) Global Database on Blood Safety (http://www.who.int/bloodsafety/GDBS_Report_2001-2002.pdf)]. In wealthy nations the other side of the coin is the occurrence of multiple immunization to red blood cell (RBC) antigens in 0.1% to 0.5% of transfused patients, leading first to difficulty in finding compatible RBC concentrates and very high costs, and eventually to full transfusion blockade. In response to these concerns, ex vivo manufacture of RBC from stem cells makes sense to enable safe and quantitatively sufficient transfusion.1 This is the concept of cultured RBC produced ex vivo from hematopoietic stem cells originating from bone marrow, peripheral blood or cord blood.2,3 Research is already focused on the feasibility of this approach at the industrial level [US Defence Advanced Research Programs Agency (USDARPA)’s ‘Blood Pharming’ program (2007) (http://www.darpa.mil/dso/solicitations/baa07-21mod6.html)]. On this background, the identification of an unlimited source of stem cells is a major objective to free the production of cultured RBC from the constraints of the supply of hematopoietic stem cells.
Human embryonic stem cells (hESC)4 are candidates as an unlimited source of cells. Several groups have explored their hematopoietic potential,5–7 although few have focused on erythroblastic differentiation,8–11 using complex protocols. One group11 was able to grow blood types A, B, O, and both Rhesus D positive (RH :1) and Rhesus D negative (RH :-1) but unable to produce the O RH :-1 blood type - the so-called “universal” donor - because none of the hESC lines approved in the USA contain the O-negative gene. The embryo, therefore, appears to have limitations with regards to the development of pluripotent stem cell lines with a broad spectrum of transfusion compatibility.
Recent advances in somatic cell reprogramming to produce human induced pluripotent stem cells (hiPSC)12–14 have enabled the generation of “hESC-like” cells, i.e cells which can indefinitely self-renew in vitro while maintaining the ability to differentiate toward all three germ layers. Although hematopoietic differentiation has been explored,15–17 no terminal erythroid differentiation has been reported to date.
In the present study we characterized for the first time the erythroid differentiation and maturation of hiPSC cell lines obtained from human fetal (IMR90) and adult fibro-blasts (FD-136) compared to those of a hESC line (H1).
Design and Methods
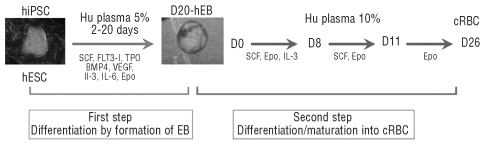
Our protocol comprised two steps: (i) differentiation of hiPSC by formation of human embryoid bodies (hEB) and (ii) differentiation/maturation to the stage of mature cultured RBC in the presence of cytokines (Figure 1). All experiments were simultaneously performed with hiPSC and hESC. The research was authorized by the French Biomedicine Agency.
Figure 1.
Schematic representation of the successive culture steps for production of cultured red blood cells (cRBC) from pluripotent stem cells. First step: clumps of undifferentiated hiPSC and hESC were cultured in “erythroid body (EB) medium” for 20 days. Second step: dissociated D20-EB were then cultured in a liquid medium for up to 25 days in the presence of sequential cocktails of cytokines (see Design and Methods section).
Human induced pluripotent stem cell generation and characterization
Human fetal lung fibroblasts IMR-90 were retrieved from the ATCC (Manassas, VA, USA) and adult hiPSC were generated using a skin primary fibroblast cell line established from a healthy 25-year old woman after informed consent (FD136 kindly provided by A. Munnich, Inserm U781, Paris, France) and plasmids pSin-EF2-Oct4-Pur, pSin-EF2-Sox2-Pur, pSin-EF2-Nanog-Pur and pSin-EF2-Lin28-Pur13 from Addgene (Cambridge, USA). Virus production was performed by Vectalys (Labège, France). hiPSC clones were obtained as previously described by Thomson’s group.13 Briefly, 200,000 fibroblasts were infected 1 day after plating with the four lentivectors at the highest possible MOI between 7 and 23 depending on the original virus preparation, in the presence of polybrene at 8 μg/mL (Sigma). Two days later, viruses were removed and medium progressively changed to hESC medium in the following week. The medium was then changed on a daily basis, as for hESC. hiPSC colonies appeared between 3 and 6 weeks after infection and were picked up and clonally amplified.
hiPSC clones were characterized using different techniques: karyotypes were determined by multi-fluorescence in situ hybridization and gene expression by either flow cytometry or by real-time polymerase chain reaction (PCR) and Taqman low density arrays. Briefly, RNA was extracted from cells using an RNeasy kit (Qiagen); 1 μg of total RNA was retrotranscribed using SuperScript (Invitrogen) enzyme and the expression of markers was analyzed using the comparative ΔΔCt method with GAPDH as the endogenous control and a human embryonic carcinoma 2102EP (hEC) or a normal hESC sample as a calibrator (HUES-24 line; kindly provided by M. Pucéat, INSERM UMR861, Evry, France). Endogenous and exogenous expression of OCT4, SOX2, LIN28 and NANOG was evaluated in hiPSC clones. In vitro differentiation of hiPSC clones was appreciated by the formation of hEB. Briefly, hiPSC were recovered from dishes using collagenase (1 mg/mL; Invitrogen) and transferred to low attachment plates (Nunc, Dutscher, Brumath, France) in the same medium without basic fibroblast growth factor. Media were changed every 2–3 days. After 10 days, hEB were retrieved and lysed for RNA extraction and quantitative real-time PCR (qRT-PCR). Markers for each embryonic layer were analyzed (ectoderm: Pax6, ck18; mesoderm: brachyury, Gata4, RunX1, CD34, Nkx2.5, KDR; endoderm: ck17, AFP).
Cells and culture conditions
hiPSC (IMR90)-16 (n=3) passages 13–21, hiPSC (FD-136)-25 (n=2) passages 25–32 and hESC H1 (n=3) (National Institute of Health code WA-01) passages 23–48 were grown on primary mouse embryonic fibroblasts treated with mitomycin (20 μg/mL) as previously described.4
Erythroid induction and differentiation
To allow hEB formation, undifferentiated hESC and hiPSC were treated with collagenase IV (1 mg/mL; Invitrogen) and transferred to low attachment plates (Nunc) in liquid culture medium (Iscove’s modified Dulbecco’s medium - glutamax, Biochrom, Berlin, Germany) containing human plasma in the presence of stem cell factor (SCF, 100 ng/mL), thrombopoietin (TPO, 100 ng/mL), FLT3 ligand (FL, 100 ng/mL), recombinant human bone morphogenetic protein 4 (BMP4; 10 ng/mL), recombinant human vascular endothelial growth factor (VEGF-A165; 5 ng/mL), interleukin-3 (IL-3; 5 ng/mL), interleukin-6 (IL-6; 5 ng/mL) (Peprotech) and erythropoietin (Epo; 3 U/mL) (Eprex, kindly provided by Janssen-Cilag, France). hEB were cultured for 20 days at 37°C in a humidified 5% CO2 atmosphere. The cells were dissociated into a single-cell suspension by incubation with collagenase B (0.4 U/mL; Roche Diagnostics, Laval, QC, Canada) for 30 min at 37°C and cell dissociation buffer (Invitrogen) for 10 min at 37°C. Then the erythroid protocol (D0 to 25) was adapted from our previous procedures.3 From day 0 to day 8 dissociated hEB were plated at a density of 106 cells/mL in liquid culture medium containing 10% human plasma, insulin 10 μg/mL (Cellgenix, France) and 3 U/mL heparin, in the presence of SCF (100 ng/mL), IL-3 (5 ng/mL) and Epo (3 U/mL). From day 8 to day 11 the cells were suspended at a density of 3×105 cells/mL and cultured in fresh medium supplemented with SCF (100 ng/mL) and Epo (3 U/mL). From day 11 to day 25 the cells were suspended at a density of 106 or 2×106 cells/mL and cultured in fresh medium supplemented with Epo (3 U/mL). The cultures were maintained at 37 °C in 5% CO2 in air.
Flow cytometric analysis
Samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San José, CA, USA). The following antibodies were used for flow cytometric analysis: SSEA4-PE (phycoerythrin) and SSEA1-PE (Clinisciences, Montrouge, France); TRA-1-60, TRA-1-81, goat anti-mouse IgM-PE and goat anti-mouse IgG-PE (Chemicon, Saint-Quentin en Yvelines, France); CD34-PE, CD45-PE, CD45-PC7, CD71-FITC, CD36-FITC and CD235a-PE (glycophorin A) (Beckman Coulter- Immunotech, Marseille, France).
Hemoglobin composition of mature cultured red blood cells, as determined by chromatography and mass spectrometry
The percentage of hemoglobin fractions was measured by cation exchange - high performance liquid chromatography (CE-HPLC) using a Bio-Rad Variant II Hb analyzer (Bio-Rad Laboratories, Hercules, CA, USA). The separation of the different globin chain fractions was done by reversed phase liquid chromatography (RP-LC) and spectral analysis, as previously described.18 RP-LC analyses were performed on a C4 Uptisphere (silica beads 5 μm; average pore size 300 Å) (Interchim, Montluçon, France) (4.6 × 250 mm). Isolated globin-chain fractions were identified by electrospray ionization-mass spectrometry, as previously described.19
Functionality of hemoglobin from cultured red blood cells
The binding of hemoglobin (Hb) at 10 μM [heme] with carbon monoxide (CO) was studied by flash photolysis using a 4×10 mm optical cuvette (4 mm for the transmitted light and 10 mm for the laser beam). We also used a 1 mm optical cuvette for HbA studied at 50 μM [heme] to avoid the presence of Hb dimer (the dimertetramer interface of the liganded form is weaker for HbA than for HbF). The method has been described elsewhere.20
Results
Human induced pluripotent stem cell generation and characterization
Human fetal fibroblasts IMR-90 and an adult skin primary fibroblast cell line from a healthy donor were used to obtain hiPSC clones as previously described by Thomson’s group.13 The fetal hiPSC IMR90-16 had a normal karyotype (Online Supplementary Figure S1A). Both fetal and adult-derived hiPSC expressed hESC markers, as determined by flow cytometry and PCR (Online Supplementary Figure S1B). hiPSC clones expressed endogenous OCT4, SOX2, LIN28 and NANOG at levels equivalent to or slightly below those of the levels in the hESC cell line H1. The expression of the exogenous genes was determined in hiPSC clones, with a well-known variability depending on the clone and the transgene (Online Supplementary Figure S1C, compare endogenous versus total, and Online Supplementary Table S1).13
After 10 days of differentiation of hEB, the cells expressed markers of endoderm, mesoderm and ectoderm. The analyses were performed by qRT-PCR or Taqman low density array (Online Supplementary Figure S1D and Online Supplementary Table S2).
Differentiation of human induced pluripotent stem cells into human erythroid bodies conditioned for erythroid commitment (first step)
We chose to induce and stimulate the erythropoietic pathway very early. While addition of BMP421 and VEGF-A16522 appears to be indispensable, we performed preliminary experiments (data not shown) to test the essential role of cytokines and the type of serum. After carrying out these experiments, we were able to define a culture medium capable of inducing hEB differentiation from hiPSC with early erythroid commitment (for hEB conditioning erythroid commitment). This culture medium contains a cocktail of eight cytokines: SCF, TPO, FLT3 ligand, rhu BMP4, rhu VEGF-A165, IL-3, IL-6 and Epo.
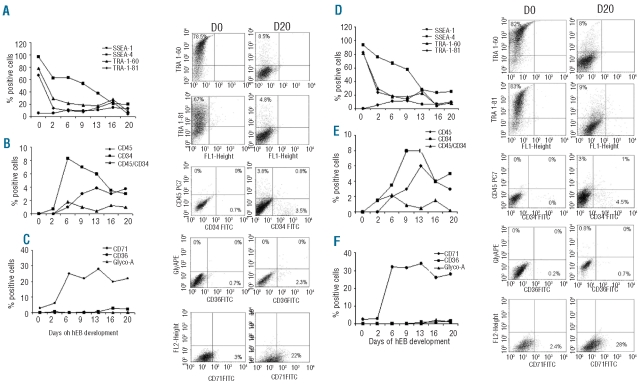
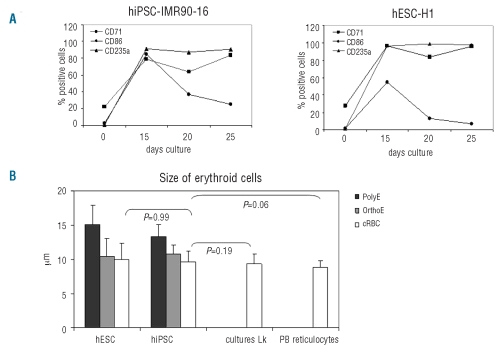
Our strategy consisted of identifying the stage or stages of differentiation of hEB with high erythroid potential. We, therefore, analyzed the kinetics of differentiation of hEB from fetal and adult hiPSC lines between days 2 and 20 of culture by following the expression of specific markers of hematopoiesis and erythropoiesis by flow cytometry. The data are summarized in Figure 2. The expression of markers of undifferentiated human cells (SSEA-4, Tra 1–60, Tra 1–81) decreased progressively until day 20 (Figure 2A). CD34 and/or CD45 were expressed from day 6 to day 20 (Figure 2B). The erythroid markers CD36 and CD235a were weakly expressed while the transferrin receptor CD71 was expressed throughout the culture period and at high levels between days 6 and 20 (Figure 2C). Overall, between days 9 and 20, hEB significantly expressed the hematopoietic and erythroid markers studied: CD45, CD34 and CD71. Thus, compared to hESC (Figure 2D, E, and F), we observed no significant differences in the expression kinetics of undifferentiated cell markers and erythroid markers with both fetal and adult hiPSC.
Figure 2.
Phenotypic analyses of hiPSC-IMR90-16 (A, B, C) and hESC-H1-derived cells (D, E, F) during EB differentiation from day 0 to day 20. (A) and (D): percentage expression of undifferentiated cell markers (SSEA-4, Tra 1–60, Tra 1–81) and representative dot plots. (B) and (E): percentage expression of the hematopoietic markers (CD45, CD34) and representative dot plots. (C) and (F): percentage expression of erythroid markers (CD71, CD36, CD235a) and representative dot plots as determined by flow cytometry in one representative experiment.
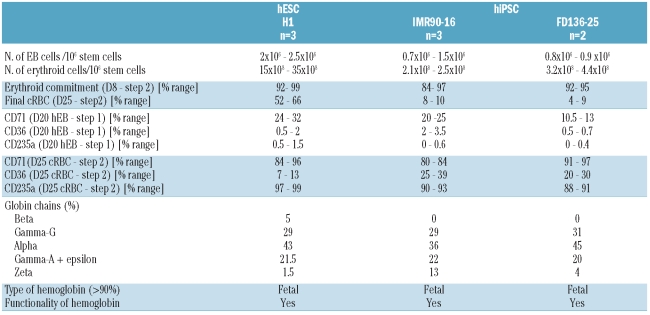
Taken all together, these results indicated that hEB from day 20 (D20-hEB) might have a high erythroid potential and we decided to pursue erythroid differentiation studies using these D20-hEB. Meanwhile the number of colony-forming units (CFU) that developed from hESC and hiPSC remained very low, being 45 and 2 B/CFU-E after 14 days for D20-hEB from hESC and hiPSC, respectively (data not shown), pointing out the weak clonogenic potential of those cells. Detailed results for each cell line are presented in Table 1.
Table 1.
Global summary of experiments with hiPSC and hESC lines.
Differentiation/maturation of human erythroid bodies into cultured red blood cells (second step)
Dissociated D20-hEB from both fetal and adult hiPSC and hESC were re-plated following the liquid culture protocol for erythroblastic differentiation/maturation i.e. in the presence of 10% human plasma and an evolving cocktail of cytokines based on SCF, IL-3 and Epo (Figure 1). Erythrocyte maturation was evaluated regularly based on cell morphology after staining with May-Grünwald-Giemsa and the expression of erythroid membrane antigens, was determined by flow cytometry.
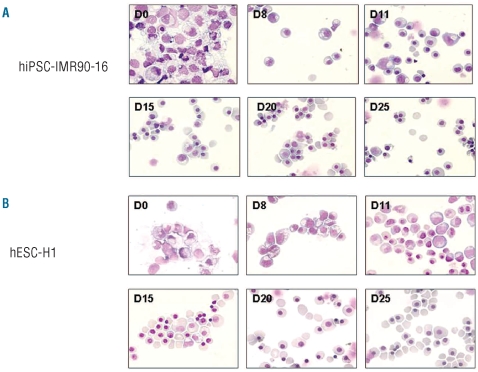
The erythroid commitment of D20-hEB was complete after 8 days of liquid culture with up to 99% production of erythroblasts for both hiPSC and hESC. At day 25, the population contained respectively 4% to 10% of enucleated cultured RBC and 90% to 96% of orthochromatic erythroblasts from hiPSC (Figure 3A), and 52% to 66% of enucleated cultured RBC and 34% to 48% of orthochromatic erythroblasts from H1-hESC (Figure 3B).
Figure 3.
Morphology of the erythroid cells generated from hiPSC-IMR90-16 (A) and hESC-H1 (B). During the second step of the protocol (differentiation and maturation to mature cultured RBC), aliquots of cells were taken at the indicated times for morphological analysis of the cells by May-Grünwald-Giemsa staining. Photographs show each stage of erythroid maturation on days 0, 8, 11, 15, 20, and 25 (magnification x 630). One representative experiment.
The amplification potential for each cell line is detailed in Table 1. Overall, 1×106 hiPSC gave rise to up to 1.5×106 D20-hEB, which generated up to 4.4×108 mature erythroid cells. In comparison, amplification of 1×106 hESC gave rise to up to 2.5×106 D20-hEB, which generated up to 35×108 mature erythroid cells.
Analysis of the cultured red blood cells
Flow cytometric analysis of the membrane antigens demonstrated the degree of maturity of the cultured RBC. At the end of culture, the RBC strongly expressed CD235a and CD71, with a low expression of CD36 which is consistent with a terminal erythroid phenotype (Figure 4A and Online Supplementary Figure S2). We observed no difference between cultured RBC derived from fetal and adult hiPSC or from hESC (Table 1).
Figure 4.
Analyses of cultured RBC maturation. (A) Kinetics of expression of antigens during erythroid differentiation from hiPSC-IMR90-16 and hESC-H1 by flow cytometry. (B) The size of mature cultured RBC (cRBC) generated from D25-hiPSC and D25 -hESC was compared to that of mature cRBC derived from CD34+ hematopoietic stem cells from leukapheresis (Lk) and control adult reticulocytes from peripheral blood (PB reticulocytes). Measures were performed in 100 cells with an optical micrometer and P values calculated by the Mann-Whitney test. One representative experiment.
The size of the cultured RBC was measured by microscopy and compared to that of cultured RBC derived from hESC, CD34+ hematopoietic stem cells from leukapheresis and control adult reticulocytes from peripheral blood. Results were comparable, with a mean diameter of 9.7±1.5, 10±2.4, 9.3±1.5 and 8.8±1.2 μm for cultured RBC from hiPSC, hESC, CD34+ hematopoietic stem cells from leukapheresis and native peripheral blood reticulocytes, respectively (P values not significant) (Figure 4B).
To analyze the type of hemoglobin synthesized at D16 of the erythroid differentiation (i.e. the orthochromatic stage) we combined the identification of the globin chains by RP-LC and spectral analysis with the study of tetrameric hemoglobin by CE-HPLC.
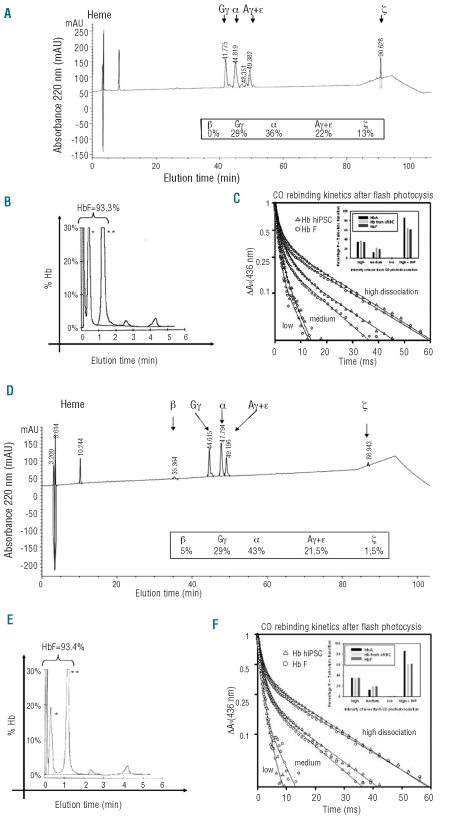
Separation of the globin chains by RP-LC permitted quantification of the hemoglobin production of the mature cultured RBC from: (i) both fetal and adult hiPSC with 29% to 31% γG, 36% to 45% α, 20% to 22% γA + ɛ and 4% to 13% ζ-chains, and (ii) hESC with 5% β, 29% γG, 43% α, 21.5% γA + ɛ and 1.5% ζ chains.
In all cases, there was a largely predominant synthesis of fetal chains (γG + γA: 50% to 51%), with 36% to 45% α chains. These results were confirmed by mass spectrometric identification of the fractions eluted by RP-LC (Figure 5A and 5D).
Figure 5.
Hemoglobin analyses and functionality of erythroid cells generated from hiPSC-IMR90-16 (A, B, C) and hESC-H1. (D, E, F). (AD) Representative RP-LC profiles of globin chains identified by mass spectrometry for D16 mature cultured RBC. (B, and E) CE-HPLC analysis of the hemoglobin for mature cultured RBC at D16. [*acetylated HbF, **non-acetylated HbF]. (C, and F) CO rebinding kinetics after flash photolysis of cultured RBC hemoglobin (black curves with triangles) and hemoglobin from control native cord blood RBC (black curves with circles). The two samples show similar binding properties, including the allosteric transition. The fraction of T-state tetramers is similar for hemoglobin from cultured RBC control, hiPSC and hESC at different intensities of laser photodissociation, The increase in allosteric transition to the low affinity T-state tetramers upon addition of 0.1 mM inositol hexaphosphate (IHP) is larger for the HbA than that for HbF and hemoglobin from cultured RBC, hiPSC and hESC with regard to the photodissociation level (about 30 % higher T-state transition). This is explained by a lower allosteric response of HbF to the IHP binding as already reported for 2,3 DPG.25
An analysis of tetrameric hemoglobin by CE-HPLC showed the synthesis of 93% HbF and the profiles were the same as those obtained for cultured RBC derived from hESC (Figure 5B and 5E). The two latter peaks which represented 2.3% and 4.3% of the total hemoglobin could be embryonic hemoglobin (HbGower1/HbGower2). These results fit with RP-LC and mass spectrometry data demonstrating the synthesis of fetal hemoglobin in its tetrameric form in cultured RBC derived from hiPSC.
The functionality of the hemoglobin generated from hiPSC and hESC was assessed by ligand binding kinetics after flash photolysis (Figures 5C and 5F). The bimolecular kinetics after photodissociation of CO provides a sensitive probe of hemoglobin function. Thus, two phases are observed which correspond to the two hemoglobin quaternary states for ligand binding. The fast component arises from the tetramers in the R-state and the slow component from the T-state tetramers. The R-state CO bimolecular rate is typically 6×106 M−1s−1 while the T-state rate is about 20 times slower. At high levels of photodissociation the main species are singly and doubly bound, while at intermediate levels it is better to probe the doubly-bound tetramers, a form difficult to study by equilibrium techniques; finally, at low levels CO binding to triply-liganded species is mainly measured. Therefore, by varying the photodissociation level one can probe the allosteric equilibrium for the different partially liganded species. The R to T transition in normal HbA occurs after binding of a second ligand to the hemoglobin tetramer. In the presence of an allosteric effector such as inositol hexaphosphate, the switch-over point occurs later and the intrinsic R and T affinities also decrease. The CO rebinding kinetics for hemoglobin from cultured RBC were almost identical to those for a sample of fetal blood, as expected from the HPLC analysis showing a large amount of HbF in the cultured RBC. After addition of inositol hexaphosphate, the R to T transition was displaced towards the low affinity tetramers to a similar extent as in fetal blood (same magnitude of the slow T-state rebinding phase). The CO flash photolysis experiments thus confirmed that the HbF in cultured RBC is functional not only under physiological conditions but also in response to a potent allosteric effector.
Discussion
The large scale ex vivo manufacture of RBC is critically dependent on the availability of simple culture protocols. In work published to date in hESC lines, some groups favor cellular co-culture on stroma and formation of colonies,9,10 while other teams focus on a first phase of embryoid body formation followed by a phase of erythroid maturation either in the presence of OP9 cells8 or by colony formation.11 The erythroid commitment is complete but maturation is blocked at the stage of acidophilic erythroblasts. Analysis of the globin chains by immunofluorescence on slides, qRT-PCR or mass spectrometry reveals the presence of ζ, ɛ, α and γ chains. However none of these techniques is suitable for development under Good Manufacturing Practice culture conditions.
Our approach differs conceptually in that it has two steps: (i) differentiation of hiPSC by formation of embryoid bodies with indispensable conditioning in the presence of cytokines and human plasma to obtain early erythroid commitment, and (ii) differentiation/maturation to the stage of RBC in the presence of cytokines. The combination of these two steps enables reconstitution of definitive erythropoiesis as it starts during the fifth week of gestation in the aorta-gonad-mesonephros region, before migrating to the fetal liver and then to the bone marrow. The erythroid cells matured little by little, leading to the production of enucleated RBC which were normocytic and contained fetal (α2γ2) and then adult (α2β2) hemoglobin.23
One group recently reported that hiPSC are capable of generating hemangioblast/blast cells, endothelial cells, hematopoietic cells and erythroblastic differentiation without terminal differentiation.17 This group mainly showed functional differences between hESC and hiPSC including significantly increased apoptosis, severely limited growth and expansion capability including hematopoietic colonies. Thanks to a simple methodology avoiding stroma and xenogenic proteins, we report, for the first time, the generation of mature cultured RBC synthesizing fetal hemoglobin in a functional tetrameric form from hiPSC. These findings provide strong evidence that hiPSC act very similarily to hESC, and differentiation protocols established for hESC can be transposable to hiPSC. Indeed, we did not observe any difference between hiPSC and hESC lines in terms of erythroid commitment and expression of erythroid markers, type of hemoglobin or functionality of hemoglobin. However, as described by Lanza’s group,17 we observed a difference between hiPSC and hESC lines regarding the amplification and percentage of enucleated RBC.
The possibility of manufacturing cultured RBC concentrates would lead to many dramatic improvements in transfusion practice. First, patients allo-immunized against RBC antigens account for 1% to 3% of the total population of transfused patients: their management by blood transfusion centers is extremely time-consuming and expensive. Furthermore, some of these patients develop antibodies that exclude the possibility of future transfusions. We are able to show, in a large series (n=16,486), that only ten clones of hiPSC representing the most useful RBC phenotype combinations would be sufficient to manage 99.43% of allo-immunized patients. Furthermore, we can anticipate that three clones would enable management of more than 99% of these situations, avoiding testing for hundred of thousands of RBC phenotypes in voluntary blood donors.
Second, avoidance of allo-immunization is the best long-term policy for frequently transfused patients such as those with sickle-cell disease or thalassemia. However, all blood transfusion services have difficulty in always providing these patients with RBC concentrates that are fully identical or at least pheno-compatible for the Rh and Kell blood groups. The ten already proposed hiPS clones would enable almost all these patients to have RBC concentrates fully compatible for Rh, Kell, Duffy and Kidd blood groups, providing them much safer transfusions than they currently receive.
Third, 165 different rare phenotypes/genotypes are known, and even Rare Blood Banks have difficulty in providing compatible RBC for the corresponding patients. As every Rare Blood Bank already has a registry of known donors, through international cooperation it would be easy to collect the cellular material necessary to prepare almost all existing rare RBC phenotypes, which would be able to be given to all patients requiring them within a minimal time, which, at present, is not at all the case for too many of them.
Finally, on a routine basis, laboratories in charge of immunological transfusion safety use RBC panels for RBC antibody screening and identification. Manufacturers of these in vitro diagnostic devices encounter difficulties in making such panels on a regular basis with an adequate combination of antigens, i.e. the best configuration of informative cells for all tested blood group antigens, as well as the maximum number of homozygous expressions for Duffy, Kidd and MNS alleles. As a result, producing the ideal RBC phenotype combination using cultured RBC from hiPSC, at least for screening, if not for identification panels, would enhance the global quality of RBC transfusion immunological surveillance.
Apart from the very specific problem of rare phenotypes, it can easily be extrapolated that no more than one-fiftieth of selected hiPS lines would deliver the required antigenic diversity for transfusion support of patients worldwide, if this possibility was considered a goal. The next steps will be to show that stem-cell-derived RBC are safe and functional in animals and ultimately in human beings. Clearly there is a long way to go and the expenses will undoubtedly be substantial. But, in addition to the improvements already mentioned, the prospect of being able to boost the global blood supply based on this concept is a most welcome one, not only to face medical crises when blood stocks run low in industrialized countries, but also to provide safe blood products adapted to the need of developing and transitional countries in which an inadequate blood supply currently results in countless deaths.
Acknowledgments
the authors would like to thank Marc Peschanski and I-STEM Institute for teaching on hESC culture methods, Michel Cailleret (I-Stem), Laurence Reutenauer (Puccio Lab, IGBMC) and Nadège Vaucamps (Puccio Lab, IGBMC) for iPS generation and characterization, and Cécile André (IGBMC) for her excellent technical assistance.
Footnotes
Funding: this work was supported by the Etablissement Français du Sang, the Association Laurette Fugain, the Association Combattre La Leucémie, the Association Française pour l’Ataxie de Friedriech (to MWD), the CEE under ERC grant 206634/ISCATAXIA (to HP) and the Association AFM.
The online version of this article has a a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Douay L, Andreu G. Ex vivo production of human red blood cells from hematopoietic stem cells: what is the future in transfusion? Transfus Med Rev. 2007;21(2):91–100. doi: 10.1016/j.tmrv.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Neildez-Nguyen TM, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarratana MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20(5):467–72. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 3.Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23(1):69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282 (5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98(19):10716–21. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108(6):2095–105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu C, Hanson E, Olivier E, Inada M, Kaufman DS, Gupta S, et al. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33(12):1450–8. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Chang KH, Nelson AM, Cao H, Wang L, Nakamoto B, Ware CB, et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108(5):1515–23. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu C, Olivier EN, Velho M, Bouhassira EE. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111(4):2400–8. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma F, Ebihara Y, Umeda K, Sakai H, Hanada S, Zhang H, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci USA. 2008;105(35):13087–92. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu SJ, Feng Q, Park JS, Vida L, Lee BS, Strausbauch M, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112(12):4475–84. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 14.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 15.Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27(3):559–67. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119(9):2818–29. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Q, Lu SJ, Klimanskaya I, Gomes I, Kim D, Chung Y, et al. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28(4):704–12. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- 18.Wajcman H, Prehu C, Bardakdjian-Michau J, Prome D, Riou J, Godart C, et al. Abnormal hemoglobins: laboratory methods. Hemoglobin. 2001;25(2):169–81. doi: 10.1081/hem-100104026. [DOI] [PubMed] [Google Scholar]
- 19.Zanella-Cleon I, Becchi M, Lacan P, Giordano PC, Wajcman H, Francina A. Detection of a thalassemic alpha-chain variant (Hemoglobin Groene Hart) by reversed-phase liquid chromatography. Clin Chem. 2008;54(6):1053–9. doi: 10.1373/clinchem.2007.097857. [DOI] [PubMed] [Google Scholar]
- 20.Marden MC, Kister J, Bohn B, Poyart C. T-state hemoglobin with four ligands bound. Biochemistry. 1988;27(5):1659–64. doi: 10.1021/bi00405a041. [DOI] [PubMed] [Google Scholar]
- 21.Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102(3):906–15. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 22.Cerdan C, Rouleau A, Bhatia M. VEGF-A165 augments erythropoietic development from human embryonic stem cells. Blood. 2004;103(7):2504–12. doi: 10.1182/blood-2003-07-2563. [DOI] [PubMed] [Google Scholar]
- 23.Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lievre F, Peault B. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996;87(1):67–72. [PubMed] [Google Scholar]